Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods
Abstract
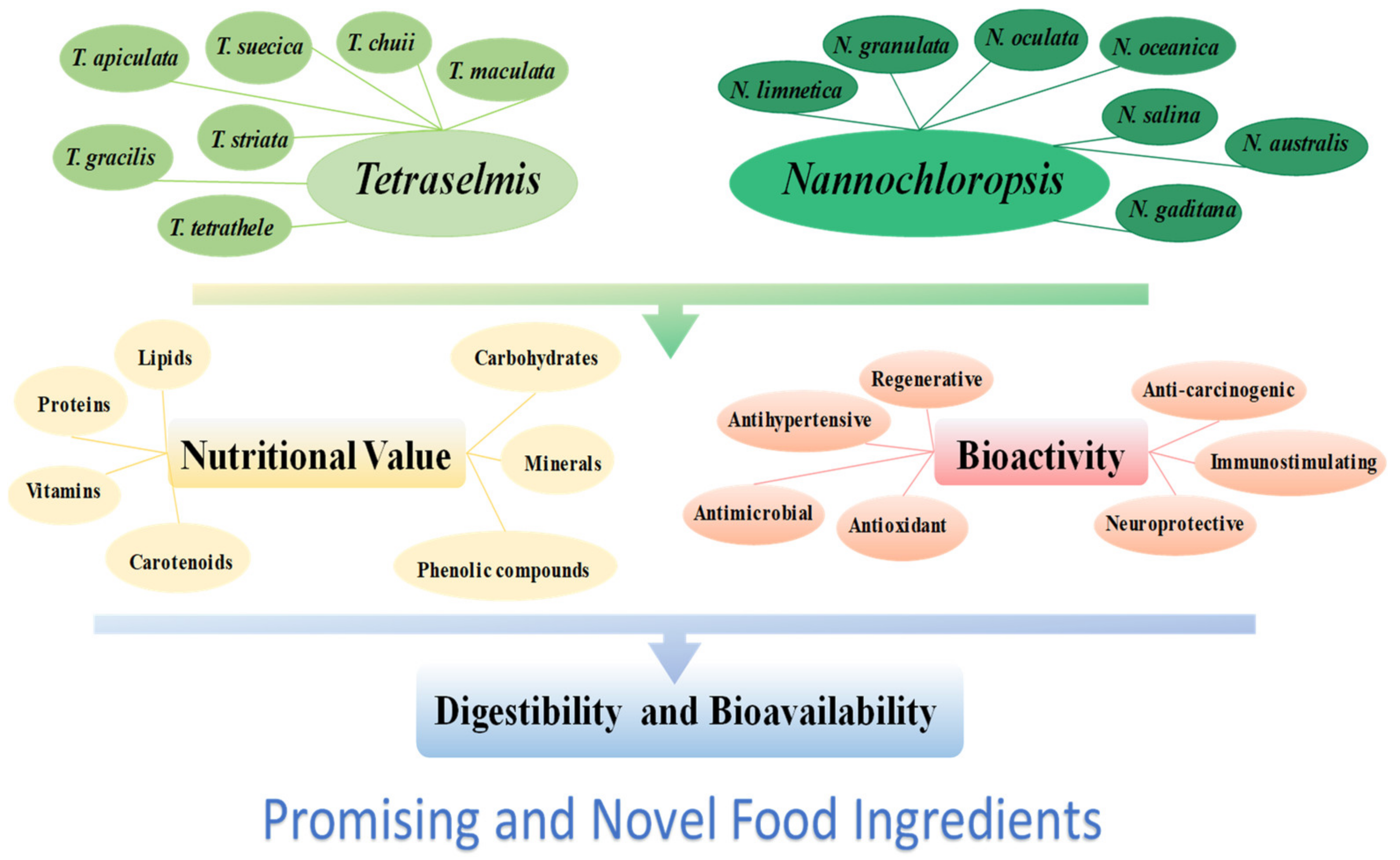
1. Introduction
2. Nutritional Value of Tetraselmis and Nannochloropsis sp. Microalgae
2.1. Proteins and Amino Acids
2.2. Lipids and Fatty Acids
2.3. Carbohydrates
2.4. Mineral Elements
2.5. Vitamins
| Vitamin | Content (mg/100 g) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. suecica1 | T. suecica2 | T. suecica3 | N. sp. CS-246 3 | T. sp. CS362 4 | T. sp. CTP4 5 | N. sp.4 | N. oculata6 | N. limnetica7 | N. salina7 | N. gaditana8 | N. oceanica9 | |
| A * | 29625 | 428000 | <25 | 220 | <4 | |||||||
| B1 | 3.23 | 62.70 | 5.10-7.00 | 10.90 | 0.18 | |||||||
| B2 | 1.91 | 4.20 | 2.50-6.20 | 2.60 | 0.53 | 2.21 | ||||||
| B3 | 8.93 | 141 | 7.98 | 11 | ||||||||
| B5 | 3.77 | 0.65 | ||||||||||
| B6 | 0.28 | 15.50 | 0.36-0.95 | 0.58 | 6.90 | |||||||
| B7 | 0.08 | 0.10-0.11 | 0.13 | |||||||||
| B9 * | 300 | 1700-2600 | 2000 | 0.02 | 2080 | |||||||
| B12 * | 50.00 | 900 | 85-170 | 195 | 7.80 | 25 | ||||||
| C | 19.10 | 49.80 | 100-320 | 300 | 79.20 | |||||||
| D2 * | 1400 | 45.0 | <35 | |||||||||
| D3 * | 35.0 | <35 | 1–48 | |||||||||
| E | 42.18 | 632 | 1980 | 18.0-35.0 | 7.00 | 20.28 | 210 | 48-233 | 2.12 | 4.41 | ||
| K * | 2800 | |||||||||||
2.6. Carotenoids
| Compound | Carotenoids (mg/g DW) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T. suecica1 | T. suecica2 | T. chuii2 | T. chuii3 | T. sp. Z3 4 | T. sp. C6 4 | N. gaditana 1 | N. gaditana5 | N. sp. BR2 2 | N. granulata3 | N. sp. 5 | N. salina6 | N. limnetica6 | |
| Carotene | |||||||||||||
| α-carotene | 0.41 | 0.20 | 0.17 | 1.70 | 0.02 | 0.08 | |||||||
| β-carotene | 0.43 | 0.62 | 0.94 | 1.00 | 0.002 | 0.01 | 1.00 | 0.45 | 1.00 | 2.22 | 0.28 | ||
| Lycopene | 1.50 | ||||||||||||
| Total Carotenes | 1.15 | 1.16 | |||||||||||
| Xanthophyll | |||||||||||||
| Cantaxanthin | 0.40 | 0.40 | 0.14 | 0.003 | |||||||||
| Zeaxanthin | 0.10 | 0.01 | 0.90 | 0.10 | 0.20 | 0.58 | 0.14 | ||||||
| Violaxanthin | 0.82 | 1.41 | 0.54 | 3.36 | 4.45 | 1.08 | 4.17 | 1.68 | 1.23 | ||||
| Lutein | 0.85 | 0.48 | 0.62 | 0.60 | 0.37 | 1.65 | 0.02 | ||||||
| Anteraxanthin | 0.83 | 0.20 | 0.17 | ||||||||||
| Astaxanthin | 2.26 | 0.10 | 0.32 | ||||||||||
| Fucoxanthin | 0.05 | 0.08 | 0.01 | 0.18 | |||||||||
| Neoxanthin | 0.12 | 0.79 | 0.01 | 0.05 | 0.42 | ||||||||
| Diadinoxanthin | 1.44 | 1.18 | 0.14 | ||||||||||
| Vaucheriaxanthin | 0.09 | 0.16 | |||||||||||
| Alloxanthin | 0.13 | ||||||||||||
| Diatoxanthin | 0.14 | ||||||||||||
2.7. Phenolic Compounds
| Microalgae | Total Content | Individual Phenolic Compounds | Ref. |
|---|---|---|---|
| Nannochloropsis sp. | 1.4 | --- | [65] |
| Nannochloropsis sp. | 0.6 | Gallic, ferulic, protocatechuic, chlorogenic, hydroxybenzoic, syringic, vanillic | [67] |
| N. gaditana | 32.0 | --- | [64] |
| 22.9 | Caffeic, caffeoylglucoside, protocatechuic, p-coumaroyl tyrosine, quercetin, rhamnosyhexosyl-methyl-quercetin, apigenin-O-rutinoside, feruloylglucaricacid | [68] | |
| N. granulata | 6.0–8.0 | --- | [48] |
| N. limnetica | 5.8 | Gallic, caffeic, ferulic, cinnamic, salycilic | [57] |
| N. oculata | 2.0 | --- | [65] |
| 4.1 | --- | [69] | |
| N. salina | 6.8 | Gallic, 3,4 dihydroxy benzoic, ferulic, p-coumaric, salycilic | [57] |
| Tetraselmis sp. | 3.7 | --- | [65] |
| Tetraselmis sp. | 25.5 | --- | [64] |
| T. sp. IMP3 | 2.3 | Gallic, gentisic, catechin hydrate, 4-hydroxybenzaldehyde, vanillic, caffeic, epicatechin, ferulic salicylic, naringenin-7-glucoside, luteolin-7-o-glucoside, rutin, ellagic, quercetin | [66] |
| T. sp. CTP4 | 0.76 | Gallic, p-hydroxybenzoic, catechin hydrate, epicatechin | [66] |
| T. chuii | 8.6 | --- | [69] |
| 20.0 | --- | [48] | |
| T. suecica | 1.7 | --- | [65] |
| 28.0 | Caffeic, caffeoylglucoside, protocatechuic, dimethoxyflavone, p-coumaroyl tyrosine, apigenin-O-rutinoside, rhamnosyhexosyl-methyl-quercetin | [68] |
3. Bioactivity of Tetraselmis sp. and Nannochloropsis sp. Microalgae
3.1. Antioxidant Activity of Microalgae and Derived Compounds
3.2. Antimicrobial Activity of Tetraselmis and Nannochloropsis Microalgae
3.3. Anti-Carcinogenic Effects of Tetraselmis and Nannochloropsis sp. Microalgae Compounds
4. Digestibility of Tetraselmis sp. and Nannochloropsis sp. Microalgae Biomass
5. Digestibility of Tetraselmis sp. and Nannochloropsis sp. in Food Products
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barros de Medeiros, V.P.; da Costa, W.K.A.; da Silva, R.T.; Pimentel, T.C.; Magnani, M. Microalgae as source of functional ingredients in new-generation foods: Challenges, technological effects, biological activity, and regulatory issues. Crit. Rev. Food Sci. Nutr. 2022, 62, 4929–4950. [Google Scholar] [CrossRef] [PubMed]
- Vieira, M.V.; Turkiewicz, I.P.; Tkacz, K.; Fuentes-Grünewald, C.; Pastrana, L.M.; Fuciños, P.; Wojdyło, A.; Nowicka, P. Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules 2021, 26, 7593. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. Biology of Microalgae. In Microalgae in Health and Disease Prevention; Levine, I.A., Fleurence, J., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 23–72. [Google Scholar]
- Adarme-Vega, T.C.; Thomas-Hall, S.R.; Lim, D.K.Y.; Schenk, P.M. Effects of Long Chain Fatty Acid Synthesis and Associated Gene Expression in Microalga Tetraselmis sp. Mar. Drugs 2014, 12, 3381–3398. [Google Scholar] [CrossRef]
- Dahmen-Ben Moussa, I.; Chtourou, H.; Karray, F.; Sayadi, S.; Dhouib, A. Nitrogen or phosphorus repletion strategies for enhancing lipid or carotenoid production from Tetraselmis marina. Bioresour. Technol. 2017, 238, 325–332. [Google Scholar] [CrossRef]
- Cameron, H.; Mata, M.T.; Riquelme, C. The effect of heavy metals on the viability of Tetraselmis marina AC16-MESO and an evaluation of the potential use of this microalga in bioremediation. Peer J. 2018, 6, 9.e5295. [Google Scholar] [CrossRef] [PubMed]
- European Union. Commission implementing regulation (EU) 2017/2470 of 20 December 2017 establishing the Union list of novel foods in accordance with Regulation (EU) 2015/2283 of the European Parliament and of the Council on novel foods. Off. J. Eur. Union 2017, L351, 72–201. [Google Scholar]
- AECOSAN. Report of the Scientific Committee of the Spanish Agency for Consumer Affairs, Food Safety and Nutrition (AECOSAN) on a request for initial assessment for marketing of the dried marine microalgae Tetraselmis chuii in food supplements under Regulation (EC) No 258/97 on novel foods and novel food ingredients. AECOSAN Sci. Comm. J. 2017, 25, 11–21. [Google Scholar]
- Al-Hoqani, U.; Young, R.; Purton, S. The biotechnological potential of Nannochloropsis. Perspect. Phycol. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Lubián, L.M.; Montero, O.; Moreno-Garrido, I.; Huertas, I.E.; Sobrino, C.; González-del Valle, M.; Parés, G. Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J. Appl. Phycol. 2000, 12, 249–255. [Google Scholar] [CrossRef]
- Olofsson, M.; Lamela, T.; Nilsson, E.; Bergé, J.-P.; Del Pino, V.; Uronen, P.; Legrand, C. Combined Effects of Nitrogen Concentration and Seasonal Changes on the Production of Lipids in Nannochloropsis oculata. Mar. Drugs 2014, 12, 1891–1910. [Google Scholar] [CrossRef] [PubMed]
- Van Vooren, G.; Le Grand, F.; Legrand, J.; Cuiné, S.; Peltier, G.; Pruvost, J. Investigation of fatty acids accumulation in Nannochloropsis oculata for biodiesel application. Bioresour. Technol. 2012, 124, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Kwon, Y.M.; Kim, K.W.; Kim, J.Y.H. Exploring the Potential of Nannochloropsis sp. Extract for Cosmeceutical Applications. Mar. Drugs 2021, 19, 690. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Hegde, A.S.; Sharma, K.; Parmar, P.; Srivatsan, V. Microalgae as a sustainable source of edible proteins and bioactive peptides—Current trends and future prospects. Food Res. Int. 2022, 157, 111338. [Google Scholar] [CrossRef] [PubMed]
- Levasseur, W.; Perré, P.; Pozzobon, V. A review of high value-added molecules production by microalgae in light of the classification. Biotechnol. Adv. 2020, 41, 107545. [Google Scholar] [CrossRef]
- Pereira, H.; Silva, J.; Santos, T.; Gangadhar, K.N.; Raposo, A.; Nunes, C.; Coimbra, M.A.; Gouveia, L.; Barreira, L.; Varela, J. Nutritional Potential and Toxicological Evaluation of Tetraselmis sp. CTP4 Microalgal Biomass Produced in Industrial Photobioreactors. Molecules 2019, 24, 3192. [Google Scholar] [CrossRef] [PubMed]
- Zanella, L.; Vianello, F. Microalgae of the genus Nannochloropsis: Chemical composition and functional implications for human nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Cavonius, L.R.; Albers, E.; Undeland, I. In vitro bioaccessibility of proteins and lipids of pH-shift processed Nannochloropsis oculata microalga. Food Funct. 2016, 7, 2016–2024. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Sáez, M.I.; Martínez, T.F.; Acién, F.G.; Alarcón, F.J. Differential hydrolysis of proteins of four microalgae by the digestive enzymes of gilthead sea bream and Senegalese sole. Algal Res. 2019, 37, 145–153. [Google Scholar] [CrossRef]
- Qazi, W.M.; Ballance, S.; Uhlen, A.K.; Kousoulaki, K.; Haugen, J.-E.; Rieder, A. Protein enrichment of wheat bread with the marine green microalgae Tetraselmis chuii—Impact on dough rheology and bread quality. LWT 2021, 143, 111115. [Google Scholar] [CrossRef]
- Sandgruber, F.; Gielsdorf, A.; Baur, A.C.; Schenz, B.; Müller, S.M.; Schwerdtle, T.; Stangl, G.I.; Griehl, C.; Lorkowski, S.; Dawczynski, C. Variability in Macro- and Micronutrients of 15 Commercially Available Microalgae Powders. Mar. Drugs 2021, 19, 310. [Google Scholar] [CrossRef] [PubMed]
- Wild, K.J.; Steingaß, H.; Rodehutscord, M. Variability in nutrient composition and in vitro crude protein digestibility of 16 microalgae products. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1306–1319. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.N.; Chen, T.P.; Yang, B.; Liu, J.; Chen, F. Lipid Production from Nannochloropsis. Mar. Drugs 2016, 14, 61. [Google Scholar] [CrossRef] [PubMed]
- Moser, G.A.O.; Barrera-Alba, J.J.; Ortega, M.J.; Alves-de-Souza, C.; Bartual, A. Comparative characterization of three Tetraselmis chui (Chlorophyta) strains as sources of nutraceuticals. J. Appl. Phycol. 2022, 34, 821–835. [Google Scholar] [CrossRef]
- Volkman, J.K.; Brown, M.R.; Dunstan, G.A.; Jeffrey, S.W. The biochemical composition of marine microalgae from the class Eustigmatophyceae. J. Phycol. 1993, 29, 69–78. [Google Scholar] [CrossRef]
- Krienitz, L.; Wirth, M. The high content of polyunsaturated fatty acids in Nannochloropsis limnetica (Eustigmatophyceae) and its implication for food web interactions, freshwater aquaculture and biotechnology. Limnologica 2006, 36, 204–210. [Google Scholar] [CrossRef]
- Verspreet, J.; Soetemans, L.; Gargan, C.; Hayes, M.; Bastiaens, L. Nutritional Profiling and Preliminary Bioactivity Screening of Five Micro-Algae Strains Cultivated in Northwest Europe. Foods 2021, 10, 1516. [Google Scholar] [CrossRef] [PubMed]
- Banskota, A.H.; Sperker, S.; Stefanova, R.; McGinn, P.J.; O’Leary, S.J.B. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019, 31, 309–318. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E.; Vogt, G.; Gislerød, H.R. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Bartley, M.L.; Boeing, W.J.; Daniel, D.; Dungan, B.N.; Schaub, T. Optimization of environmental parameters for Nannochloropsis salina growth and lipid content using the response surface method and invading organisms. J. Appl. Phycol. 2016, 28, 15–24. [Google Scholar] [CrossRef]
- Rebolloso-Fuentes, M.M.; Navarro-Pérez, A.; García-Camacho, F.; Ramos-Miras, J.J.; Guil-Guerrero, J.L. Biomass nutrient profiles of the microalga Nannochloropsis. J. Agric. Food Chem. 2001, 49, 2966–2972. [Google Scholar] [CrossRef]
- Van Wagenen, J.; Miller, T.W.; Hobbs, S.; Hook, P.; Crowe, B.; Huesemann, M. Effects of Light and Temperature on Fatty Acid Production in Nannochloropsis Salina. Energies 2012, 5, 731–740. [Google Scholar] [CrossRef]
- Ahmmed, M.K.; Ahmmed, F.; Tian, H.; Carne, A.; Bekhit, A.E.-D. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food Sci. Food Saf. 2020, 19, 64–123. [Google Scholar] [CrossRef] [PubMed]
- Couto, D.; Conde, T.A.; Melo, T.; Neves, B.; Costa, M.; Cunha, P.; Guerra, I.; Correia, N.; Silva, J.T.; Pereira, H.; et al. Effects of outdoor and indoor cultivation on the polar lipid composition and antioxidant activity of Nannochloropsis oceanica and Nannochloropsis limnetica: A lipidomics perspective. Algal Res. 2022, 64, 102718. [Google Scholar] [CrossRef]
- Mitra, M.; Patidar, S.K.; Mishra, S. Integrated process of two stage cultivation of Nannochloropsis sp. for nutraceutically valuable eicosapentaenoic acid along with biodiesel. Bioresour. Technol. 2015, 193, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Grubišic, M.; Šantek, B.; Zoric, Z.; Cošic, Z.; Vrana, I.; Gašparovic, B.; Což-Rakovac, R.; Šantek, M.I. Bioprospecting of Microalgae Isolated from the Adriatic Sea: Characterization of Biomass, Pigment, Lipid and Fatty Acid Composition, and Antioxidant and Antimicrobial Activity. Molecules 2022, 27, 1248. [Google Scholar] [CrossRef]
- Banskota, A.H.; Stefanova, R.; Sperker, S.; McGinn, P.J. New diacylglyceryltrimethylhomoserines from the marine microalga Nannochloropsis granulata and their nitric oxide inhibitory activity. J. Appl. Phycol. 2013, 25, 1513–1521. [Google Scholar] [CrossRef]
- Kagan, M.L.; West, A.L.; Zante, C.; Calder, P.C. Acute appearance of fatty acids in human plasma—A comparative study between polar-lipid rich oil from the microalgae Nannochloropsis oculata and krill oil in healthy young males. Lipids Health Dis. 2013, 12, 102. [Google Scholar] [CrossRef]
- Rao, A.; Briskey, D.; Nalley, J.O.; Ganuza, E. Omega-3 Eicosapentaenoic Acid (EPA) Rich Extract from the Microalga Nannochloropsis Decreases Cholesterol in Healthy Individuals: A Double-Blind, Randomized, Placebo-Controlled, Three-Month Supplementation Study. Nutrients 2020, 12, 1869. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.R. The amino-acid and sugar composition of 16 species of microalgae used in mariculture. J. Exp. Mar. Biol. Ecol. 1991, 145, 79–99. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Sanchez del Pulgar, J.; Aguzzi, A.; Caproni, R.; Gabrielli, P.; Lombardi-Boccia, G. Chemical characterization and nutritional evaluation of microalgal biomass from large-scale production: A comparative study of five species. Eur. Food Res. Technol. 2020, 246, 323–332. [Google Scholar] [CrossRef]
- Vogler, B.W.; Brannum, J.; Chung, J.W.; Seger, M.; Posewitz, M.C. Characterization of the Nannochloropsis gaditana storage carbohydrate: A 1,3-beta glucan with limited 1,6-branching. Algal Res. 2018, 36, 152–158. [Google Scholar] [CrossRef]
- Espinoza, D.; Contreras-Porcia, L.; Ehrenfeld, N. B-glucans, production and properties in microalgae with emphasis on Nannochloropsis genus (Ochrophyta, Eustigmatales). Rev. Biol. Mar. Oceanogr. 2017, 52, 33–49. [Google Scholar]
- Becker, B.; Becker, D.; Kamerling, J.P.; Melkonian, M. 2-Keto-sugar acids in green flagellates: A chemical marker for Prasinophycean scales. J. Phycol. 1991, 27, 498–504. [Google Scholar] [CrossRef]
- Kermanshahi-Pour, A.; Sommer, T.J.; Anastas, P.T.; Zimmerman, J.B. Enzymatic and acid hydrolysis of Tetraselmis suecica for polysaccharide characterization. Bioresour. Technol. 2014, 173, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Templeton, D.W.; Quinn, M.; Van Wychen, S.; Hyman, D.; Laurens, L.M.L. Separation and quantification of microalgal carbohydrates. J. Chromatogr. A 2012, 1270, 225–234. [Google Scholar] [CrossRef]
- Tibbetts, S.M.; Milley, J.E.; Lall, S.P. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J. Appl. Phycol. 2015, 27, 1109–1119. [Google Scholar] [CrossRef]
- Ampofo, J.; Abbey, L. Microalgae: Bioactive Composition, Health Benefits, Safety and Prospects as Potential High-Value Ingredients for the Functional Food Industry. Foods 2022, 11, 1744. [Google Scholar] [CrossRef] [PubMed]
- Del Mondo, A.; Smerilli, A.; Sane, E.; Sansone, C.; Brunet, C. Challenging microalgal vitamins for human health. Microb. Cell Factories 2020, 19, 201. [Google Scholar] [CrossRef]
- De Roeck-Holtzhauer, Y.; Quere, I.; Claire, C. Vitamin analysis of five planktonic microalgae and one macroalga. J. Appl. Phycol. 1991, 3, 259–264. [Google Scholar] [CrossRef]
- Fabregas, J.; Herrero, C. Vitamin content of four marine microalgae. Potential use as source of vitamins in nutrition. J. Ind. Microbiol. 1990, 5, 259–264. [Google Scholar] [CrossRef]
- Santiago-Morales, I.S.; Trujillo-Valle, L.; Márquez-Rocha, F.J.; López Hernández, J.F. Tocopherols, Phycocyanin and Superoxide Dismutase from Microalgae: As Potential Food Antioxidants. Appl. Food Biotechnol. 2018, 5, 19–27. [Google Scholar] [CrossRef]
- Brown, M.R.; Mular, M.; Miller, I.; Farmer, C.; Trenerry, C. The vitamin content of microalgae used in aquaculture. J. Appl. Phycol. 1999, 11, 247–255. [Google Scholar] [CrossRef]
- Edelmann, M.; Aalto, S.; Chamlagain, B.; Kariluoto, S.; Piironen, V. Riboflavin, niacin, folate and vitamin B12 in commercial microalgae powders. J. Food Comp. Anal. 2019, 82, 103226. [Google Scholar] [CrossRef]
- Durmaz, Y. Vitamin E (α-tocopherol) production by the marine microalgae Nannochloropsis oculata (Eustigmatophyceae) in nitrogen limitation. Aquaculture 2007, 272, 717–722. [Google Scholar] [CrossRef]
- Safafar, H.; van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, Phenolic Compounds and Tocopherols Contribute to the Antioxidative Properties of Some Microalgae Species Grown on Industrial Wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef]
- Ljubic, A.; Jacobsen, C.; Holdt, S.L.; Jakobsen, J. Microalgae Nannochloropsis oceanica as a future new natural source of vitamin D3. Food Chem. 2020, 320, 126627. [Google Scholar] [CrossRef]
- Goiris, K.; Van Colen, W.; Wilches, I.; Leon-Tamariz, F.; De Cooman, L.; Muylaert, K. Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Res. 2015, 7, 51–57. [Google Scholar] [CrossRef]
- Di Lena, G.; Casini, I.; Lucarini, M.; Lombardi-Boccia, G. Carotenoid profiling of five microalgae species from large-scale production. Food Res. Int. 2019, 120, 810–818. [Google Scholar] [CrossRef]
- Sathasivam, R.; Ki, J.S. A Review of the Biological Activities of Microalgal Carotenoids and Their Potential Use in Healthcare and Cosmetic Industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 2014, 165, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Ryckebosch, E.; Bruneel, C.; Termote-Verhalle, R.; Muylaert, K.; Foubert, I. Influence of extraction solvent system on extractability of lipid components from different microalgae species. Algal Res. 2014, 3, 36–43. [Google Scholar] [CrossRef]
- Maadane, A.; Merghoub, N.; Ainane, T.; El Arroussi, H.; Benhima, R.; Amzazi, S.; Bakri, Y.; Wahby, I. Antioxidant activity of some Moroccan marine microalgae: Pufa profiles, carotenoids and phenolic content. J. Biotechnol. 2015, 215, 13–19. [Google Scholar] [CrossRef]
- Goiris, K.; Muylaert, K.; Fraeye, I.; Foubert, I.; De Brabanter, J.; De Cooman, L. Antioxidant potential of microalgae in relation to their phenolic and carotenoid content. J. Appl. Phycol. 2012, 24, 1477–1486. [Google Scholar] [CrossRef]
- Cardoso, C.; Pereira, H.; Franca, J.; Matos, J.; Monteiro, I.; Pousao-Ferreira, P.; Gomes, A.; Barreira, L.; Varela, J.; Neng, N.; et al. Lipid composition and some bioactivities of 3 newly isolated microalgae (Tetraselmis sp. IMP3, Tetraselmis sp. CTP4, and Skeletonema sp.). Aquac. Int. 2020, 28, 711–727. [Google Scholar] [CrossRef]
- Scaglioni, P.T.; Quadros, L.; de Paula, M.; Furlong, V.B.; Abreu, P.C.; Badiale-Furlong, E. Inhibition of Enzymatic and Oxidative Processes by Phenolic Extracts from Spirulina sp. and Nannochloropsis sp. Food Technol. Biotechnol. 2018, 56, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Haoujar, I.; Cacciola, F.; Abrini, J.; Mangraviti, D.; Giuffrida, D.; El Majdoub, Y.O.; Kounnoun, A.; Miceli, N.; Taviano, M.F.; Mondello, L.; et al. The Contribution of Carotenoids, Phenolic Compounds, and Flavonoids to the Antioxidative Properties of Marine Microalgae Isolated from Mediterranean Morocco. Molecules 2019, 24, 4037. [Google Scholar] [CrossRef] [PubMed]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Albericio, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Dolganyuk, V.; Belova, D.; Babich, O.; Prosekov, A.; Ivanova, S.; Katserov, D.; Patyukov, N.; Sukhikh, S. Microalgae: A Promising Source of Valuable Bioproducts. Biomolecules 2020, 10, 1153. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for High-Value Products Towards Human Health and Nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Listing the World’s Algae. Available online: www.algaebase.org (accessed on 18 October 2022).
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar]
- Sen, S.; Chakraborty, R.; Sridhar, C.; Reddy, Y.; De, B. Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int. J. Pharm. Sci. Rev. Res. 2010, 3, 91–100. [Google Scholar]
- Pashkow, F.J. Oxidative Stress and Inflammation in Heart Disease: Do Antioxidants Have a Role in Treatment and/or Prevention? Int. J. Inflamm. 2011, 2011, 514623. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef] [PubMed]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef] [PubMed]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Custódio, L.; Soares, F.; Pereira, H.; Barreira, L.; Vizetto-Duarte, C.; Rodrigues, M.J.; Rauter, A.P.; Alberício, F.; Varela, J. Fatty acid composition and biological activities of Isochrysis galbana T-ISO, Tetraselmis sp. and Scenedesmus sp.: Possible application in the pharmaceutical and functional food industries. J. Appl. Phycol. 2014, 26, 151–161. [Google Scholar] [CrossRef]
- Coulombier, N.; Nicolau, E.; Le Déan, L.; Antheaume, C.; Jauffrais, T.; Lebouvier, N. Impact of Light Intensity on Antioxidant Activity of Tropical Microalgae. Mar. Drugs 2020, 18, 122. [Google Scholar] [CrossRef]
- Amna Kashif, S.; Hwang, Y.J.; Park, J.K. Potent biomedical applications of isolated polysaccharides from marine microalgae Tetraselmis species. Bioprocess Biosyst. Eng. 2018, 41, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Gam, D.H.; Yi Kim, S.; Kim, J.W. Optimization of Ultrasound-Assisted Extraction Condition for Phenolic Compounds, Antioxidant Activity, and Epigallocatechin Gallate in Lipid-Extracted Microalgae. Molecules 2020, 25, 454. [Google Scholar] [CrossRef] [PubMed]
- Kokkali, M.; Martí-Quijal, F.J.; Taroncher, M.; Ruiz, M.J.; Kousoulaki, K.; Barba, F.J. Improved Extraction Efficiency of Antioxidant Bioactive Compounds from Tetraselmis chuii and Phaedoactylum tricornutum Using Pulsed Electric Fields. Molecules 2020, 25, 3921. [Google Scholar] [CrossRef]
- Silva, M.; Kamberovic, F.; Uota, S.T.; Kovan, I.-M.; Viegas, C.S.B.; Simes, D.C.; Gangadhar, K.N.; Varela, J.; Barreira, L. Microalgae as Potential Sources of Bioactive Compounds for Functional Foods and Pharmaceuticals. Appl. Sci. 2022, 12, 5877. [Google Scholar] [CrossRef]
- Trentin, R.; Custódio, L.; Rodrigues, M.J.; Moschin, E.; Sciuto, K.; da Silva, J.P.; Moro, I. Total Phenolic Levels, In Vitro Antioxidant Properties, and Fatty Acid Profile of Two Microalgae, Tetraselmis marina Strain IMA043 and Naviculoid Diatom Strain IMA053, Isolated from the North Adriatic Sea. Mar. Drugs 2022, 20, 207. [Google Scholar] [CrossRef]
- Lee, K.H.; Jang, Y.W.; Kim, H.; Ki, J.-S.; Yoo, H.Y. Optimization of Lutein Recovery from Tetraselmis suecica by Response Surface Methodology. Biomolecules 2021, 11, 182. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Mexía, A.; Ulloa-Mercado, G.; Gortáres-Moroyoqui, P.; González-Mercado, A.; Sánchez, M.; Sineiro, J.; Núñez, M.J. Antioxidant potential and antiangiogenic activity of Tetraselmis suecica grown in a semicontinuous culture. J. Chem. Technol. Biotechnol. 2022, 97, 2528–2536. [Google Scholar] [CrossRef]
- Sansone, C.; Galasso, C.; Orefice, I.; Nuzzo, G.; Luongo, E.; Cutignano, A.; Romano, G.; Brunet, C.; Fontana, A.; Esposito, F.; et al. The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci. Rep. 2017, 7, 41215. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, C.; Andersen, J.H.; Hansen, E.; Albrigtsen, M.; Escalera, L.; Esposito, F.; Helland, K.; Hanssen, K.Ø.; Romano, G.; Ianora, A. Bioactivity Screening of Microalgae for Antioxidant, Anti-Inflammatory, Anticancer, Anti-Diabetes, and Antibacterial Activities. Front. Mar. Sci. 2016, 3, 68. [Google Scholar] [CrossRef]
- Norzagaray-Valenzuela, C.D.; Valdez-Ortiz, A.; Shelton, L.M.; Jiménez-Edeza, M.; Rivera-López, J.; Valdez-Flores, M.A.; Germán-Báez, L.J. Residual biomasses and protein hydrolysates of three green microalgae species exhibit antioxidant and anti-aging activity. J. Appl. Phycol. 2017, 29, 189–198. [Google Scholar] [CrossRef]
- Parra-Riofrío, G.; García-Márquez, J.; Casas-Arrojo, V.; Uribe-Tapia, E.; Abdala-Díaz, R.T. Antioxidant and Cytotoxic Effects on Tumor Cells of Exopolysaccharides from Tetraselmis suecica (Kylin) Butcher Grown Under Autotrophic and Heterotrophic Conditions. Mar. Drugs 2020, 18, 534. [Google Scholar] [CrossRef] [PubMed]
- Natrah, F.M.I.; Yusoff, F.M.; Shariff, M.; Abas, F.; Mariana, N.S. Screening of Malaysian indigenous microalgae for antioxidant properties and nutritional value. J. Appl. Phycol. 2007, 19, 711–718. [Google Scholar] [CrossRef]
- Deepa, P.K.; Subramanian, A.; Manjusha, W.A. Evaluation of antioxidant potential and bioactive metabolites of Nannochloropsis sp. J. Adv. Appl. Sci. Res. 2021, 2, 1–9. [Google Scholar] [CrossRef]
- Khatib, S.; Artoul, F.; Paluy, I.; Boluchevsky, L.; Kvitnitsky, E.; Vaya, J. Nannochloropsis sp. ethanol extract prevents macrophage and LDL oxidation and enhances PON1 activity through the principal active compound lyso-diacylglyceryltrimethylhomoserine (lyso-DGTS). J. Appl. Phycol. 2018, 30, 1679–1689. [Google Scholar] [CrossRef]
- Santhar, D.T.; Haq, M.A.B.; Marudhupandi, T.; Vaseeharan, B.; Rajan, D.K.; Moovendhan, M. Evaluation of chemical compositions and antioxidant potential of marine microalgae of the genus Nannochloropsis. Biomass Convers. Biorefinery 2021. [Google Scholar] [CrossRef]
- Goh, S.H.; Yusoff, F.M.; Loh, S.P. A comparison of the antioxidant properties and total phenolic content in a Diatom, Chaetoceros sp. and a green microalga, Nannochloropsis sp. J. Agric. Sci. 2010, 2, 123–130. [Google Scholar] [CrossRef]
- Guedes, A.C.; Gião, M.S.; Seabra, R.; Ferreira, A.C.S.; Tamagnini, P.; Moradas-Ferreira, P.; Malcata, F.X. Evaluation of the Antioxidant Activity of Cell Extracts from Microalgae. Mar. Drugs 2013, 11, 1256–1270. [Google Scholar] [CrossRef] [PubMed]
- Pereira, H.; Custódio, L.; Rodrigues, M.J.; De Sousa, C.B.; Oliveira, M.; Barreira, L.; Neng, N.D.R.; Nogueira, J.M.F.; Alrokayan, S.A.; Mouffouk, F.; et al. Biological Activities and Chemical Composition of Methanolic Extracts of Selected Autochthonous Microalgae Strains from the Red Sea. Mar. Drugs 2015, 13, 3531–3549. [Google Scholar] [CrossRef] [PubMed]
- Martínez, R.; García-Beltrán, A.; Kapravelou, G.; Mesas, C.; Cabeza, L.; Perazzoli, G.; Guarnizo, P.; Rodríguez-López, A.; Andrés Vallejo, R.; Galisteo, M.; et al. In Vivo Nutritional Assessment of the Microalga Nannochloropsis gaditana and Evaluation of the Antioxidant and Antiproliferative Capacity of Its Functional Extracts. Mar. Drugs 2022, 20, 318. [Google Scholar] [CrossRef] [PubMed]
- Medina, C.; Rubilar, M.; Shene, C.; Torres, S.; Verdugo, M. Protein Fractions with Techno-Functional and Antioxidant Properties from Nannochloropsis gaditana Microalgal Biomass. J. Biobased Mater. Bioenergy 2015, 9, 417–425. [Google Scholar] [CrossRef]
- Millao, S.; Uquiche, E. Antioxidant activity of supercritical extracts from Nannochloropsis gaditana: Correlation with its content of carotenoids and tocopherols. J. Supercrit. Fluids 2016, 111, 143–150. [Google Scholar] [CrossRef]
- Ebrahimzadeh, M.A.; Khalili, M.; Dehpour, A.A. Antioxidant activity of ethyl acetate and methanolic extracts of two marine algae, Nannochloropsis oculata and Gracilaria gracilis—An in vitro assay. Braz. J. Pharm. Sci. 2018, 54, e17280. [Google Scholar] [CrossRef]
- Feller, R.; Matos, Â.P.; Mazzutti, S.; Moecke, E.H.S.; Tres, M.V.; Derner, R.B.; Oliveira, J.V.; Junior, A.F. Polyunsaturated ω-3 and ω-6 fatty acids, total carotenoids and antioxidant activity of three marine microalgae extracts obtained by supercritical CO2 and subcritical n-butane. J. Supercrit. Fluids 2018, 133, 437–443. [Google Scholar] [CrossRef]
- Ben Hafsa, M.; Ben Ismail, M.; Garrab, M.; Aly, R.; Gagnon, J.; Naghmouchi, K. Antimicrobial, antioxidant, cytotoxic and anticholinesterase activities of water-soluble polysaccharides extracted from microalgae Isochrysis galbana and Nannochloropsis oculata. J. Serbian Chem. Soc. 2017, 82, 509–522. [Google Scholar] [CrossRef]
- Hussein, H.A.; Mohamad, H.; Mohd Ghazaly, M.; Laith, A.A.; Abdullah, M.A. Anticancer and antioxidant activities of Nannochloropsis oculata and Chlorella sp. extracts in co-application with silver nanoparticle. J. King Saud Univ. Sci. 2020, 32, 3486–3494. [Google Scholar] [CrossRef]
- Wali, A.F.; Al Dhaheri, Y.; Ramakrishna Pillai, J.; Mushtaq, A.; Rao, P.G.M.; Rabbani, S.A.; Firdous, A.; Elshikh, M.S.; Farraj, D.A.A. LC-MS Phytochemical Screening, In Vitro Antioxidant, Antimicrobial and Anticancer Activity of Microalgae Nannochloropsis oculata Extract. Separations 2020, 7, 54. [Google Scholar] [CrossRef]
- Stahl, W.; Sies, H. Photoprotection by dietary carotenoids: Concept, mechanisms, evidence and future development. Mol. Nutr. Food Res. 2012, 56, 287–295. [Google Scholar] [CrossRef]
- El-Agamey, A.; McGarvey, D.J. Carotenoid Radicals and Radical Ions. In Carotenoids: Volume 4: Natural Functions; Britton, G., Liaaen-Jensen, S., Pfander, H., Eds.; Birkhäuser Basel: Basel, Switzerland, 2008; pp. 119–154. [Google Scholar]
- Ochoa Becerra, M.; Mojica Contreras, L.; Hsieh Lo, M.; Mateos Díaz, J.; Castillo Herrera, G. Lutein as a functional food ingredient: Stability and bioavailability. J. Funct. Foods 2020, 66, 103771. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; de Morais, A.M.M.B.; de Morais, R.M.S.C. Bioactivity and Applications of Polysaccharides from Marine Microalgae. In Polysaccharides: Bioactivity and Biotechnology; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–38. [Google Scholar]
- Qiao, H.; Hu, D.; Ma, J.; Wang, X.; Wu, H.; Wang, J. Feeding effects of the microalga Nannochloropsis sp. on juvenile turbot (Scophthalmus maximus L.). Algal Res. 2019, 41, 101540. [Google Scholar] [CrossRef]
- Rahman, N.A.; Khatoon, H.; Yusuf, N.; Banerjee, S.; Haris, N.A.; Lananan, F.; Tomoyo, K. Tetraselmis chuii biomass as a potential feed additive to improve survival and oxidative stress status of Pacific white-leg shrimp Litopenaeus vannamei postlarvae. Int. Aquat. Res. 2017, 9, 235–247. [Google Scholar] [CrossRef]
- Nacer, W.; Baba Ahmed, F.Z.; Merzouk, H.; Benyagoub, O.; Bouanane, S. Evaluation of the anti-inflammatory and antioxidant effects of the microalgae Nannochloropsis gaditana in streptozotocin-induced diabetic rats. J. Diabetes Metab. Disord. 2020, 19, 1483–1490. [Google Scholar] [CrossRef]
- Abu-Ghannam, N.; Rajauria, G. Antimicrobial activity of compounds isolated from algae. In Functional Ingredients from Algae for Foods and Nutraceuticals; Domínguez, H., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 287–306. [Google Scholar]
- Herrero, M.; Ibáñez, E.; Cifuentes, A.; Reglero, G.; Santoyo, S. Dunaliella salina Microalga Pressurized Liquid Extracts as Potential Antimicrobials. J. Food Prot. 2006, 69, 2471–2477. [Google Scholar] [CrossRef]
- Klejdus, B.; Lojková, L.; Plaza, M.; Šnóblová, M.; Štěrbová, D. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. J. Chromat. A 2010, 1217, 7956–7965. [Google Scholar] [CrossRef]
- Bhagavathy, S.; Sumathi, P.; Jancy Sherene Bell, I. Green algae Chlorococcum humicola-a new source of bioactive compounds with antimicrobial activity. Asian Pac. J. Trop. Biomed. 2011, 1, S1–S7. [Google Scholar] [CrossRef]
- Maadane, A.; Merghoub, N.; El Mernissi, N.; Ainane, T.; Amzazi, S.; Wahby, I.; Bakri, Y. Antimicrobial activity of marine microalgae isolated from Moroccan coastlines. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1257–1260. [Google Scholar] [CrossRef]
- Jusidin, M.R.; Othman, R.; Shaleh, S.R.M.; Ching, F.F.; Senoo, S.; Oslan, S.N.H. In Vitro Antibacterial Activity of Marine Microalgae Extract against Vibrio harveyi. Appl. Sci. 2022, 12, 1148. [Google Scholar] [CrossRef]
- Austin, B.; Baudet, E.; Stobie, M. Inhibition of bacterial fish pathogens by Tetraselmis suecica. J. Fish Dis. 1992, 15, 55–61. [Google Scholar] [CrossRef]
- Khavari, F.; Saidijam, M.; Taheri, M.; Nouri, F. Microalgae: Therapeutic potentials and applications. Mol. Biol. Rep. 2021, 48, 4757–4765. [Google Scholar] [CrossRef]
- Guzman, F.; Wong, G.; Roman, T.; Cardenas, C.; Alvarez, C.; Schmitt, P.; Albericio, F.; Rojas, V. Identification of Antimicrobial Peptides from the Microalgae Tetraselmis suecica (Kylin) Butcher and Bactericidal Activity Improvement. Mar. Drugs 2019, 17, 453. [Google Scholar] [CrossRef]
- Hoernke, M.; Schwieger, C.; Kerth, A.; Blume, A. Binding of cationic pentapeptides with modified side chain lengths to negatively charged lipid membranes: Complex interplay of electrostatic and hydrophobic interactions. Biochim. Biophys. Acta Biomembr. BBA 2012, 1818, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Yang, Y.; Yang, G.; Yu, L. Studies on antibacterial activity and antibacterial mechanism of a novel polysaccharide from Streptomyces virginia H03. Food Control 2010, 21, 1257–1262. [Google Scholar] [CrossRef]
- Ratovitski, E.A. Anticancer Natural Compounds as Epigenetic Modulators of Gene Expression. Curr. Genom. 2017, 18, 175–205. [Google Scholar] [CrossRef]
- Hussein, H.A.; Mohamad, H.; Ghazaly, M.M.; Laith, A.A.; Abdullah, M.A. Cytotoxic effects of Tetraselmis suecica chloroform extracts with silver nanoparticle co-application on MCF-7, 4 T1, and Vero cell lines. J. Appl. Phycol. 2020, 32, 127–143. [Google Scholar] [CrossRef]
- Hussein, H.A.; Kassim, M.N.I.; Maulidiani, M.; Abas, F.; Abdullah, M.A. Cytotoxicity and 1H NMR metabolomics analyses of microalgal extracts for synergistic application with Tamoxifen on breast cancer cells with reduced toxicity against Vero cells. Heliyon 2022, 8, e09192. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.K.A.; Fernando, I.P.S.; Samarakoon, K.W.; Lakmal, H.H.C.; Kim, E.-A.; Kwon, O.N.; Dilshara, M.G.; Lee, J.-B.; Jeon, Y.-J. Anti-inflammatory and anti-cancer activities of sterol rich fraction of cultured marine microalga Nannochloropsis oculata. Algae 2016, 31, 277–287. [Google Scholar] [CrossRef]
- Castejón, N.; Marko, D. Fatty Acid Composition and Cytotoxic Activity of Lipid Extracts from Nannochloropsis gaditana Produced by Green Technologies. Molecules 2022, 27, 3710. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Reinado, R.; Escobar-Niño, A.; Fajardo, C.; Morano, I.M.; Amil-Ruiz, F.; Martinez-Rodríguez, G.; Fuentes-Almagro, C.; Capilla, V.; Tomás-Cobos, L.; Soriano-Romaní, L.; et al. Development of New Antiproliferative Compound against Human Tumor Cells from the Marine Microalgae Nannochloropsis gaditana by Applied Proteomics. Int. J. Mol. Sci. 2021, 22, 96. [Google Scholar] [CrossRef] [PubMed]
- Letsiou, S.; Kalliampakou, K.; Gardikis, K.; Mantecon, L.; Infante, C.; Chatzikonstantinou, M.; Labrou, N.E.; Flemetakis, E. Skin protective effects of Nannochloropsis gaditana extract on H2O2-stressed human dermal fibroblasts. Front. Mar. Sci. 2017, 4, 221. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Verstreken, H.; Dejonghe, C.; Gheysen, L.; Foubert, I.; Grauwet, T.; Van Loey, A.M. Cell disruption of Nannochloropsis sp. improves in vitro bioaccessibility of carotenoids and ω3-LC-PUFA. J. Funct. Foods 2020, 65, 103770. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 11: Suitability of taxonomic units notified to EFSA until September 2019. EFSA J. 2020, 18, 5965. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Jamsazzadeh Kermani, Z.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Mantecón, L.; Moyano, R.; Cameán, A.M.; Jos, A. Safety assessment of a lyophilized biomass of Tetraselmis chuii (TetraSOD®) in a 90 day feeding study. Food Chem. Toxicol. 2019, 133, 110810. [Google Scholar] [CrossRef]
- Nunes, M.C.; Fernandes, I.; Vasco, I.; Sousa, I.; Raymundo, A. Tetraselmis chuii as a Sustainable and Healthy Ingredient to Produce Gluten-Free Bread: Impact on Structure, Colour and Bioactivity. Foods 2020, 9, 579. [Google Scholar] [CrossRef] [PubMed]
- Qazi, W.M.; Ballance, S.; Kousoulaki, K.; Uhlen, A.K.; Kleinegris, D.M.M.; Skjånes, K.; Rieder, A. Protein Enrichment of Wheat Bread with Microalgae: Microchloropsis gaditana, Tetraselmis chui and Chlorella vulgaris. Foods 2021, 10, 3078. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Bursic, I.; Sousa, I.; Raymundo, A.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae as Functional Ingredients in Savory Food Products: Application to Wheat Crackers. Foods 2019, 8, 611. [Google Scholar] [CrossRef] [PubMed]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
| Amino Acid | Content (g/16 g Nitrogen) | |||||
|---|---|---|---|---|---|---|
| FAO | Nannochloropsis1 | N. granulata2 | N. oculata2 | T. chuii2 | T. suecica2 | |
| Essential | ||||||
| Isoleucine | 4.0 | 3.6–4.2 | 5.6 | 4.8 | 3.5 | 3.5 |
| Leucine | 7.0 | 7.9–8.4 | 11.0 | 7.8 | 7.5 | 8.0 |
| Valine | 5.0 | 4.8–5.4 | 7.1 | 6.5 | 5.8 | 5.7 |
| Lysine | 5.5 | 5.2–6.0 | 8.5 | 6.1 | 5.7 | 6.0 |
| Phenylalanine | 6.0 | 4.4–4.8 | 6.2 | 6.2 | 5.4 | 5.9 |
| Methionine | 3.5 | 1.7–2.1 | 3.5 | 1.6 | 1.9 | 2.3 |
| Tryptophan | 1.0 | 1.5–1.8 | 2.8 | 1.6 | 3.7 | 3.8 |
| Threonine | 4.0 | 4.5–4.9 | 5.4 | 5.5 | 4.2 | 4.1 |
| Non-essential | ||||||
| Tyrosine | 2.8–3.2 | 4.2 | 4.2 | 3.7 | 3.8 | |
| Cysteine | 0.6–0.8 | 1.6 | 0.4 | 0.6 | 0.7 | |
| Alanine | 6.2–6.9 | 7.1 | 7.4 | 6.8 | 6.9 | |
| Arginine | 5.1–5.4 | 7.4 | 7.3 | 13.5 | 13.2 | |
| Aspartic acid | 8.2–8.6 | 11.4 | 7.6 | 9.4 | 8.9 | |
| Glutamic acid | 9.9–12.0 | 14.1 | 10.1 | 12.4 | 11.2 | |
| Glycine | 5.1–5.4 | 7.5 | 5.5 | 5.9 | 5.9 | |
| Histidine | 2.0–2.1 | 2.3 | 2.1 | 1.8 | 1.8 | |
| Proline | 4.3–7.8 | 11.2 | 9.3 | 5.1 | 4.7 | |
| Serine | 4.2–4.3 | 5.6 | 5.0 | 4.3 | 4.6 | |
| TAA | 82.0–94.1 | 122.5 | 99.0 | 101.2 | 101.0 | |
| HAA | 37.8–45.4 | 60.3 | 49.8 | 44.0 | 45.3 | |
| AAA | 8.7–9.8 | 13.2 | 12.0 | 12.8 | 13.5 | |
| Fatty Acid | Fatty Acids (mg/g DW) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N. oculata1 | N. limnetica2 | N. gaditana3 | N. granulata4 | N. oceanica5 | N. oceanica6 | T. sp. 5 | T. suecica6 | T. chuii4 | T. chuii7 | |
| SFA | ||||||||||
| 12:0 | --- | --- | 0.4 | 1.4 | 1.2 | 3.5 | --- | --- | --- | 0.02 |
| 14:0 | 2.7 | 6.3 | 4.1 | 11.3 | 16.9 | 9.3 | 0.5 | --- | --- | 0.2 |
| 16:0 | 14.6 | 36.1 | 17.9 | 27.7 | 17.2 | 67.3 | 6.3 | 13.3 | 13.9 | 14.9 |
| 18:0 | 0.7 | 0.3 | --- | 1.8 | 3.5 | 1.2 | --- | --- | 0.4 | |
| MUFA | ||||||||||
| 16:1 | 21.8 | 37.1 | 31.5 | 41.5 | 18.2 | 58.2 | 1.3 | 0.4 | --- | 0.4 |
| cis-9 18:1 | 6.3 | 30.1 | 1.9 | 11 | 4.1 | 30.6 | 10.7 | 19.5 | 6 | 18.4 |
| 20:1 | --- | --- | --- | --- | 0.5 | --- | 0.9 | --- | 1.2 | 1.7 |
| PUFA | ||||||||||
| 16:2 n-4 | --- | --- | --- | --- | --- | --- | --- | --- | 1.8 | --- |
| 18:2 n-6 | 2.4 | 3.8 | 1.4 | 7.7 | 9.7 | 2.8 | 2.5 | 11.9 | 6.2 | 4.8 |
| 18:3 n-6 | --- | 0.2 | 0.8 | --- | --- | 0.7 | --- | --- | --- | 0.5 |
| 20:2 n-6 | --- | --- | --- | --- | 0.5 | 0.4 | --- | --- | --- | 0.04 |
| 20:4 n-6 | 5.8 | 5.3 | 3.2 | --- | 3.7 | 4.8 | 0.6 | --- | --- | 0.4 |
| 18:3 n-3 | --- | 0.4 | 0.1 | --- | 0.5 | 0.2 | 6.4 | 17.6 | 10.8 | 9.7 |
| 18:4 n-3 | --- | --- | --- | --- | --- | --- | 4.1 | --- | --- | --- |
| 20:5 n-3 | 23.3 | 28.1 | 54.9 | 31.7 | 23.4 | 31.5 | 4.8 | --- | 3.8 | 4.1 |
| 22:6 n-3 | --- | 0.1 | 0.20 | --- | --- | --- | 0.2 | --- | --- | --- |
| Mineral | Content (mg/100 g DW) | |||||||
|---|---|---|---|---|---|---|---|---|
| N. sp. 1 | N. sp. 2 | N. granulata3 | N. gaditana4 | T. chuii3 | T. chuii4 | T. CTP4 5 | T. suecica6 | |
| Major elements | ||||||||
| Calcium | 972 | 590 | 90 | 631 | 2990 | 185 | 1190 | 1791 |
| Magnesium | 316 | 530 | 260 | 370 | 430 | 305 | 2080 | 601 |
| Phosphorus | 1300 | 730 | 887 | 1460 | 710 | 964 | ||
| Potassium | 533 | 1500 | 1500 | 1261 | 1860 | 4200 | 1349 | |
| Sodium | 659 | 2500 | 1030 | 2022 | 890 | 1180 | 3223 | |
| Sulphur | 610 | 580 | 1380 | |||||
| Trace elements | ||||||||
| Copper | 35 | 1.5 | 1. 8 | 2.9 | 10.2 | 0.5 | 1.1 | 1.1 |
| Iron | 136 | 62.7 | 139.5 | 48.9 | 177.4 | 51.8 | 32.3 | 30.9 |
| Iodine | 0.2 | 0.3 | 0.1 | |||||
| Manganese | 3.4 | 6.9 | 15.1 | 9.3 | 19.1 | 0.1 | 3.5 | |
| Selenium | 0.01 | 0.1 | 0.1 | |||||
| Zinc | 103 | 5.5 | 3.2 | 10.1 | 6.4 | 2.1 | 2.9 | 6.6 |
| Nickel | 0.01 | |||||||
| Molybdenum | 0.05 | |||||||
| Microalgae | Pretreatment/Extraction Conditions | Mechanisms | Responsible Compound | Ref. |
|---|---|---|---|---|
| Tetraselmis sp. | (1) Hexane (2) Sequential extraction with ether, acetone and water | DPPH radical scavenging, and iron and copper chelating | PUFAs and phenolic compounds | [79] |
| Ethanol and ethanol/water or ultrasound + water | DPPH radical scavenging | --- | [64] | |
| Methanol/dichloromethane + ultrasound or acetone + ultrasound | ORAC radical scavenging, and inhibition of lipid peroxidation (TBARS) | Xanthophylls | [80] | |
| Ethanol/water or hexane + ethyl acetate + hot water | ABTS radical scavenging, FRAP, and inhibition of linoleic acid oxidation | Phenolic compounds | [65] | |
| Methanol/dichloromethane + ultrasound | Inhibition of lipid peroxidation (TBARS) | --- | [77] | |
| T. sp. KCTC12236BP and KCTC12432BP | (1) 1 N H2SO4, 1 M NaOH and distilled water (2) Ethanol precipitation | ABTS and DPPH radical scavenging and FRAP | Polysaccharides | [81] |
| T. sp. KCTC12236BP | Ultrasound + water:ethanol | DPPH radical scavenging | Total phenolic compounds and epigallocatechin gallate | [82] |
| T. sp. M8 | Water, hexane or ethyl acetate | ORAC | Carotenoids | [62] |
| T. sp. IMP3, and T. sp. CTP4 | Water or ethanol 96% | ABTS and DPPH radical scavenging and FRAP | Phenolic compounds and PUFAs | [66] |
| T. chuii | Methanol or (1) hexane/dichloromethane and (2) acetone/water/acetic acid | ORAC and DPPH radical scavenging | PUFAs and phenolic compounds | [28] |
| Dichloromethane (Folch method) | ABTS and DPPH radical scavenging | PUFAs and other compounds | [76] | |
| (1) Pulsed electric field (2) DMSO or distilled water | ABTS radical scavenging | Polyphenols and pigments | [83] | |
| Ethanol, ethyl acetate, or water | DPPH radical scavenging, and iron and cupper chelating | --- | [84] | |
| Water, hexane or ethyl acetate | ORAC | Carotenoids | [62] | |
| T. marina IMA043 | (1) Ultrasound (2) Water, methanol or dichloromethane | DPPH and ABTS radical scavenging, iron/cupper chelating, and FRAP | SFA, MUFAs and phenolic compounds | [85] |
| T. suecica | Distilled water, hexane, heptane, ethyl acetate, acetone, ethanol or methanol | ABTS radical scavenging, and FRAP | Lutein | [86] |
| Ultrasound + methanol:water, methanol or ethanol | DPPH and ABTS radical scavenging, and β-carotene bleaching | Multiple compounds | [87] | |
| Ethanol:water | DPPH radical scavenging | Carotenoids | [88] | |
| Ethanol/water or hexane + ethyl acetate + hot water | ABTS radical scavenging, FRAP, and inhibition of linoleic acid oxidation | Phenolic compounds | [65] | |
| Water, hexane or ethyl acetate | ORAC | Carotenoids | [62] | |
| Water + ultrasound + acetone | Cellular antioxidant and lipid peroxidation inhibition in HepG2 cells | --- | [89] | |
| T. suecica TES2 | (1) Ultrasound + chloroform:methanol or ethanol (2) Enzymatic hydrolysis | ORAC, DPPH, and ABTS radical scavenging | Protein/peptide fractions | [90] |
| T. suecica (Kylin) Butcher | (1) Phenolics removal with polyvinylpyrrolidone (2) Ethanol or N-cetylpyridinium bromide extraction | ABTS and DPPH radical scavenging | Exopolysaccharides | [91] |
| T. striata CTP4 | Ethanol, ethyl acetate, or water | DPPH radical scavenging, and iron and cupper chelating | --- | [84] |
| T. tetrathele | Methanol | DPPH radical scavenging, and inhibition of lipid and linoleic acid peroxidation | --- | [92] |
| Nannochloropsis sp. | Ethyl acetate, methanol, water, isopropanol or petroleum ether | Total capacity, hydroxyl and NO radical scavenging, metal chelating, and FRAP | Fatty acids (hexadecanoic acid) | [93] |
| Ethanol:water | Inhibition of LDL oxidation and ROS inhibition in H2O2-induced macrophages J-774A | Lyso-diacetylglyceryltrimethylhomoserine | [94] | |
| Methanol | Total capacity; DPPH, superoxide, and hydroxyl radical scavenging | --- | [95] | |
| Ethanol, ethyl acetate, or water | DPPH radical scavenging, and iron and cupper chelating | --- | [84] | |
| Methanol + ultrasound | ABTS and DPPH radical scavenging, FRAP, and ferrous ion-chelating ability | Phenolic compounds and carotenoids | [57] | |
| Water, hexane or ethyl acetate | ORAC | Carotenoids | [62] | |
| Hexane, dichloromethane, chloroform or methanol | DPPH, superoxide anion radical scavenging, FRAP, and ferrous-ion chelating | --- | [96] | |
| Ethanol + water | ABTS radical scavenging | --- | [97] | |
| N. sp. NNX1 | (1) Ultrasound + chloroform:methanol or ethanol (2) Enzymatic hydrolysis | ORAC, DPPH, and ABTS radical scavenging | Protein/peptide fractions | [90] |
| N. sp. SBL1 and SBL4 | Methanol | DPPH radical scavenging and iron and cupper chelating | Phenolic compounds | [98] |
| N. gaditana | Ethanol and ethanol/water or ultrasound + water | DPPH radical scavenging | --- | [64] |
| Ethanol:water:12N HCl | ABTS and DPPH radical scavenging, and lipid peroxidation inhibition | --- | [99] | |
| (1) High pressure disruption (2) Alkaline pH and isoelectric protein precipitation (3) Papain hydrolysis | ORAC radical scavenging | Protein/peptide fractions | [100] | |
| Supercritical CO2 | DPPH radical scavenging, β-carotene bleaching, and FRAP | Lipids | [101] | |
| N. granulata | Methanol or (1) hexane/dichloromethane and (2) acetone/water/acetic acid | ORAC and DPPH radical scavenging | PUFAs and phenolic compounds | [28] |
| N. limnetica 0065NA | Chloroform:methanol | DPPH radical scavenging | Polar lipids | [35] |
| N. oceanica | Dichloromethane (Folch method) | ABTS and DPPH radical scavenging | PUFAs and other compounds | [76] |
| N. oceanica 0011NN | Chloroform:methanol | DPPH radical scavenging | Polar lipids | [35] |
| N. oculata | Ethyl acetate or methanol | DPPH and NO radical scavenging, metal chelating, and FRAP | Phenolic and flavonoid compounds | [102] |
| (1) Supercritical CO2 (2) Subcritical n-butane extracts | DPPH radical scavenging | Carotenoids | [103] | |
| (1) Methanol (2) Water | DPPH radical scavenging | Polysaccharides | [104] | |
| Methanol, ethanol:water, chloroform, or hexane (Soxhlet) | DPPH radical scavenging | Hexanedioic acid, bis (2-ethylhexyl)ester | [105] | |
| Methanol:water:acetic acid:ascorbic acid + ultrasound or methanol:acetone:hexane or ethyl acetate:hexane + ultrasound or methanol:water:HCl + ultrasound | ABTS and ORAC radical scavenging, and FRAP | Polyphenols, carotenoids, chlorophylls, and triterpenoids | [2] | |
| Methanol + ultrasound | DPPH, H2O2, and ABTS radical scavenging | --- | [106] | |
| Ethanol/water or hexane + ethyl acetate + hot water | ABTS radical scavenging, FRAP, and inhibition of linoleic acid oxidation | Phenolic compounds | [65] | |
| Hexane or methanol | DPPH radical scavenging, and iron and copper chelating | Phenolic compounds and PUFAs | [69] | |
| Methanol | DPPH radical scavenging, and inhibition of lipid and linoleic acid peroxidation | --- | [92] |
| Microalgae Specie/Strain | Antimicrobial Activity (Microorganism) | Anti-Carcinogenic Activity (Cell Model) | Other Bioactivities | Ref. |
|---|---|---|---|---|
| Tetraselmis sp. | --- | Cell viability inhibition (HepG2) | Acetylcholinesterase inhibition | [79] |
| T. sp. KCTC12236BP and KCTC12432BP | Antifungal (Candida albicans and Penicillium italicum) | --- | Tyrosinase inhibition | [94] |
| T. chuii | --- | Cell viability inhibition (HepG2) | Calcium chelating | [84] |
| T. suecica | --- | Cell viability inhibition and apoptosis induction (MCF-7 and 4T1) | --- | [127] |
| Antibacterial (Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus) Antifungal (Candida albicans and Aspergillus niger) | --- | --- | [118] | |
| --- | Anti-angiogenic through over-expression of VEGF (PC-3 cells) | --- | [87] | |
| --- | Repairing effects through increasing expression of DHCR24 and PTGR1 genes and proteins and reducing PGE2 (A549) | Repairing effects on reconstructed human epidermal tissue cells (EpiDermTM) | [88] | |
| T. suecica CCAP904 | Antibacterial (Escherichia coli, Salmonella typhimurium, Pseudomonas aeruginosa, Bacillus cereus, Staphylococcus aureus, Listeria monocytogenes, Micrococcus luteus) | --- | --- | [122] |
| T. suecica TES2 | --- | --- | Anti-aging potential through inhibition of elastase and hyaluronidase | [90] |
| T. suecica (Kylin) Butcher | --- | Cell viability inhibition (MCF-7, HL-60, and NCI-H460) | --- | [91] |
| T. striata CTP4 | --- | --- | Calcium chelating Anti-inflammatory through decreasing TNF-α in LPS-induced macrophages THP-1 | [84] |
| Nannochloropsis sp. | --- | --- | Increase in anti-atherogenic paraoxonase 1 activity | [94] |
| --- | --- | Calcium chelating Anti-inflammatory through decreasing TNF-α in LPS-induced macrophages THP-1 | [84] | |
| N. sp. NNX1 | --- | --- | Anti-aging potential through inhibition of elastase and hyaluronidase | [90] |
| N. gaditana | --- | Cell viability inhibition (Caco-2 and HepG2) | --- | [130] |
| --- | Cell viability inhibition (HCT-116) | --- | [129] | |
| --- | --- | Skin protection properties by mediating oxidative responses and apoptosis (H2O2-challenged dermal fibroblasts) | [131] | |
| N. oceanica | Antibacterial (Vibrio harveyi) | --- | --- | [119] |
| N. oculata | Antibacterial (Enterococcus faecalis, Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa) Antifungal (Candida albicans, Candida glabrata, Candida kreusei, and Candida parapsilosis) | Cell proliferation inhibition (HeLa) | Anti-cholinesterase activity | [104] |
| --- | Cell viability inhibition and apoptosis induction (MCF-7 and 4T1) | --- | [105] | |
| --- | Cell viability inhibition and apoptosis induction (MCF-7 and 4T1) | --- | [127] | |
| --- | Cell viability inhibition and apoptosis induction (HL-60) | Anti-inflammatory through decreasing LPS-induced iNOS and COX-2 protein levels (RAW264.7) | [128] | |
| --- | --- | Anti-aging through inhibition of acetylcholinesterase and butyrylcholinesterase Anti-diabetic through inhibition of α-amylase Anti-obesity through inhibition of pancreatic lipase | [2] | |
| Antibacterial (Staphylococcus aureus, Pseudomonas aeruginosa, and Escherichia coli) Anti-fungal (Candida albicans) | Cell viability inhibition (MDA-MB-231) | --- | [106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paterson, S.; Gómez-Cortés, P.; de la Fuente, M.A.; Hernández-Ledesma, B. Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods. Nutrients 2023, 15, 477. https://doi.org/10.3390/nu15020477
Paterson S, Gómez-Cortés P, de la Fuente MA, Hernández-Ledesma B. Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods. Nutrients. 2023; 15(2):477. https://doi.org/10.3390/nu15020477
Chicago/Turabian StylePaterson, Samuel, Pilar Gómez-Cortés, Miguel Angel de la Fuente, and Blanca Hernández-Ledesma. 2023. "Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods" Nutrients 15, no. 2: 477. https://doi.org/10.3390/nu15020477
APA StylePaterson, S., Gómez-Cortés, P., de la Fuente, M. A., & Hernández-Ledesma, B. (2023). Bioactivity and Digestibility of Microalgae Tetraselmis sp. and Nannochloropsis sp. as Basis of Their Potential as Novel Functional Foods. Nutrients, 15(2), 477. https://doi.org/10.3390/nu15020477







