Peptidomic Characterization and Amino Acid Availability after Intake of Casein vs. a Casein Hydrolysate in a Pig Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment
2.2. Nitrogen Analysis and SDS-PAGE
2.3. Peptidomic Analysis
2.4. Statistical Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.-P.; Maubois, J.-L.; Beaufrère, B. Slow and Fast Dietary Proteins Differently Modulate Postprandial Protein Accretion. Proc. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef] [PubMed]
- Barbé, F.; Ménard, O.; Gouar, Y.L.; Buffière, C.; Famelart, M.-H.; Laroche, B.; Feunteun, S.L.; Rémond, D.; Dupont, D. Acid and Rennet Gels Exhibit Strong Differences in the Kinetics of Milk Protein Digestion and Amino Acid Bioavailability. Food Chem. 2014, 143, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vandenplas, Y.; Alarcon, P.; Fleischer, D.; Hernell, O.; Kolacek, S.; Laignelet, H.; Lönnerdal, B.; Raman, R.; Rigo, J.; Salvatore, S.; et al. Should Partial Hydrolysates Be Used as Starter Infant Formula? A Working Group Consensus. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Jonker, J.T.; Wijngaarden, M.A.; Kloek, J.; Groeneveld, Y.; Gerhardt, C.; Brand, R.; Kies, A.K.; Romijn, J.A.; Smit, J.W.A. Effects of Low Doses of Casein Hydrolysate on Post-Challenge Glucose and Insulin Levels. Eur. J. Intern. Med. 2011, 22, 245–248. [Google Scholar] [CrossRef]
- Drummond, E.; Flynn, S.; Whelan, H.; Nongonierma, A.B.; Holton, T.A.; Robinson, A.; Egan, T.; Cagney, G.; Shields, D.C.; Gibney, E.R.; et al. Casein Hydrolysate with Glycemic Control Properties: Evidence from Cells, Animal Models, and Humans. J. Agric. Food Chem. 2018, 66, 4352–4363. [Google Scholar] [CrossRef]
- Shen, J.; Mu, C.; Wang, H.; Huang, Z.; Yu, K.; Zoetendal, E.G.; Zhu, W. Stimulation of Gastric Transit Function Driven by Hydrolyzed Casein Increases Small Intestinal Carbohydrate Availability and Its Microbial Metabolism. Mol. Nutr. Food Res. 2020, 64, 2000250. [Google Scholar] [CrossRef]
- Dalziel, J.; Young, W.; McKenzie, C.; Haggarty, N.; Roy, N. Gastric Emptying and Gastrointestinal Transit Compared among Native and Hydrolyzed Whey and Casein Milk Proteins in an Aged Rat Model. Nutrients 2017, 9, 1351. [Google Scholar] [CrossRef]
- Mihatsch, W.; Högel, J.; Pohlandt, F. Hydrolysed Protein Accelerates the Gastrointestinal Transport of Formula in Preterm Infants. Acta Paediatr. 2001, 90, 196–198. [Google Scholar] [CrossRef]
- Mihatsch, W.A.; Franz, A.R.; Kuhnt, B.; Högel, J.; Pohlandt, F. Hydrolysis of Casein Accelerates Gastrointestinal Transit via Reduction of Opioid Receptor Agonists Released from Casein in Rats. Neonatology 2005, 87, 160–163. [Google Scholar] [CrossRef]
- Picariello, G.; Mamone, G.; Nitride, C.; Addeo, F.; Ferranti, P. Protein Digestomics: Integrated Platforms to Study Food-Protein Digestion and Derived Functional and Active Peptides. TrAC Trends Anal. Chem. 2013, 52, 120–134. [Google Scholar] [CrossRef]
- Caira, S.; Picariello, G.; Renzone, G.; Arena, S.; Troise, A.D.; De Pascale, S.; Ciaravolo, V.; Pinto, G.; Addeo, F.; Scaloni, A. Recent Developments in Peptidomics for the Quali-Quantitative Analysis of Food-Derived Peptides in Human Body Fluids and Tissues. Trends Food Sci. Technol. 2022, 126, 41–60. [Google Scholar] [CrossRef]
- Basilicata, M.G.; Pepe, G.; Sommella, E.; Ostacolo, C.; Manfra, M.; Sosto, G.; Pagano, G.; Novellino, E.; Campiglia, P. Peptidome Profiles and Bioactivity Elucidation of Buffalo-Milk Dairy Products after Gastrointestinal Digestion. Food Res. Int. 2018, 105, 1003–1010. [Google Scholar] [CrossRef] [PubMed]
- Grosvenor, A.J.; Haigh, B.J.; Dyer, J.M. Digestion Proteomics: Tracking Lactoferrin Truncation and Peptide Release during Simulated Gastric Digestion. Food Funct. 2014, 5, 2699–2705. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rivera, L.; Ménard, O.; Recio, I.; Dupont, D. Peptide Mapping during Dynamic Gastric Digestion of Heated and Unheated Skimmed Milk Powder. Food Res. Int. 2015, 77, 132–139. [Google Scholar] [CrossRef]
- Cattaneo, S.; Stuknytė, M.; Masotti, F.; De Noni, I. Protein Breakdown and Release of β-Casomorphins during in Vitro Gastro-Intestinal Digestion of Sterilised Model Systems of Liquid Infant Formula. Food Chem. 2017, 217, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Boutrou, R.; Gaudichon, C.; Dupont, D.; Jardin, J.; Airinei, G.; Marsset-Baglieri, A.; Benamouzig, R.; Tomé, D.; Leonil, J. Sequential Release of Milk Protein Derived Bioactive Peptides in the Jejunum in Healthy Humans. Am. J. Clin. Nutr. 2013, 97, 1314–1323. [Google Scholar] [CrossRef]
- Sanchón, J.; Fernández-Tomé, S.; Miralles, B.; Hernández-Ledesma, B.; Tomé, D.; Gaudichon, C.; Recio, I. Protein Degradation and Peptide Release from Milk Proteins in Human Jejunum. Comparison with in Vitro Gastrointestinal Simulation. Food Chem. 2018, 239, 486–494. [Google Scholar] [CrossRef]
- Egger, L.; Schlegel, P.; Baumann, C.; Stoffers, H.; Guggisberg, D.; Brügger, C.; Dürr, D.; Stoll, P.; Vergères, G.; Portmann, R. Physiological Comparability of the Harmonized INFOGEST in Vitro Digestion Method to in Vivo Pig Digestion. Food Res. Int. 2017, 102, 567–574. [Google Scholar] [CrossRef]
- Contreras, M.d.M.; Sevilla, M.A.; Monroy-Ruiz, J.; Amigo, L.; Gómez-Sala, B.; Molina, E.; Ramos, M.; Recio, I. Food-Grade Production of an Antihypertensive Casein Hydrolysate and Resistance of Active Peptides to Drying and Storage. Int. Dairy J. 2011, 21, 470–476. [Google Scholar] [CrossRef]
- Miralles, B.; Sanchón, J.; Sánchez-Rivera, L.; Martínez-Maqueda, D.; Le Gouar, Y.; Dupont, D.; Amigo, L.; Recio, I. Digestion of Micellar Casein in Duodenum Cannulated Pigs. Correlation between in Vitro Simulated Gastric Digestion and in Vivo Data. Food Chem. 2021, 343, 128424. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2 Elegant Graphics for Data Analysis; Use R! Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Wagih, O. Ggseqlogo: A Versatile R Package for Drawing Sequence Logos. Bioinformatics 2017, 33, 3645–3647. [Google Scholar] [CrossRef]
- Caraux, G.; Pinloche, S. PermutMatrix: A Graphical Environment to Arrange Gene Expression Profiles in Optimal Linear Order. Bioinformatics 2005, 21, 1280–1281. [Google Scholar] [CrossRef]
- Mahe, S.; Roos, N.; Benamouzig, R.; Rautureau, J.; Tome, D. Gastrojejunal Kinetics and the Digestion of [15N] F3- Lactoglobulin and Casein in Humans: The Influence of the Nature and Quantity of The. Am. J. Clin. Nutr. 1996, 63, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Barbé, F.; Le Feunteun, S.; Rémond, D.; Ménard, O.; Jardin, J.; Henry, G.; Laroche, B.; Dupont, D. Tracking the in Vivo Release of Bioactive Peptides in the Gut during Digestion: Mass Spectrometry Peptidomic Characterization of Effluents Collected in the Gut of Dairy Matrix Fed Mini-Pigs. Food Res. Int. 2014, 63 Pt B, 147–156. [Google Scholar] [CrossRef]
- Picariello, G.; Miralles, B.; Mamone, G.; Sánchez-Rivera, L.; Recio, I.; Addeo, F.; Ferranti, P. Role of Intestinal Brush Border Peptidases in the Simulated Digestion of Milk Proteins. Mol. Nutr. Food Res. 2015, 59, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Asledottir, T.; Le, T.T.; Petrat-Melin, B.; Devold, T.G.; Larsen, L.B.; Vegarud, G.E. Identification of Bioactive Peptides and Quantification of β-Casomorphin-7 from Bovine β-Casein A1, A2 and I after Ex Vivo Gastrointestinal Digestion. Int. Dairy J. 2017, 71, 98–106. [Google Scholar] [CrossRef]
- Brantl, V.; Teschemacher, H.; Henschen, A.; Lottspeich, F. Novel Opioid Peptides Derived from Casein (Beta-Casomorphins). I. Isolation from Bovine Casein Peptone. Hoppe Seylers Z Physiol. Chem. 1979, 360, 1211–1216. [Google Scholar] [CrossRef] [PubMed]
- Jinsmaa, Y.; Yoshikawa, M. Enzymatic Release of Neocasomorphin and [Beta]-Casomorphin from Bovine [Beta]-Casein. Peptides 1999, 20, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Loukas, S.; Varoucha, D.; Zioudrou, C.; Streaty, R.A.; Klee, W.A. Opioid Activities and Structures of.Alpha.-Casein-Derived Exorphins. Biochemistry 1983, 22, 4567–4573. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Martínez-Maqueda, D.; Girón, R.; Goicoechea, C.; Miralles, B.; Recio, I. Novel Peptides Derived from As1-Casein with Opioid Activity and Mucin Stimulatory Effect on HT29-MTX Cells. J. Funct. Foods 2016, 25, 466–476. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yamamoto, N.; Sakai, K.; Takano, T. Antihypertensive Effect of Sour Milk and Peptides Isolated from It That Are Inhibitors to Angiotensin I-Converting Enzyme. J. Dairy Sci. 1995, 78, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Deglaire, A.; Fromentin, C.; Fouillet, H.; Airinei, G.; Gaudichon, C.; Boutry, C.; Benamouzig, R.; Moughan, P.J.; Tomé, D.; Bos, C. Hydrolyzed Dietary Casein as Compared with the Intact Protein Reduces Postprandial Peripheral, but Not Whole-Body, Uptake of Nitrogen in Humans. Am. J. Clin. Nutr. 2009, 90, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Deglaire, A.; Moughan, P.J.; Airinei, G.; Benamouzig, R.; Tomé, D. Intact and Hydrolyzed Casein Lead to Similar Ileal Endogenous Protein and Amino Acid Flows in Adult Humans. Am. J. Clin. Nutr. 2020, 111, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Horstman, A.M.H.; Ganzevles, R.A.; Kudla, U.; Kardinaal, A.F.M.; van den Borne, J.J.G.C.; Huppertz, T. Postprandial Blood Amino Acid Concentrations in Older Adults after Consumption of Dairy Products: The Role of the Dairy Matrix. Int. Dairy J. 2021, 113, 104890. [Google Scholar] [CrossRef]
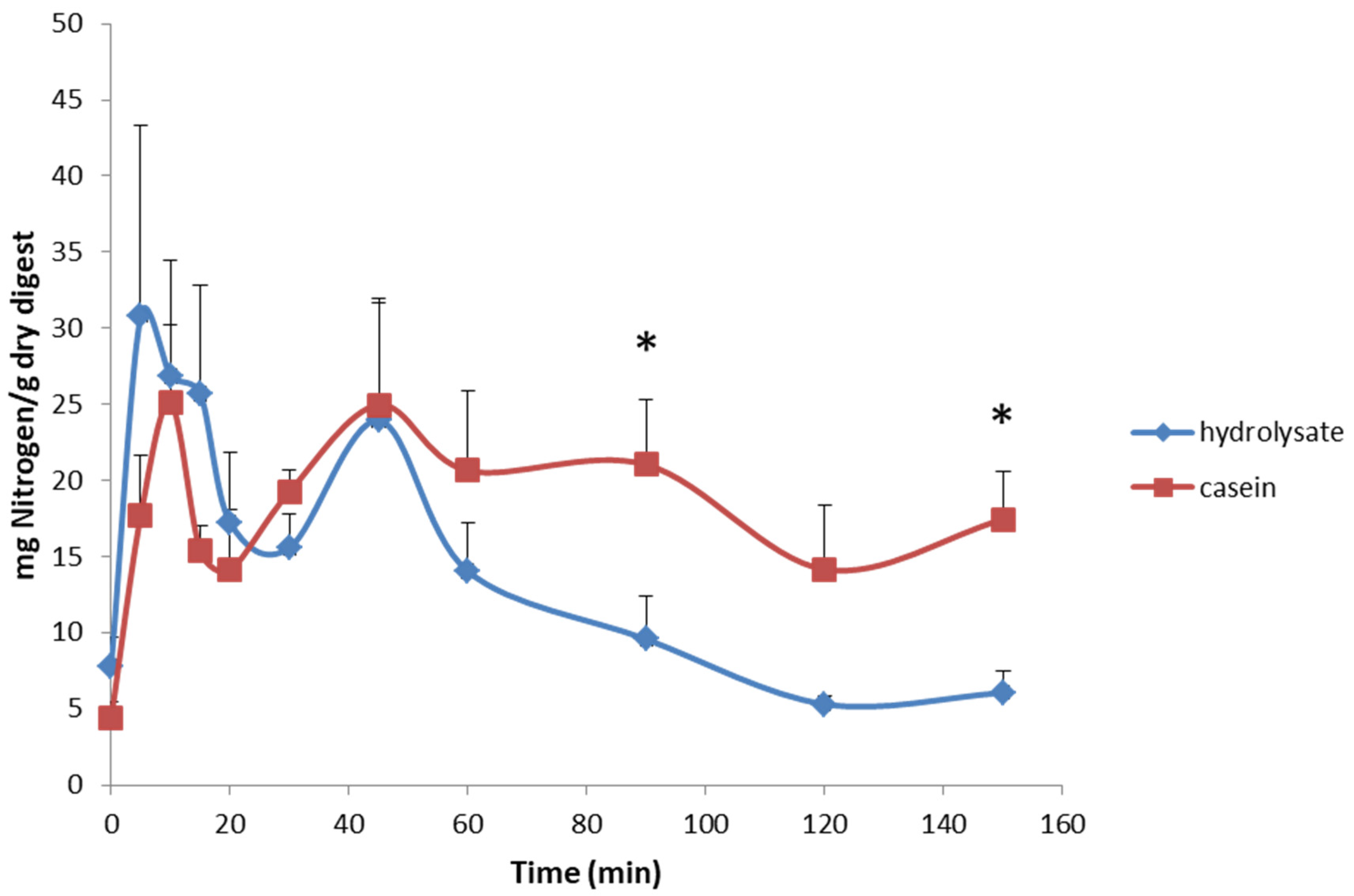

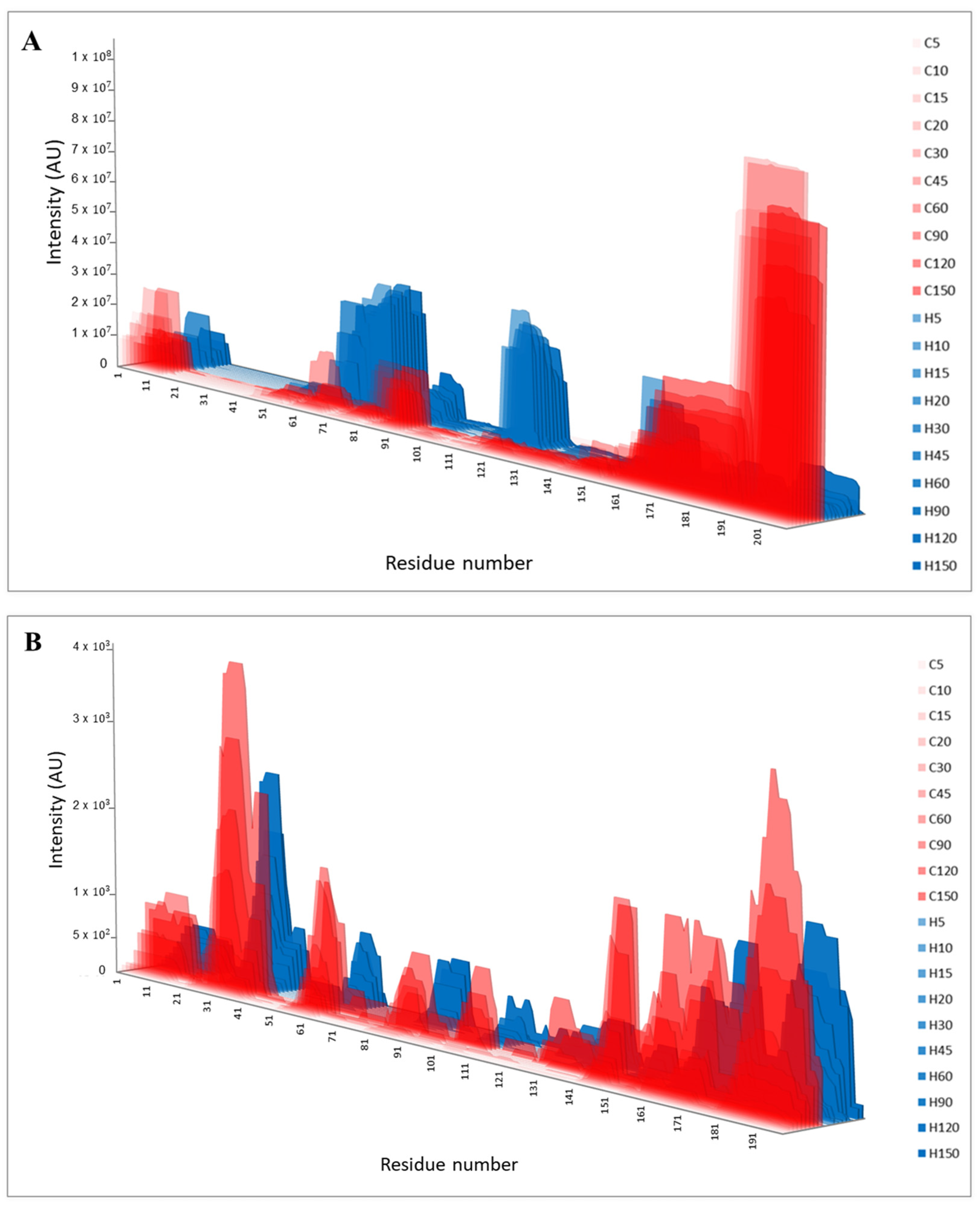
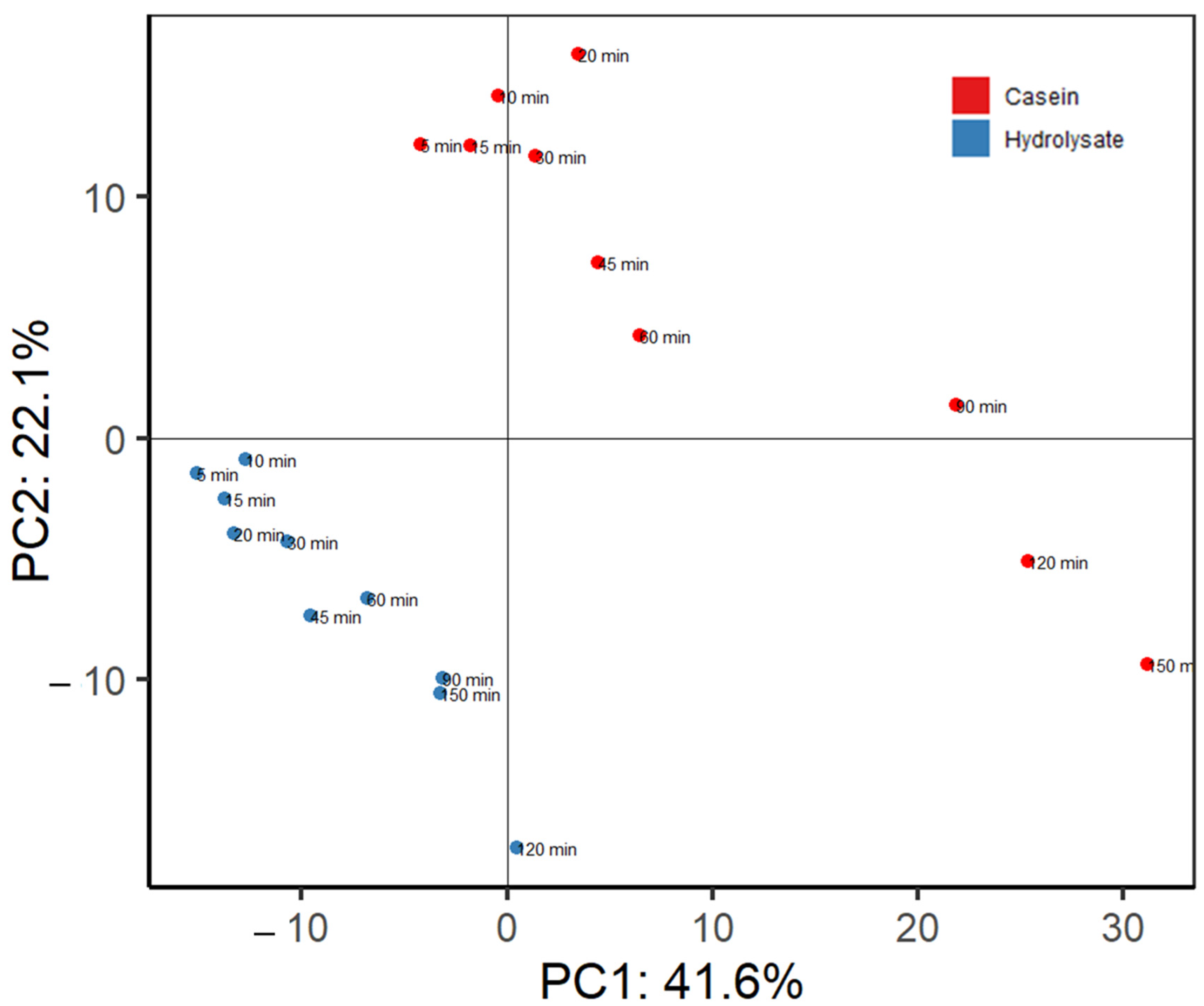
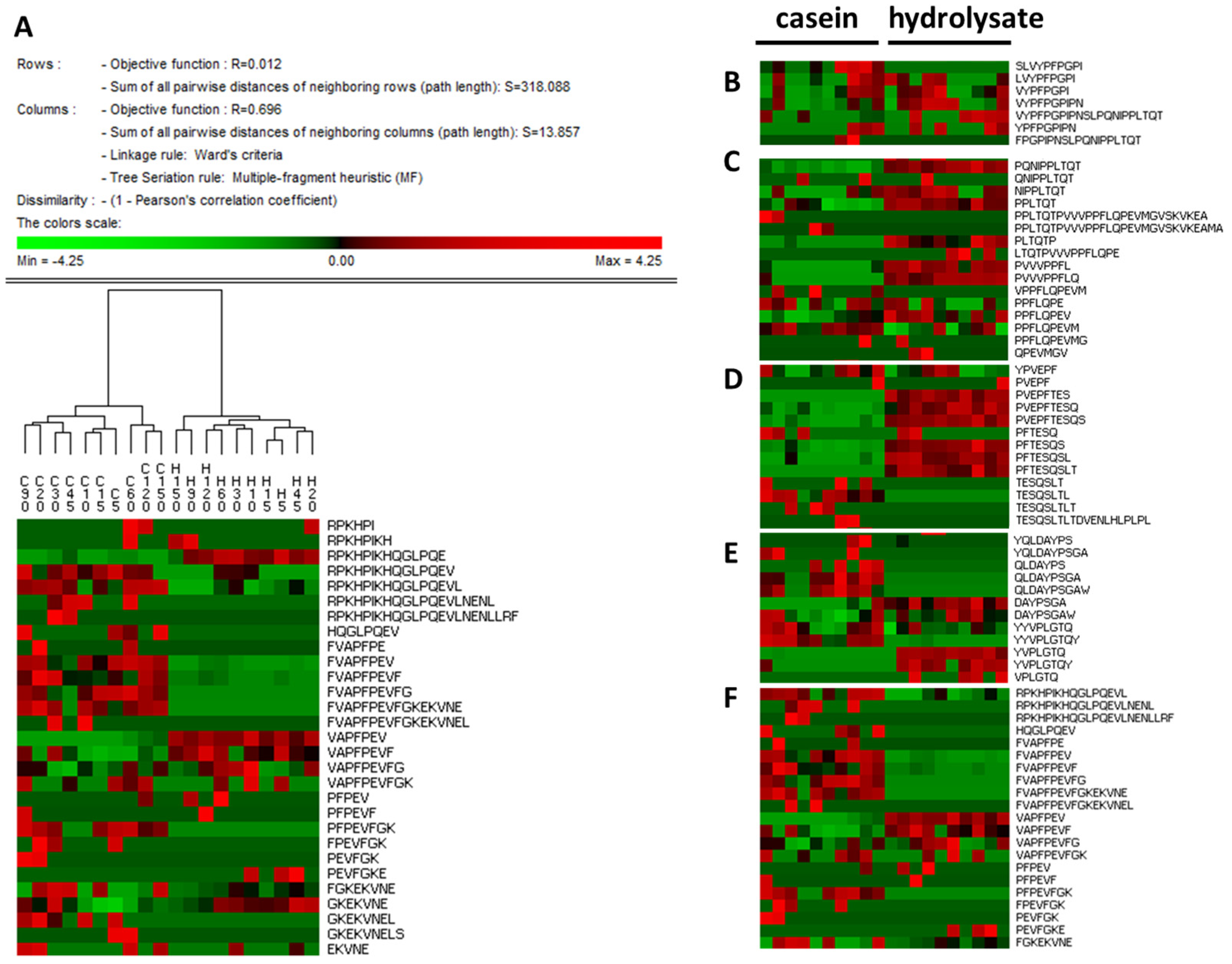

| Time | Casein | Hydrolysate | ||
|---|---|---|---|---|
| Total Peptide Number | Common Peptide Number 1 | Total Peptide Number | Common Peptide Number 1 | |
| 5 | 444 | 270 | 258 | 98 |
| 10 | 369 | 170 | 294 | 115 |
| 15 | 312 | 140 | 237 | 91 |
| 20 | 419 | 211 | 281 | 137 |
| 30 | 303 | 145 | 299 | 140 |
| 45 | 312 | 148 | 274 | 137 |
| 60 | 418 | 221 | 283 | 142 |
| 90 | 427 | 187 | 290 | 136 |
| 120 | 425 | 222 | 338 | 158 |
| 150 | 434 | 217 | 258 | 137 |
| Total 2 | 3863 | 1931 | 2812 | 1291 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Barrios, P.; Sánchez-Rivera, L.; Martínez-Maqueda, D.; Le Gouar, Y.; Dupont, D.; Miralles, B.; Recio, I. Peptidomic Characterization and Amino Acid Availability after Intake of Casein vs. a Casein Hydrolysate in a Pig Model. Nutrients 2023, 15, 1065. https://doi.org/10.3390/nu15051065
Jiménez-Barrios P, Sánchez-Rivera L, Martínez-Maqueda D, Le Gouar Y, Dupont D, Miralles B, Recio I. Peptidomic Characterization and Amino Acid Availability after Intake of Casein vs. a Casein Hydrolysate in a Pig Model. Nutrients. 2023; 15(5):1065. https://doi.org/10.3390/nu15051065
Chicago/Turabian StyleJiménez-Barrios, Pablo, Laura Sánchez-Rivera, Daniel Martínez-Maqueda, Yann Le Gouar, Didier Dupont, Beatriz Miralles, and Isidra Recio. 2023. "Peptidomic Characterization and Amino Acid Availability after Intake of Casein vs. a Casein Hydrolysate in a Pig Model" Nutrients 15, no. 5: 1065. https://doi.org/10.3390/nu15051065
APA StyleJiménez-Barrios, P., Sánchez-Rivera, L., Martínez-Maqueda, D., Le Gouar, Y., Dupont, D., Miralles, B., & Recio, I. (2023). Peptidomic Characterization and Amino Acid Availability after Intake of Casein vs. a Casein Hydrolysate in a Pig Model. Nutrients, 15(5), 1065. https://doi.org/10.3390/nu15051065








