Abstract
Emerging studies in the literature describe an association between high-fat, low-carbohydrate diets and severe hypercholesterolemia consistent with the levels observed in patients with (homozygous) familial hypercholesterolemia (FH). High levels of low-density lipoprotein cholesterol (LDL-C) may result from the reduced clearance of LDL particles from the circulation, the increased production of their precursor, or a combination of both. The increased intake of (saturated) fat and cholesterol, combined with limited to no intake of carbohydrates and fiber, are the main features of diets linked to hypercholesterolemia. However, several observations in previous studies, together with our observations from our lipid clinic, do not provide a definitive pathophysiological explanation for severe hypercholesterolemia. Therefore, we review these findings and possible pathophysiological explanations as well as opportunities for future research. Altogether, clinicians should rule out high-fat, low-carbohydrate diets as a possible cause for hypercholesterolemia in patients presenting with clinical FH in whom no mutation is found and discuss dietary modifications to durably reduce LDL-C levels and cardiovascular disease risk.
1. Introduction
The notion that circulating low-density lipoprotein cholesterol (LDL-C) causes atherosclerotic cardiovascular disease (ASCVD) is firmly rooted in evidence from genetic, epidemiological, and clinical studies [1]. Interestingly, some of the earliest lines of evidence were derived from experimental studies involving the modification of plasma cholesterol levels through diet. The pioneering groundwork for cholesterol’s involvement in atherosclerosis can be traced back to St. Petersburg in 1913, when a young experimental pathologist named Anitschkow induced atherosclerosis in rabbits by feeding them purified cholesterol dissolved in sunflower oil. Control animals fed only the sunflower oil showed no lesions [2]. In the following decades, evidence accumulated that unraveled the relationship between (dietary) cholesterol, atherosclerosis, and potential options for treatment [3]. Over a century after Anitchkow’s seminal observations, the interplay between diet and (severe) dyslipidemia is still of special scientific interest, specifically concerning high-fat, low-carbohydrate diets, which is the topic of the narrative review presented here.
Epidemiological observations in large, cross-cultural prospective studies from the general population have been instrumental in linking the intake of fat and cholesterol with plasma cholesterol levels and the incidence of coronary events [4,5]. The first recommendations on dietary intervention to prevent cardiovascular disease were proposed by large professional medical associations in the 1960s [6], and it has now been widely acknowledged that diet has a modest yet meaningful influence on LDL-C levels on a population level [7,8]. The first data to show that cholesterol uptake in the intestine was amenable to a pharmacotherapeutic intervention were derived from the Coronary Primary Prevention Trial, published in 1984. This landmark placebo-controlled randomized clinical trial showed that the bile acid sequestrant cholestyramine, which helps remove cholesterol from the enterohepatic circulation, lowered LDL-C levels and reduced cardiovascular events in 3806 asymptomatic middle-aged men with primary hypercholesterolemia [9].
Bile acid sequestrants have since then been surpassed by statins and other more effective pharmacological lipid-lowering therapies, but dietary modifications continue to be highlighted in the most recent clinical guidelines for the prevention of cardiovascular disease [10,11,12], partly due to their LDL-lowering effect [7,13]. More specifically, guidelines recommend plant-based and Mediterranean-type diets containing vegetables, nuts, whole grains, and fish rich in unsaturated fats and dietary fibers, while the intake of processed meat, deep-fried foods, ice cream, high-fat dairy products, refined carbohydrates, and sweetened beverages high in saturated fatty acids (SFAs), cholesterol, trans-fatty acids (TFAs), sodium, and glucose should be avoided or kept to a minimum.
Besides observational and interventional data on cholesterol intake or reuptake in large study populations, understanding the link between plasma LDL-C, atherosclerosis, and treatment thereof has hugely progressed through the study of patients with extremely elevated plasma LDL-C levels due to genetic causes [3,14]. Patients with familial hypercholesterolemia (FH), an autosomal dominant inherited disorder caused by variants in the genes involved in lipoprotein metabolism [15], have elevated LDL-C levels from birth, which are more than double the LDL-C levels observed in patients from the general population [16,17]. In general, such high LDL-C levels are not observed in healthy individuals who adhere to a dietary pattern rich in fat and cholesterol.
Sparked by striking observations from our own clinical practice, we review the available evidence of high-fat, low-carbohydrate diets linked to severe dyslipidemia that may be mistaken for (clinical) FH. We discuss this phenomenon in light of possible pathophysiological explanations, point out knowledge gaps for future research, and provide practical recommendations to clinicians who encounter such patients.
2. High-Fat, Low-Carbohydrate Diets: Examples from the Lipid Clinic
2.1. Carnivorous Diet
Two brothers aged 33 years old (patient 1) and 28 years old (patient 2) were referred by their general practitioner to a university lipid clinic because of severe hypercholesterolemia. Upon referral, the LDL-C levels reported by the general practitioner were 15 and 12 mmol/L, respectively.
Both patients had an unremarkable medical history, used no medication, and had no family history of dyslipidemia or (premature) cardiovascular disease. Patients reported exercising regularly (approximately 4×/week, including resistance training) and had a muscular physique with a body mass index (BMI) of 26.2 and 27.3, respectively.
Physical examination was normal and did not specifically show visible cholesterol depositions in the form of xanthomas, corneal arcus, or xanthelasmata.
A fasting blood sample was obtained, and laboratory evaluations showed elevated LDL-C of 15.02 mmol/L and 8.59 mmol/L (patients 1 and 2, respectively, Table 1). We ruled out hypothyroidism and proteinuria as secondary causes of hypercholesterolemia. Liver enzymes were within the upper limits of normal. We suspected familial hypercholesterolemia (FH), possibly even in a homozygous form, as the underlying genetic cause and performed the next-generation sequencing of 27 lipid-related genes [16]. However, no pathogenic variants in any of these genes were found.
Dietary anamnesis revealed that both patients had started a carnivorous diet approximately one year prior to presentation to the lipid clinic. This diet consists solely of meat (mostly red) and high-fat dairy products. The calculated energy intake from carbohydrates on a typical day was <3 E-% (6 g/d), meaning that this diet was strongly ketogenic. The intake from protein and fat was 37 E-% and 61 E-%, respectively (see Appendix A).
Although no lipid profiles were available prior to the initiation of the carnivore diet, we suspected this diet to cause an abnormal lipid profile. We strongly advised the patients to adopt a regular, balanced dietary pattern (including carbohydrates and vegetables) and explained the risks of prolonged exposure to such elevated LDL-C levels. However, both decided to continue the carnivore diet and decided against starting lipid-lowering therapy as an alternative way to reduce the elevated LDL-C levels.
We performed fast protein liquid chromatography (FPLC) using stored plasma. This showed disproportionately elevated very-low-density lipoprotein (VLDL) and intermediate-density lipoprotein (IDL) fractions, which suggested that the overproduction of VLDL contributed to hypercholesterolemia (Figure 1).
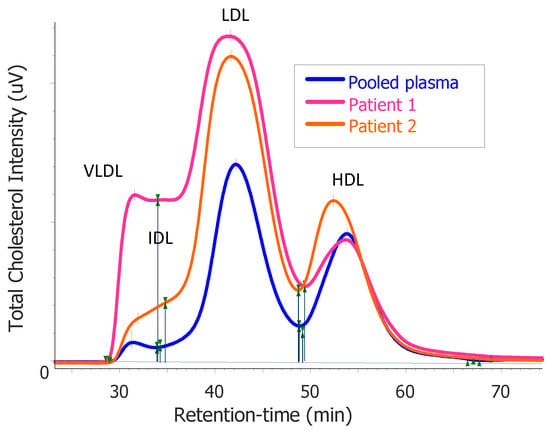
Figure 1.
Results of the total cholesterol distribution in plasma after fast protein liquid chromatography (FPLC) profiling from patient 1 and patient 2 compared with that of pooled plasma from healthy subjects. The elution of the main lipoprotein classes is indicated for very-low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), low-density lipoprotein (LDL), and high-density lipoprotein (HDL), respectively.
To better understand the effect of this diet on glucose metabolism, we measured fasting levels of glucose, insulin, and c-peptide and calculated the HOMA-IR to gauge the level of insulin sensitivity. Insulin and c-peptide were close to the lower limit of normal, and HOMA-IR was found to approximate >95th percentile, compared with a relevant reference population [18], which suggested good to very good insulin sensitivity.
We investigated whether a copious intake of dietary fatty acids and cholesterol would overwhelm the hepatic triglyceride and cholesterol pools and manifest as hepatic steatosis. We performed vibration-controlled transient elastography (VCTE) using FibroScan® 530 Compact (Echosens, France) estimated liver stiffness measurement (LSM) and controlled attenuation parameter (CAP) in the right liver lobe of both patients but found no signs of liver fibrosis or steatosis. Liver MR spectroscopy subsequently confirmed the near absence of liver fat in both patients.
We performed a carotid ultrasound, which revealed intima–media thickening suggestive of the early development of atherosclerosis.


Table 1.
Summary of laboratory and imaging results derived from patients 1 and 2 adhering to a carnivore diet.
Table 1.
Summary of laboratory and imaging results derived from patients 1 and 2 adhering to a carnivore diet.
| Patient 1 | Patient 2 | Reference Range | |
|---|---|---|---|
| BMI (kg/m2) | 26.2 | 27.3 | 18.5–25 |
| Total cholesterol (mmol/L) | 16.81 | 10.88 | |
| HDL cholesterol (mmol/L) | 1.53 | 2.00 | |
| LDL cholesterol (mmol/L) | 15.02 | 8.59 | |
| Triglycerides (mmol/L) | 0.57 | 0.65 | <1.80 |
| ApoB (mg/dL) | 321 | 186 | <120 |
| ApoA1 (mg/dL) | 156 | 188 | |
| Lp(a) (nmol/L) | 18 | 7 | <105 |
| Glucose (mmol/L) | 4.3 | 5.0 | <6.0 |
| Insulin (pmol/L) | 16.4 | 17.5 | 12–96 |
| C-peptide (nmol/L) | 0.19 | 0.19 | 0.2–0.8 |
| HOMA-IR | 0.5 | 0.6 | <2.0 |
| FibroScan CAP (dB/m) | 185 | 185 | 156–288 |
| FibroScan LSM (kPa) | 6.2 | 6.2 | <7 |
| Liver fat content (%) | <2 | 2.7 | <5 |
| Carotid IMT thickness (percentile) | >97th | 90th | Based on age and sex, according to [19] |
2.2. Journey from Zero-Carb to Raw-Food Diet
Patient 3 was a 23-year-old male referred to the academic hospital lipid clinic for the analysis of severe hypercholesterolemia, with an LDL-C level of 12.2 mmol/L reported by his general practitioner.
The patient had no relevant medical history and used no medication. Besides his maternal grandmother, who had started statin therapy at an elderly age, it was unknown if family members had hypercholesterolemia. The family history of cardiovascular disease was negative. The patient told us he had specially requested a lipid panel measurement from his general practitioner because he was interested to learn his blood cholesterol levels after having recently adopted a new diet. The patient reported that, since his youth, he had struggled to maintain a healthy and balanced diet and that he had experienced that his dietary pattern was closely linked to his psychological well-being. The patient had, therefore, experimented with progressively eliminating food products from his diet. This led to the point that, at the time of referral, the patient was on a zero-carb diet rich in animal proteins and fats in the form of eggs and bacon, supplemented with raw minced meat, raw liver, and a limited amount of vegetables.
Physical examination showed a lean young male (BMI 21 kg/m2) without signs of xanthoma, xanthelasmata, or corneal arcus, and was otherwise unremarkable.
A repeat lipid profile was obtained, which showed somewhat lower, albeit still severely elevated, levels of LDL-C at 7.9 mmol/L, which could well be consistent with heterozygous FH. However, genetic analysis using next-generation sequencing showed no FH-causing variant. It was suspected that the patient’s diet contributed to the elevated LDL-C levels. Dietary changes as well as the lowering of LDL-C by starting statin therapy were discussed, and the patient opted to modify his diet first.
Three months later, the patient had significantly reduced his intake of animal fats (mostly cut his intake of eggs and bacon), and a repeat lipid panel showed a near normalization of LDL-C levels (Table 2), after which he was referred back to the care of the general practitioner. The most recent lipid panel was completely normal for age and gender. At this time, the patient reported being on a balanced raw food diet with approximately 400 g of raw meat or fish daily, supplemented with leafy vegetables, nuts, and fruit (usually 2 or 3 mangos daily). The patient reported no intake of starchy carbohydrates.

Table 2.
Lipid panels of patient 3.
2.3. Heterozygous Familial Hypercholesterolemia Derailed
Patient 4 was a male diagnosed with FH (variant p.R3500Q in APOB) at the age of 31 years old, with no relevant medical history. Following the diagnosis of FH, a statin combined with ezetimibe had been prescribed as lipid-lowering therapy, and the patient was regularly followed up by his internist at the lipid clinic.
At the age of 41 years old, the patient started a low-carb high-fat diet along with his spouse, who intended to lose weight. At the latest lipid panel obtained before the patient had started this diet, LDL-C levels were relatively well controlled at 2.9 mmol/L, with the patient taking rosuvastatin 40 mg and ezetimibe 10 mg daily. However, when the patient presented for his next yearly follow-up visit after having adopted his new diet, LDL-C levels were severely elevated at 8.39 mmol/L (Table 3). Adherence to lipid-lowering medication was unchanged, and secondary causes of hypercholesterolemia (hypothyroidism and proteinuria) were ruled out. The estimated untreated LDL-C levels would well be within the range observed in patients with homozygous FH.

Table 3.
Lipid panels of patient 4.
The patient was referred to a dietician for dietary assessment and counseling. His diet was shown to be low in carbohydrates and high in dairy fats (Appendix B), and recommendations were made for a more balanced diet. His BMI dropped from 23.4 to 21.4 kg/m2 with diet. With the help of his dietician, the patient modified his diet by, amongst others, reducing his intake of dairy fat. At the next follow-up visit, LDL-C had completely normalized to pre-diet levels (Table 3), with unchanged adherence to lipid-lowering therapy.
3. Mechanism of Dietary Induced Hypercholesterolemia
Recent observations from our clinical practice add to the emerging literature describing the association between high-fat, low-carbohydrate diets and severe hypercholesterolemia. We reviewed these findings and possible pathophysiological explanations for this phenomenon as well as opportunities for future research. We argue that clinicians should rule out adherence to a high-fat, low-carbohydrate diet in patients presenting with clinical FH.
Several other studies have reported patients consuming a high-fat ketogenic diet who present with high LDL-C levels consistent with FH, which was shown to be reversible with the normalization of the diet. Goldberg and colleagues reported five cases of LDL-C levels ranging from 6.3 to as high as 17.7 mmol/L. Genetic analysis revealed no pathogenic FH variants in any of the patients, although the patient with the highest cholesterol levels was found to have dysbetalipoproteinemia (confirmed by identification of APOE E2/E2 genotype) [20]. Schaffer et al. reported three patients with severe hypercholesterolemia (LDL-C levels of 8.9, 11.5, and 15.5 mmol/L), whose LDL-C levels decreased after making dietary modifications [21]. Norwitz and colleagues reported a series of five patients on a ketogenic diet whose LDL-C levels ranged from 6.2 to up to 17.2 mmol/L. All tested negative for an FH-causing variant and after the reported moderate reintroduction of carbohydrates (50–100 g/day), hypercholesterolemia attenuated and even normalized in one patient [22]. The same authors also recently described a 26-year-old male with ulcerative colitis who, in an attempt to relieve its symptoms, initiated a ketogenic diet on which LDL-C levels peaked at 14.1 mmol/L [23]. Interestingly, a coronary CT angiography did not show (non)calcified plaque after two years of exposure to LDL-C levels in a similar range, as seen in patients with homozygous FH [23,24].
The fact that this phenomenon is more widespread than the subject of academic curiosity, only described in rare case descriptions, is exemplified by a recent online survey that collected patient-reported data, including lipid panels, from several hundred adults following a carbohydrate-restricted diet [22]. Of all the 903 participants who participated in the survey, 42% and 22% reported having LDL-C levels higher than 6.5 mmol/L and 8.5 mmol/L, respectively. These values are thresholds in the Dutch Lipid Clinic Network (DLCN) criteria used in the (clinical) diagnosis of FH. Notably, 5% of the respondents entered LDLC levels > 13 mmol/L, which is considered to be consistent with homozygous FH, the most severe form of inherited dyslipidemia, which can cause cardiovascular mortality as early as in childhood if left untreated [24,25].
These observations, combined with the cases we described, are examples that both individuals adhering to these diets as well as their clinicians, should be cautious of their potential to cause or exacerbate severe hypercholesterolemia. High-fat, low-carbohydrate (‘keto’) diets may be considered in a line of fad diets known for exaggerated health claims and are frequently propagated through social media, where health misinformation is widespread, and the quality is difficult to assess [26]. Support for these diets is often provided in the form of opinionated, absolute statements that lack the backing of good-quality evidence highlighting harms and benefits [27,28]. Nevertheless, the number of individuals who follow a high-fat, low-carbohydrate diet is considerable and increasing. This diet is reported among the most frequently followed dietary patterns in the United States, with comparable prevalence to the commonly recommended Mediterranean and dietary approaches to stopping hypertension (DASH) diet [29,30]. There is currently no evidence to support that prolonged exposure to high levels of LDL-C in the context of high-fat, low-carbohydrate diets is not atherogenic (and therefore ‘safe’), which should be balanced against the totality of evidence on atherogenic lipoproteins and their causal role in the development of ASCVD [1].
The observations of extreme hypercholesterolemia described here and by others beg a discussion of possible pathophysiological explanations for this phenomenon. High levels of LDL-C may result from the reduced clearance of LDL particles from the circulation, the increased production of their VLDL precursor, or a combination of both. The increased intake of dietary fat and cholesterol, combined with limited to no intake of carbohydrates, are salient features of the diets consumed by the patients we described. In the following sections, we discuss these factors and their potential roles in the pathophysiology of hypercholesterolemia individually, but it is likely that these factors act in concert.
3.1. Increased Intake of Dietary Cholesterol
Cholesterol stores in the human body are in a constant state of flux. Cholesterol in LDL particles either originates from intestinal absorption or de novo synthesis in the liver. After an LDL particle is taken up by the liver, its cholesterol content enters the hepatic cholesterol pool and may either be secreted back into the bloodstream packed in lipoproteins or be excreted in bile directly or after conversion to bile acid. Combined with the intake from diet, this cholesterol is partly taken up by the intestine and transported back to the liver and partly exits the body through feces [31]. It could be hypothesized that the hypercholesterolemia observed in our patients is partly due to their cholesterol-rich diet.
However, cholesterol absorption, synthesis, and biliary excretion appear to be balanced in a way that the dietary intake of cholesterol, under normal circumstances, only modestly translates into the cholesterol levels found in the circulation. For example, vegans consuming 90% less dietary cholesterol than omnivores were found to have plasma LDL-C levels that were only 13% lower [32]. A meta-analysis of intervention studies that supplemented cholesterol through diet showed only moderate increases in LDL-C levels (up to 0.22 mmol/L) [33]. The apparent ‘resilience’ of the plasma cholesterol to high dietary cholesterol intake is exemplified by a case report of an 88-year-old male who ate 25 eggs per day but had a normal plasma LDL-C level of 3.68 mmol/L and no clinically important atherosclerosis. Isotope studies revealed that, compared with 10 healthy controls, the subject had a markedly reduced rate of cholesterol absorption and hepatic cholesterol synthesis but greatly increased synthesis of bile acids [34].
In healthy adults, the average fractional absorption rate of cholesterol in the intestine is approximately 50% but with great inter-individual variation ranging from 20% to 80% [35,36]. Although stable isotope studies were beyond the scope of our study, it is possible that the increased dietary intake of cholesterol exceeded the relative capacity to downregulate its intestinal absorption in our patients. The overload of dietary cholesterol delivered to hepatocytes may contribute to the downregulation of the LDL receptor (LDLR) and thus increase the circulating LDL-C levels [37,38,39].
The influence of gut microbiota on bile acid metabolism is another factor influencing cholesterol absorption [40]. Gut microbiota influence the circulating LDL-C levels via the conjugation of primary bile acids to secondary bile acids [41] and by facilitating bile acid excretion into feces [40]. Specific bacterial phyla, such as Lactobacillus and Clostridium, can prevent the reabsorption of bile acids into the enterohepatic circulation through the deconjugation of bile acids. Since most bile acids are taken up in a conjugated form, deconjugation by gut microbes may be an important factor in mediating cholesterol levels. It is possible that a high-fat, low-carbohydrate diet alters gut microbiota composition in an unfavorable way so that the intestinal reabsorption of bile acids and cholesterol is enhanced, and hence, cholesterol accumulates more quickly in the circulation. Unfortunately, fecal samples were unavailable from the cases we described.
3.2. Increased Intake of Dietary Fatty Acids
In the second half of the 20th century, large epidemiological studies linked the composition of dietary fat with plasma LDL-C levels and cardiovascular outcomes [42]. It is now clearly established that the intake of saturated fatty acids (SFAs) increases the circulating LDL-C levels, although the overall effect of limiting SFA intake on cardiovascular health remains controversial [43,44]. Meta-regression analysis of 84 feeding studies showed that replacing the daily energy intake from SFAs with carbohydrates or unsaturated fatty acids considerably lowers LDL-C levels [45]. The LDL-increasing mechanism of SFAs was investigated in various in vitro and in vivo models [46] and likely involves decreased mRNA and protein expression of LDLR, as well as decreased LDLR activity [47]. It has to be noted that this effect depends on SFA type: 12–16 carbon fatty acids, found in dairy and red meat, have the largest effect to raise LDL-C [43,44]. This notion is supported by feeding trials in which increased SFA intake in the form of red meat resulted in higher LDL-C levels than the same SFA intake through nonmeat protein sources [48]. High SFA intake by our patients likely contributed to their hypercholesterolemia, and the fact that LDL-C levels dropped when patient 4 exchanged his intake of butter, meat, and dairy products for more vegetables and vegetable oils supports this notion.
Furthermore, an increased flux of fatty acids to the liver stimulates the assembly and secretion of VLDL particles [49], which would be in line with the abundance of VLDL-C observed with FPLC analysis in patients 1 and 2. However, the absence of triglycerides suggests other factors are also at play, such as extremely efficient lipolytic activity or the active exchange of cholesteryl esters.
3.3. Decreased Intake of Carbohydrates
Diets are generally considered very low in carbohydrates when the energy intake from carbohydrates is <10% of the total energy intake or <50 g per day [50]. These diets have been recommended as a treatment for patients with specific medical conditions, such as rare metabolic diseases or epilepsy, where hyperlipidemia is a well-known side effect of the ketogenic diet [51,52]. The ketogenic diet is gaining increasing scientific attention in the field of sports medicine, where multiple feeding trials have been conducted in healthy athletes that consistently show increases in LDL-C levels [53,54,55,56,57]. However, individual responses are variable, and LDL-C levels do not reach the levels consistent with homozygous FH, as observed in the extreme cases we presented. Carbohydrate-restricted diets have further gained attention through popular scientific news outlets and social media channels to reduce weight, as was the case for patient 4.
A decreased intake of carbohydrates inadvertently means that the relative intake of protein and/or fat is increased, but the relative importance of ‘low-carbohydrate’ vs. ‘high-fat’ diet intake in causing hypercholesterolemia remains to be established. It has been hypothesized that carbohydrate restriction leads to increased dependence on fat as a metabolic substrate, which drives the increased hepatic secretion of triglyceride rich VLDL. It is thought that triglycerides are taken up very rapidly by peripheral tissues, which have come to rely heavily on fats as their metabolic substrate. The resulting lipid profile is characterized by markedly elevated levels of LDL-C and HDL-C, yet low triglycerides [58]. Our observation of elevated VLDL-C, LDL-C, and HDL-C, combined with relatively low levels of triglycerides found in patients 1 and 2, are in support of this ‘lipid energy model’. The authors further hypothesize that low levels of insulin, as we observed in patients 1 and 2, in combination with low hepatic glycogen stores (not measured by us), contribute to increased VLDL secretion rates. There is anecdotal evidence that the reintroduction of carbohydrates reverses the hypercholesterolemia seen in these subjects [22,23], but this could not be accurately assessed in our patients. Future studies, such as stable isotope studies under controlled feeding conditions, are required to unravel the relative importance of carbohydrate restriction on the observed increases in LDL-C levels. Such studies can also be used to determine both the excretion and absorption rates of cholesterol in the gut, as well as the production and uptake of lipoproteins by the liver in the context of high-fat, low-carbohydrate diets.
4. Conclusions and Future Research
In conclusion, mounting evidence describes the relationship between high-fat, low-carbohydrate diets and severely elevated plasma LDL-C levels generally considered to be consistent with (homozygous) FH. This phenomenon provides unique opportunities to study the fundamental (patho)physiological mechanisms involving cholesterol and lipoprotein homeostasis. Clinicians should rule out high-fat, low-carbohydrate diets as a possible cause for hypercholesterolemia in patients presenting with clinical FH in whom no mutation is found and discuss dietary modifications to durably reduce LDL-C levels and ASCVD risk.
Author Contributions
Conceptualization, T.R.T., V.H. and A.K.G.; resources, E.S.G.S.; data curation, T.R.T., J.H.M.L. and J.R.v.L.; writing—original draft preparation, T.R.T. and V.H.; writing—review and editing, A.G., D.M.C., J.R.v.L., A.K.G. and E.S.G.S.; visualization, T.R.T. and V.H.; supervision, A.K.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by research grants from the Netherlands Organization for Scientific Research (vidi 016.156.445) and Klinkerpad funds. VH is appointed by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie Grant Agreement No. 813781 ITN BestTreat.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Amsterdam UMC, location AMC (protocol code NL62407.018.17) for studies involving humans.
Informed Consent Statement
Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank the participants for their contribution to this research.
Conflicts of Interest
V.H., A.G., D.M.C., J.H.M.L., A.K.G. and T.R.T. declare no conflict of interest. J.R.v.L. reports research grants from Amryt and Novartis (all paid to the institution). E.S.G.S. reports consulting fees from Amgen, Sanofi, Regeneron, Esperion, Novartis, and Ionis/Akcea (all paid to the institution).
Appendix A
Dietary Pattern of Patient 1 on an Example Day
- Breakfast
- –
- Black coffee (filtered).
- Lunch
- –
- Eggs (6 to 8) and bacon fried in butter.
- Dinner
- –
- Approximately 750 g of meat, such as red meat steak pork chops (fat still on), chicken thighs, fatty fish, meatballs (three parts 18% minced meat mixed with one part minced liver), fried in butter.
- Beverages
- –
- Water with meals, black coffee, approximately two beers weekly;
- –
- No protein shakes/beverages or sugar-sweetened beverages.
- Cooking/baking
- –
- 500 g of butter weekly used for baking;
- –
- Explicitly no vegetables oils or margarines; no vegetables or fruits; no rice, pasta, or potatoes.

Table A1.
Calculated energy (kcal) and macronutrient (g and E-%), fiber (g), salt (g), and sodium (mg) intake on an example day of case 1 on a carnivore diet.
Table A1.
Calculated energy (kcal) and macronutrient (g and E-%), fiber (g), salt (g), and sodium (mg) intake on an example day of case 1 on a carnivore diet.
| Patient 1 | |
|---|---|
| Energy (kcal/d) | 3275 |
| Fat (g/d) | 220.5 |
| Fat (E-%) | 60.6 |
| Saturated fat (g/d) | 99.0 |
| Saturated fat (E-%) | 27.2 |
| Protein (g/d) | 304.1 |
| Protein E-% | 37.1 |
| Carbohydrate (g/d) | 5.5 |
| Carbohydrate (E-%) | 0.7 |
| Alcohol (g/d) | 6 |
| Fiber (g/d) | 0.4 |
| Salt (g/d) | 12.8 |
| Sodium (mg/d) | 5097 |
E-%, percentage of energy intake; g/d, grams per day; kcal/d, kilocalories per day; mg/d, milligrams per day.
Appendix B
Dietary Pattern of Patient 4 on Example Days
- Breakfast
- –
- 10% Greek yogurt with home-baked seeds in a little coconut fat, honey, and cinnamon (no other sugar or sweeteners);
- –
- Three eggs and bacon fried in butter;
- –
- Oven-baked ricotta cheese with raspberries, oranges, pecan nuts, and an egg.
- Lunch
- –
- Alternatives for lunch were beet salad with herring in olive oil or fatty mayonnaise; chicory salad with salmon and a scoop of 10% Greek yogurt; Cesar salad with fatty mayonnaise; zucchini soup with fatty cooking cream; OR low-carbohydrate sandwich with fried egg, cheese, and raw vegetables.
- Dinner
- –
- Alternatives for dinner were leek disk codfish in high-fat cooking cream; chicory dish in cheese and ham, in butter; spinach with egg and cream; Nasi (typical Indonesian dish) of cauliflower rice with rice and chicken fried in coconut fat; lightly cooked beans with chicken, tomato and some extra herbs, stir-fried in butter; OR pizza made with chicken breast with lots of vegetables covered in tomato sauce and mozzarella.
- Cooking/baking
- –
- Nearly all dishes are cooked based on recipes in the books by “The New Food”: https://thenewfood.nl/ (accessed on 26 November 2021)

Table A2.
Calculated energy (kcal) and macronutrient (g and E-%), fiber (g), salt (g), and sodium (mg) intake on an example day of case 4 on a low-carbohydrate, high-fat diet.
Table A2.
Calculated energy (kcal) and macronutrient (g and E-%), fiber (g), salt (g), and sodium (mg) intake on an example day of case 4 on a low-carbohydrate, high-fat diet.
| Patient 4 | |
|---|---|
| Energy (kcal/d) | 1912 |
| Fat (g/d) | 147.2 |
| Fat (E-%) | 69.3 |
| Saturated fat (g/d) | 74.1 |
| Saturated fat (E-%) | 34.9 |
| Protein (g/d) | 85.2 |
| Protein (E-%) | 17.8 |
| Carbohydrate (g/d) | 55.3 |
| Carbohydrate (E-%) | 11.6 |
| Alcohol (g/d) | 0.0 |
| Fiber (g/d) | 17.2 |
| Salt (g/d) | 3.9 |
| Sodium (mg/d) | 1558 |
E-%, percentage of energy intake; g/d, grams per day; kcal/d, kilocalories per day; mg/d, milligrams per day.
References
- Ference, B.A.; Ginsberg, H.N.; Graham, I.; Ray, K.K.; Packard, C.J.; Bruckert, E.; Hegele, R.A.; Krauss, R.M.; Raal, F.J.; Schunkert, H.; et al. Low-density lipoproteins cause atherosclerotic cardiovascular disease. 1. Evidence from genetic, epidemiologic, and clinical studies. A consensus statement from the European Atherosclerosis Society Consensus Panel. Eur. Heart J. 2017, 38, 2459–2472. [Google Scholar] [CrossRef]
- Anitschkow, N.N.; Chalatov, S. Ueber experimentelle Choleserinsteatose und ihre Bedeutung fur die Entstehung einiger pathologischer Prozesse. Zentralbl. Allg. Pathol. 1913, 24, 1–9. [Google Scholar]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Kromhout, D.; Menotti, A.; Bloemberg, B.; Aravanis, C.; Blackburn, H.; Buzina, R.; Dontas, A.S.; Fidanza, F.; Giaipaoli, S.; Jansen, A.; et al. Dietary saturated and trans fatty acids and cholesterol and 25-year mortality from coronary heart disease: The Seven Countries Study. Prev. Med. 1995, 24, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Menotti, A.; Puddu, P.E.; Adachi, H.; Tolonen, H.; Kafatos, A. Association of serum cholesterol with coronary heart disease mortality during 50-year follow-up in ten cohorts of the seven countries study. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1337–1346. [Google Scholar] [CrossRef]
- Steinberg, D. An interpretive history of the cholesterol controversy: Part I. J. Lipid. Res. 2004, 45, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Poli, A.; Barbagallo, C.M.; Cicero, A.F.G.; Corsini, A.; Manzato, E.; Trimarco, B.; Bernini, F.; Visioli, F.; Bianchi, A.; Canzone, G.; et al. Nutraceuticals and functional foods for the control of plasma cholesterol levels. An intersociety position paper. Pharmacol. Res. 2018, 134, 51–60. [Google Scholar] [CrossRef]
- Schoeneck, M.; Iggman, D. The effects of foods on LDL cholesterol levels: A systematic review of the accumulated evidence from systematic reviews and meta-analyses of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1325–1338. [Google Scholar] [CrossRef]
- The Lipid Research Clinics Coronary Primary Prevention Trial results. I. Reduction in incidence of coronary heart disease. JAMA 1984, 251, 351–364. [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, e177–e232. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Hollænder, P.L.B.; Ross, A.B.; Kristensen, M. Whole-grain and blood lipid changes in apparently healthy adults: A systematic review and meta-analysis of randomized controlled studies1-3. Am. J. Clin. Nutr. 2015, 102, 556–572. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L. Familial hypercholesterolemia: Defective binding of lipoproteins to cultured fibroblasts associated with impaired regulation of 3 hydroxy 3 methylglutaryl coenzyme A reductase activity. Proc. Natl. Acad. Sci. USA 1974, 71, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Humphries, S.E.; Ginsberg, H.N.; Masana, L.; Descamps, O.S.; Wiklund, O.; Hegele, R.A.; Raal, F.J.; Defesche, J.C.; et al. Familial hypercholesterolaemia is underdiagnosed and undertreated in the general population: Guidance for clinicians to prevent coronary heart disease: Consensus Statement of the European Atherosclerosis Society. Eur. Heart J. 2013, 34, 3478–3490. [Google Scholar] [CrossRef]
- Reeskamp, L.F.; Tromp, T.R.; Defesche, J.C.; Grefhorst, A.; Stroes, E.S.; Hovingh, G.K.; Zuurbier, L. Next-generation sequencing to confirm clinical familial hypercholesterolemia. Eur. J. Prev. Cardiol. 2020, 28, 875–883. [Google Scholar] [CrossRef]
- Balder, J.W.; de Vries, J.K.; Nolte, I.M.; Lansberg, P.J.; Kuivenhoven, J.A.; Kamphuisen, P.W. Lipid and lipoprotein reference values from 133,450 Dutch Lifelines participants: Age- and gender-specific baseline lipid values and percentiles. J. Clin. Lipidol. 2017, 11, 1055–1064.e6. [Google Scholar] [CrossRef]
- Matli, B.; Schulz, A.; Koeck, T.; Falter, T.; Lotz, J.; Rossmann, H.; Pfeiffer, N.; Beutel, M.; Münzel, T.; Strauch, K.; et al. Distribution of HOMA-IR in a population-based cohort and proposal for reference intervals. Clin. Chem. Lab. Med. 2021, 59, 1844–1851. [Google Scholar] [CrossRef]
- Engelen, L.; Ferreira, I.; Stehouwer, C.D.; Boutouyrie, P.; Laurent, S. Reference intervals for common carotid intima-medi thickness measured with echotracking: Relation with risk factors. Eur. Heart J. 2013, 34, 2368–2380. [Google Scholar] [CrossRef]
- Goldberg, I.J.; Ibrahim, N.; Bredefeld, C.; Foo, S.; Lim, V.; Gutman, D.; Huggins, L.-A.; Hegele, R.A. Ketogenic diets, not for everyone. J. Clin. Lipidol. 2021, 15, 61–67. [Google Scholar] [CrossRef]
- Schaffer, A.E.; D’Alessio, D.A.; Guyton, J.R. Extreme elevations of low-density lipoprotein cholesterol with very low carbohydrate, high fat diets. J. Clin. Lipidol. 2021, 15, 525–526. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Feldman, D.; Soto-Mota, A.; Kalayjian, T.; Ludwig, D.S. Elevated LDL Cholesterol with a Carbohydrate-Restricted Diet: Evidence for a “lean Mass Hyper-Responder” Phenotype. Curr. Dev. Nutr. 2022, 6, nzab144. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, N.G.; Soto-Mota, A.; Feldman, D.; Parpos, S.; Budoff, M. Case Report: Hypercholesterolemia “Lean Mass Hyper-Responder” Phenotype Presents in the Context of a Low Saturated Fat Carbohydrate-Restricted Diet. Front. Endocrinol. 2022, 13, 830325. [Google Scholar] [CrossRef] [PubMed]
- Tromp, T.R.; Hartgers, M.L.; Hovingh, G.K.; Vallejo-Vaz, A.J.; Ray, K.K.; Soran, H.; Freiberger, T.; Bertolini, S.; Harada-Shiba, M.; Blom, D.J.; et al. Worldwide experience of homozygous familial hypercholesterolaemia: Retrospective cohort study. Lancet 2022, 399, 719–728. [Google Scholar] [CrossRef]
- Cuchel, M.; Bruckert, E.; Ginsberg, H.N.; Raal, F.J.; Santos, R.D.; Hegele, R.A.; Kuivenhoven, J.A.; Nordestgaard, B.G.; Descamps, O.S.; Steinhagen-Thiessen, E.; et al. Homozygous familial hypercholesterolaemia: New insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur. Heart J. 2014, 35, 2146–2157. [Google Scholar] [CrossRef]
- Suarez-Lledo, V.; Alvarez-Galvez, J. Prevalence of health misinformation on social media: Systematic review. J. Med. Internet Res. 2021, 23, e17187. [Google Scholar] [CrossRef] [PubMed]
- Tahreem, A.; Rakha, A.; Rabail, R.; Nazir, A.; Socol, C.T.; Maerescu, C.M.; Aadil, R.M. Fad Diets: Facts and Fiction. Front. Nutr. 2022, 9, 960922. [Google Scholar] [CrossRef]
- O’Neill, B.; Raggi, P. The ketogenic diet: Pros and cons. Atherosclerosis 2020, 292, 119–126. [Google Scholar] [CrossRef]
- International Food Information Council. 2021 Food & Health Survey. Available online: https://foodinsight.org/wp-content/uploads/2021/05/IFIC-2021-Food-and-Health-Survey.May-2021-1.pdf (accessed on 26 November 2022).
- International Food Information Council. 2022 Food and Health Survey. 18 May 2022. Available online: https://foodinsight.org/wp-content/uploads/2022/05/IFIC-2022-Food-and-Health-Survey-Report.pdf (accessed on 26 November 2022).
- Stellaard, F. From Dietary Cholesterol to Blood Cholesterol, Physiological Lipid Fluxes, and Cholesterol Homeostasis. Nutrients 2022, 14, 1643. [Google Scholar] [CrossRef]
- Lütjohann, D.; Meyer, S.; von Bergmann, K.; Stellaard, F. Cholesterol Absorption and Synthesis in Vegetarians and Omnivores. Mol. Nutr. Food Res. 2018, 62, 1700689. [Google Scholar] [CrossRef]
- Berger, S.; Raman, G.; Vishwanathan, R.; Jacques, P.F.; Johnson, E.J. Dietary cholesterol and cardiovascular disease: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2015, 102, 276–294. [Google Scholar] [CrossRef] [PubMed]
- Kern, F. Normal Plasma Cholesterol in an 88-Year-Old Man Who Eats 25 Eggs a Day. N. Engl. J. Med. 1991, 324, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Bosner, M.S.; Ostlund, R.E.; Osofisan, O.; Grosklos, J.; Fritschle, C.; Lange, L.G. Assessment of percent cholesterol absorption in humans with stable isotopes. J. Lipid. Res. 1993, 34, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Lutjohann, D.; Meese, C.O.; Crouse, J.R.; Von Bergmann, K. Evaluation of deuterated cholesterol and deuterated sitostanol for measurement of cholesterol absorption in humans. J. Lipid. Res. 1993, 34, 1039–1046. [Google Scholar] [CrossRef]
- Grundy, S.M. Does Dietary Cholesterol Matter? Curr. Atheroscler. Rep. 2016, 18, 68. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. The LDL receptor. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 431–438. [Google Scholar] [CrossRef]
- Dandan, M.; Han, J.; Mann, S.; Kim, R.; Mohammed, H.; Nyangau, E.; Hellerstein, M. Turnover Rates of the Low-Density Lipoprotein Receptor and PCSK9: Added Dimension to the Cholesterol Homeostasis Model. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2866–2876. [Google Scholar] [CrossRef]
- Vourakis, M.; Mayer, G.; Rousseau, G. The role of gut microbiota on cholesterol metabolism in atherosclerosis. Int. J. Mol. Sci. 2021, 22, 8074. [Google Scholar] [CrossRef]
- Russell, D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu. Rev. Biochem. 2003, 72, 137–174. [Google Scholar] [CrossRef]
- Menotti, A.; Kromhout, D.; Puddu, P.E.; Alberti-Fidanza, A.; Hollman, P.; Kafatos, A.; Tolonen, H.; Adachi, H.; Jacobs, D.R., Jr. Baseline fatty acids, food groups, a diet score and 50-year all-cause mortality rates. An ecological analysis of the Seven Countries Study. Ann. Med. 2017, 49, 718–727. [Google Scholar] [CrossRef]
- Maki, K.C.; Dicklin, M.R.; Kirkpatrick, C.F. Saturated fats and cardiovascular health: Current evidence and controversies. J. Clin. Lipidol. 2021, 15, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Astrup, A.; Magkos, F.; Bier, D.M.; Brenna, J.T.; De Oliveira Otto, M.C.; Hill, J.O.; King, J.C.; Mente, A.; Ordovas, J.M.; Volek, J.S.; et al. Saturated Fats and Health: A Reassessment and Proposal for Food-Based Recommendations: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 76, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; World Health Organization. Effects of Saturated Fatty Acids on Serum Lipids and Lipoproteins: A Systematic Review and Regression Analysis; World Health Organization: Geneva, Switzerland, 2016; pp. 1–63. [Google Scholar]
- Fernandez, M.L.; West, K.L. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005, 135, 2075–2078. [Google Scholar] [CrossRef]
- Woollett, L.A.; Spady, D.K.; Dietschy, J.M. Saturated and unsaturated fatty acids independently regulate low density lipoprotein receptor activity and production rate. J. Lipid. Res. 1992, 33, 77–88. [Google Scholar] [CrossRef]
- Bergeron, N.; Chiu, S.; Williams, P.T.; MKing, S.; Krauss, R.M. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 24–33. [Google Scholar] [CrossRef]
- Ginsberg, H.N. Insulin resistance and cardiovascular disease. J. Clin. Invest. 2000, 106, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Kirkpatrick, C.F.; Bolick, J.P.; Kris-Etherton, P.M.; Sikand, G.; Aspry, K.E.; Soffer, D.E.; Willard, K.-E.; Maki, K.C. Review of current evidence and clinical recommendations on the effects of low-carbohydrate and very-low-carbohydrate (including ketogenic) diets for the management of body weight and other cardiometabolic risk factors: A scientific statement from the Nati. J. Clin. Lipidol. 2019, 13, 689–711.e1. [Google Scholar] [CrossRef]
- Kossoff, E.H.; Zupec-Kania, B.A.; Auvin, S.; Ballaban-Gil, K.R.; Christina Bergqvist, A.G.; Blackford, R.; Buchhalter, J.R.; Caraballo, R.H.; Cross, J.H.; Dahlin, M.G.; et al. Optimal clinical management of children receiving dietary therapies for epilepsy: Updated recommendations of the International Ketogenic Diet Study Group. Epilepsia Open. 2018, 3, 175–192. [Google Scholar] [CrossRef]
- Kwiterovich, P.O.; Vining, E.P.G.; Pyzik, P.; Skolasky, R.; Freeman, J.M. Effect of a High-Fat Ketogenic Diet on Plasma Levels of Lipids, Lipoproteins, and Apolipoproteins in Children. J. Am. Med. Assoc. 2003, 290, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Lee, J. Influences of ketogenic diet on body fat percentage, respiratory exchange rate, and total cholesterol in athletes: A systematic review and meta-analysis. Int. J. Environ. Res. Public Health 2021, 18, 2912. [Google Scholar] [CrossRef]
- Buga, A.; Welton, G.L.; Scott, K.E.; Atwell, A.D.; Haley, S.J.; Esbenshade, N.J.; Abraham, J.; Buxton, J.D.; Ault, D.L.; Raabe, A.S.; et al. The Effects of Carbohydrate versus Fat Restriction on Lipid Profiles in Highly Trained, Recreational Distance Runners: A Randomized, Cross-Over Trial. Nutrients 2022, 14, 1135. [Google Scholar] [CrossRef]
- Creighton, B.C.; Hyde, P.N.; Maresh, C.M.; Kraemer, W.J.; Phinney, S.D.; Volek, J.S. Paradox of hypercholesterolaemia in highly trained, keto-adapted athletes. BMJ Open Sport Exerc. Med. 2018, 4, e000429. [Google Scholar] [CrossRef] [PubMed]
- Burén, J.; Ericsson, M.; Damasceno, N.; Sjödin, A. A Ketogenic Low-Carbohydrate High-Fat Diet Increases LDL Cholesterol in Healthy, Young, Normal-Weight Women: A Randomized Controlled Feeding Trial. Nutrients 2021, 13, 814. [Google Scholar] [CrossRef] [PubMed]
- Retterstøl, K.; Svendsen, M.; Narverud, I.; Holven, K.B. Effect of low carbohydrate high fat diet on LDL cholesterol and gene expression in normal-weight, young adults: A randomized controlled study. Atherosclerosis 2018, 279, 52–61. [Google Scholar] [CrossRef]
- Norwitz, N.G.; Soto-Mota, A.; Kaplan, B.; Ludwig, D.S.; Budoff, M.; Kontush, A.; Feldman, D. The Lipid Energy Model: Reimagining Lipoprotein Function in the Context of Carbohydrate-Restricted Diets. Metabolites 2022, 12, 460. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).