Effect of Vitamin D Supplementation on Depression in Adults: A Systematic Review of Randomized Controlled Trials (RCTs)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Registration and Study Design
2.2. Eligibility Assessment and Inclusion/Exclusion Procedure
- study conducted in adults;
- studied population of patients with depression diagnosed;
- study presenting oral vitamin D supplementation of known dose;
- depression monitored within the study using a valid mental health outcome measure;
- study described as RCT;
- study published as an article in a peer-reviewed journal.
- animal model study;
- study presenting influence of multiple nutrients combined;
- study conducted in subjects with any concurrent physical disease or disability;
- study conducted in pregnant women;
- study conducted in subjects with concurrent eating disorders;
- study conducted in subjects with concurrent intellectual disabilities;
- study not published in English.
2.3. Searching Procedure
2.4. Data Extraction Procedure and Study Assessment Procedure
- the general description of the study and studied population (including authors and year of the study; country/detailed location; studied population; period of the study);
- the description of the studied population (including number of participants; female/male proportions; age; inclusion and exclusion criteria);
- the description of the supplementation of vitamin D (including dosage regimen; intervention duration) and of the assessment of depression status (including psychological measure);
- the observations and conclusions (based on those formulated by authors of the study).
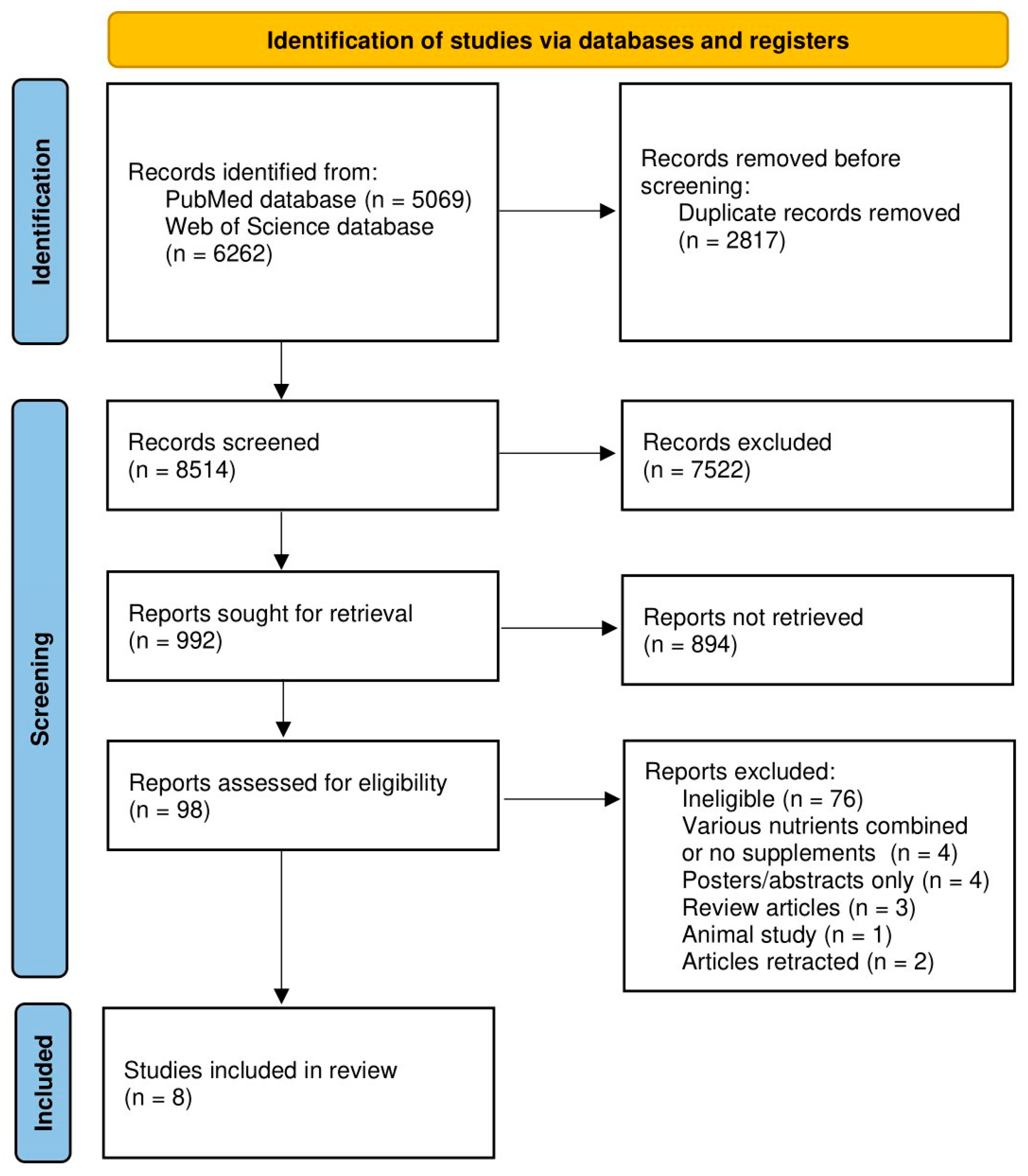
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Chand, S.P.; Arif, H. Depression. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- The American Psychiatric Association’s Diagnostic Statistical Manual of Mental Disorders, Fifth Edition (DSM-5). Available online: https://dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596?cookieSet=1 (accessed on 27 December 2022).
- National Research Council (US); Institute of Medicine (US) Committee on Depression, Parenting Practices; The Healthy Development of Children. Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention. In The Etiology of Depression; England, M.J., Sim, L.J., Eds.; National Academies Press (US): Washington, DC, USA, 2009; Volume 3. [Google Scholar]
- Lim, G.Y.; Tam, W.W.; Lu, Y.; Ho, C.S.; Zhang, M.W.; Ho, R.C. Prevalence of Depression in the Community from 30 Countries between 1994 and 2014. Sci. Rep. 2018, 12, 2861. [Google Scholar] [CrossRef]
- Bueno-Notivol, J.; Gracia-García, P.; Olaya, B.; Lasheras, I.; López-Antón, R.; Santabárbara, J. Prevalence of depression during the COVID-19 outbreak: A meta-analysis of community-based studies. Int. J. Clin. Health Psychol. 2021, 21, 100196. [Google Scholar] [CrossRef] [PubMed]
- WHO. Comprehensive Mental Health Action Plan 2013–2030. Available online: https://www.who.int/publications/i/item/9789240031029 (accessed on 27 December 2022).
- InformedHealth. Treatments for Depression; Institute for Quality and Efficiency in Health Care (IQWiG): Cologne, Germany, 2006. [Google Scholar]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Lang, U.E.; Beglinger, C.; Schweinfurth, N.; Walter, M.; Borgwardt, S. Nutritional aspects of depression. Cell. Physiol. Biochem. 2015, 37, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.R.M.; Barros, W.M.A.; Silva, M.L.D.; Silva, J.M.L.D.; Souza, A.P.D.S.; Silva, A.B.J.D.; Fernandes, M.S.S.; Souza, S.L.; Souza, V.O.N. Relationship between vitamin D deficiency and psychophysiological variables: A systematic review of the literature. Clinics 2021, 76, e3155. [Google Scholar] [CrossRef]
- Gowda, U.; Mutowo, M.P.; Smith, B.J.; Wluka, A.E.; Renzaho, A.M. Vitamin D supplementation to reduce depression in adults: Meta-analysis of randomized controlled trials. Nutrition 2015, 31, 421–429. [Google Scholar] [CrossRef]
- Li, G.; Mbuagbaw, L.; Samaan, Z.; Falavigna, M.; Zhang, S.; Adachi, J.D.; Cheng, J.; Papaioannou, A.; Thabane, L. Efficacy of vitamin D supplementation in depression in adults: A systematic review. J. Clin. Endocrinol. Metab. 2014, 99, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J.A.; Edmondson, D.; Taggart Wasson, L.; Falzon, L.; Homma, K.; Ezeokoli, N.; Li, P.; Davidson, K.W. Vitamin D supplementation for depressive symptoms: A systematic review and meta-analysis of randomized controlled trials. Psychosom. Med. 2014, 76, 190–196. [Google Scholar] [CrossRef]
- Spedding, S. Vitamin D and depression: A systematic review and meta-analysis comparing studies with and without biological flaws. Nutrients 2014, 6, 1501–1518. [Google Scholar] [CrossRef]
- Vellekkatt, F.; Menon, V. Efficacy of vitamin D supplementation in major depression: A meta-analysis of randomized controlled trials. J. Postgrad. Med. 2019, 65, 74–80. [Google Scholar]
- Mikola, T.; Marx, W.; Lane, M.M.; Hockey, M.; Loughman, A.; Rajapolvi, S.; Rocks, T.; O’Neil, A.; Mischoulon, D.; Valkonen-Korhonen, M.; et al. The effect of vitamin D supplementation on depressive symptoms in adults: A systematic review and meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. 2022, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Albuloshi, T.; Dimala, C.A.; Kuhnle, G.G.C.; Bouhaimed, M.; Dodd, G.F.; Spencer, J.P.E. The Effectiveness of Vitamin D Supplementation in Reducing Depressive Symptoms: A Systematic Review and Meta-analysis of Randomized Controlled Trials (RCTs). Nutr. Healthy Aging 2021, 6, 301–318. [Google Scholar] [CrossRef]
- Bains, N.; Abdijadid, S. Major Depressive Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Sepehrmanesh, Z.; Kolahdooz, F.; Abedi, F.; Mazroii, N.; Assarian, A.; Asemi, Z.; Esmaillzadeh, A. Vitamin D Supplementation Affects the Beck Depression Inventory, Insulin Resistance, and Biomarkers of Oxidative Stress in Patients with Major Depressive Disorder: A Randomized, Controlled Clinical Trial. J. Nutr. 2016, 146, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. The Influence of Vitamin D Intake and Status on Mental Health in Children: A Systematic Review. Nutrients 2021, 13, 952. [Google Scholar] [CrossRef]
- Guzek, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Głąbska, D. Association between Vitamin D Supplementation and Mental Health in Healthy Adults: A Systematic Review. J. Clin. Med. 2021, 10, 5156. [Google Scholar] [CrossRef]
- Guzek, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Głąbska, D. Influence of Vitamin D Supplementation on Mental Health in Diabetic Patients: A Systematic Review. Nutrients 2021, 13, 3678. [Google Scholar] [CrossRef]
- Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. Vitamin D Supplementation and Mental Health in Multiple Sclerosis Patients: A Systematic Review. Nutrients 2021, 24, 4207. [Google Scholar] [CrossRef]
- Głąbska, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Guzek, D. Vitamin D Supplementation and Mental Health in Inflammatory Bowel Diseases and Irritable Bowel Syndrome Patients: A Systematic Review. Nutrients 2021, 13, 3662. [Google Scholar] [CrossRef]
- Assessing Risk of Bias in Non-Randomized Studies. Chapter 13.5.2.3. Available online: http://handbook-5-1.cochrane.org/ (accessed on 29 December 2022).
- RoB 2: A Revised Cochrane Risk-of-Bias Tool for Randomized Trials. Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 29 December 2022).
- Minozzi, S.; Cinquini, M.; Gianola, S.; Gonzalez-Lorenzo, M.; Banzi, R. The revised Cochrane risk of bias tool for randomized trials (RoB 2) showed low interrater reliability and challenges in its application. J. Clin. Epidemiol. 2020, 126, 37–44. [Google Scholar] [CrossRef]
- Khoraminya, N.; Tehrani-Doost, M.; Jazayeri, S.; Hosseini, A.; Djazayery, A. Therapeutic effects of vitamin D as adjunctive therapy to fluoxetine in patients with major depressive disorder. Aust. N. Z. J. Psychiatry 2013, 47, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Marsh, W.K.; Penny, J.L.; Rothschild, A.J. Vitamin D supplementation in bipolar depression: A double blind placebo controlled trial. J. Psychiatr. Res. 2017, 95, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.P.; Pareek, M.; Hvolby, A.; Schmedes, A.; Toft, T.; Dahl, E.; Nielsen, C.T. Vitamin D3 supplementation and treatment outcomes in patients with depression (D3-vit-dep). BMC Res. Notes 2019, 3, 203. [Google Scholar] [CrossRef] [PubMed]
- Alavi, N.M.; Khademalhoseini, S.; Vakili, Z.; Assarian, F. Effect of vitamin D supplementation on depression in elderly patients: A randomized clinical trial. Clin. Nutr. 2019, 38, 2065–2070. [Google Scholar] [CrossRef]
- Kaviani, M.; Nikooyeh, B.; Zand, H.; Yaghmaei, P.; Neyestani, T.R. Effects of vitamin D supplementation on depression and some involved neurotransmitters. J. Affect. Disord. 2020, 15, 28–35. [Google Scholar] [CrossRef]
- Amini, S.; Amani, R.; Jafarirad, S.; Cheraghian, B.; Sayyah, M.; Hemmati, A.A. The effect of vitamin D and calcium supplementation on inflammatory biomarkers, estradiol levels and severity of symptoms in women with postpartum depression: A randomized double-blind clinical trial. Nutr. Neurosci. 2022, 25, 22–32. [Google Scholar] [CrossRef]
- Alghamdi, S.; Alsulami, N.; Khoja, S.; Alsufiani, H.; Tayeb, H.O.; Tarazi, F.I. Vitamin D Supplementation Ameliorates Severity of Major Depressive Disorder. J. Mol. Neurosci. 2020, 70, 230–235. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, Y.; Wang, T.; Lin, Y.; Yu, J.; Xia, Q.; Zhu, P.; Zhu, D.M. Vitamin D supplementation improves anxiety but not depression symptoms in patients with vitamin D deficiency. Brain Behav. 2020, 10, e01760. [Google Scholar] [CrossRef]
- Menon, V.; Kar, S.K.; Suthar, N.; Nebhinani, N. Vitamin D and Depression: A Critical Appraisal of the Evidence and Future Directions. Indian J. Psychol. Med. 2020, 42, 11–21. [Google Scholar] [CrossRef]
- Eyles, D.W.; Smith, S.; Kinobe, R.; Hewison, M.; McGrath, J.J. Distribution of the vitamin D receptor and one alpha-hydroxylase in human brain. J. Chem. Neuroanat. 2005, 29, 21–30. [Google Scholar] [CrossRef]
- Diesel, B.; Radermacher, J.; Bureik, M.; Bernhardt, R.; Seifert, M.; Reichrath, J.; Fischer, U.; Meese, E. Vitamin D(3) metabolism in human glioblastoma multiforme: Functionality of CYP27B1 splice variants, metabolism of calcidiol, and effect of calcitriol. Clin. Cancer Res. 2005, 11, 5370–5380. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Shaikh, A.S.; Han, W.; Chen, D.; Guo, Y.; Jiang, P. Vitamin D and depression: Mechanisms, determination and application. Asia Pac. J. Clin. Nutr. 2019, 28, 689–694. [Google Scholar] [PubMed]
- Croll, P.H.; Boelens, M.; Vernooij, M.W.; van de Rest, O.; Zillikens, M.C.; Ikram, M.A.; Voortman, T. Associations of vitamin D deficiency with MRI markers of brain health in a community sample. Clin. Nutr. 2021, 40, 72–78. [Google Scholar] [CrossRef]
- Garcion, E.; Wion-Barbot, N.; Montero-Menei, C.N.; Berger, F.; Wion, D. New clues about vitamin D functions in the nervous system. Trends Endocrinol. Metab. TEM 2002, 13, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Paravati, S.; Rosani, A.; Warrington, S.J. Physiology, Catecholamines. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Cass, W.A.; Smith, M.P.; Peters, L.E. Calcitriol protects against the dopamine- and serotonin-depleting effects of neurotoxic doses of methamphetamine. Ann. N. Y. Acad. Sci. 2006, 1074, 261–271. [Google Scholar] [CrossRef]
- Smith, M.P.; Fletcher-Turner, A.; Yurek, D.M.; Cass, W.A. Calcitriol protection against dopamine loss induced by intracerebroventricular administration of 6-hydroxydopamine. Neurochem. Res. 2006, 31, 533–5339. [Google Scholar] [CrossRef]
- Xie, F.; Huang, T.; Lou, D.; Fu, R.; Ni, C.; Hong, J.; Ruan, L. Effect of vitamin D supplementation on the incidence and prognosis of depression: An updated meta-analysis based on randomized controlled trials. Front. Public Health 2022, 10, 903547. [Google Scholar] [CrossRef]
- Libuda, L.; Timmesfeld, N.; Antel, J.; Hirtz, R.; Bauer, J.; Führer, D.; Zwanziger, D.; Öztürk, D.; Langenbach, G.; Hahn, D.; et al. Effect of vitamin D deficiency on depressive symptoms in child and adolescent psychiatric patients: Results of a randomized controlled trial. Eur. J. Nutr. 2020, 59, 3415–3424. [Google Scholar] [CrossRef]
- Jurinec, N.; Schienle, A. Utilizing placebos to leverage effects of cognitive-behavioral therapy in patients with depression. J. Affect. Disord. 2020, 277, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Peiris, N.; Blasini, M.; Wright, T.; Colloca, L. The Placebo Phenomenon: A Narrow Focus on Psychological Models. Perspect. Biol. Med. 2018, 61, 388–400. [Google Scholar] [CrossRef]
- Campbell, M.; McKenzie, J.E.; Sowden, A.; Katikireddi, S.V.; Brennan, S.E.; Ellis, S.; Hartmann-Boyce, J.; Ryan, R.; Shepperd, S.; Thomas, J.; et al. Synthesis without meta-analysis (SWiM) in systematic reviews: Reporting guideline. Br. Med. J. 2020, 368, l6890. [Google Scholar] [CrossRef] [PubMed]
| PICOS Criterion | Inclusion | Exclusion |
|---|---|---|
| Population | Adult patients with depression diagnosed | Pregnant women, patients with any concurrent physical disease or disability, patients with concurrent eating disorders, patients with concurrent intellectual disabilities |
| Intervention/exposure | Vitamin D oral supplementation of known dose | Multiple nutrients supplementation |
| Comparison | Compared with control group | No comparison with control group without vitamin D supplementation |
| Outcome | Depression monitored | No valid mental health outcome measure applied |
| Study design | Randomized Controlled Trials (RCTs) published as articles in peer-reviewed journals | Studies not published in English, animal model studies |
| Ref. | Authors, Year | Country/Detailed Location | Studied Population | Period of the Study |
|---|---|---|---|---|
| [29] | Khoraminya et al., 2013 | Iran/Tehran | Patients with major depressive disorder from the Roozbeh Psychiatry Hospital, Tehran University of Medical Sciences, Tehran | From November 2010 to December 2011 |
| [30] | Marsh et al., 2017 | United States of America (USA)/Massachusetts | Patients with bipolar depression and vitamin D deficiency from central Massachusetts, USA | From June 2013 to April 2015 |
| [31] | Hansen et al., 2019 | Denmark/Esbjerg, Odense and Svendborg | Patients with depressive episode from the mood disorder clinic in the Region of Southern Denmark | From November 2010 to June 2014 |
| [32] | Alavi et al., 2019 | Iran | Older patients with moderate to severe depression from three psychiatric clinics | From March 2016 to February 2017 |
| [33] | Kaviani et al., 2020 | Iran/Tehran | Patients with mild to moderate depression referred to the outpatient clinics of Baharloo Hospital | From May 2018 to June 2019 |
| [34] | Amini te al., 2020 | Iran/Ahvaz | Female patients with postpartum depression from the outpatient clinic of Ahvaz Jundishapur University of Medical Sciences | From June to November 2017 |
| [35] | Alghamdi et al., 2020 | Saudi Arabia/Jeddah | Patients with major depressive disorder from the psychiatry clinic at the King Abdulaziz University Hospital | Not specified (3 months) |
| [36] | Zhu et al., 2020 | China/Anhui | Patients with depression, anxiety and low 25(OH)D levels recruited through advertisements from Anhui Mental Health Center | From November 2015 to September 2019 |
| Ref. | Number of Participants (Female) | Age (Mean with SD/Range) | Inclusion Criteria | Exclusion Criteria |
|---|---|---|---|---|
| [29] | 40 (34) | 38.1 ± 10.1 years (vitamin D group) 39.6 ± 8.3 years (control group) | Inclusion: 18–65 years; diagnosis of major depressive disorder without psychotic features based on DSM-IV criteria; score of ≥15 on the 17-item HDRS; not taking any antidepressant or dietary supplements during the previous 2 months; being free from other psychiatric diagnoses, significant medical illnesses, or suicidal thoughts | Exclusion: substance abuse; pregnancy; lactation; occurrence of important adverse effects from medications |
| [30] | 33 (16) for baseline | 45.2 ± 13.3 years (vitamin D group) 43.3 ± 12.9 years (control group) | Inclusion: 18–70 years; bipolar disorder spectrum diagnosis (bipolar I, II, not otherwise specified); experiencing depressive symptoms rating ≥7 on the MADRS; having psychiatric care provider; if on psychotropic medication—a stable dose for the previous 2 weeks and remained on a current medication regime; serum 25-hydroxyvitamin D levels ≤ 30 ng/mL or insufficient | Exclusion: insulin dependent diabetes mellitus; liver and kidney diseases; parathyroid disorder; disorders of vitamin D metabolism; abnormal parathyroid hormone, calcium, or phosphorous level; taking Vitamin D replacement therapy; fat digestion disorder; gastrointestinal surgery; active suicidality; acute psychosis; active substance use <3 months |
| [31] | 62 (47) for baseline | 39.6 ± 13.5 years (vitamin D group) 38.7 ± 11.4 years (control group) | Inclusion: 18–65 years; depressive episode according to the ICD-10—mild to severe depression | Exclusion: bipolar affective disorder; any form of schizophrenia; tuberculosis; sarcoidosis; pregnancy; intake of more than 400 IU vitamin D daily; known allergy/intolerance to the content of the capsules; women in potential of childbearing if they did not utilize effective contraception; serum 25(OH)D < 10 nmol/L or > 100 nmol/L; serum calcium (ionized) > 1.40 mmol/L; estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2; serum phosphate < 1.50 mmol/L (females) or < 1.60 mmol/L (males aged 18–49 years) or < 1.35 mmol/L (males > 49 years), or serum PTH > 9.2 pmol/L |
| [32] | 78 (39) | 68.7 ± 7.0 years (vitamin D group) 67.0 ± 6.3 years (control group) | Inclusion: >60 years; GDS score > 5—moderate to severe depression; treatment for depression; Iranian citizenship; ability to speak Farsi and answer the questions | Exclusion: history of mental illness other than depression; history of physical disability; uncooperative; severe stress such as hospitalization or death of relatives |
| [33] | 56 (50) | 43.1 ± 9.2 years (vitamin D group) 42.8 ± 8.0 years (control group) | Inclusion: 18–60 years; BDI-II score of 13–29—mild to moderate depression | Exclusion: other psychiatric disease according to the psychiatrist’s assessments; history of heart infarction, angina pectoris, stroke, kidney stones, high blood pressure, liver disease, hyperparathyroidism; pregnancy and/or lactation; women <50 years not receiving adequate contraception; consuming nutritional supplements containing vitamin D from two months prior to the intervention |
| [34] | 76 (76) | 26.9 ± 1.0 years (vitamin D and calcium group) 29.2 ± 1.4 years (vitamin D group) 28.9 ± 1.6 years (control group) | Inclusion: women; 18–45 years; EPDS score >12—postpartum depression score; postpartum period from 1 to 6 months; BMI < 35 kg/m2 | Exclusion: serum vitamin D value >75 nmol/L; birth abnormalities; taking contraceptive agents; endocrine disorders; history of severe depression and/or other mental disorders; using antidepressants; serum calcium concentration >2.65 mmol/L; intake of vitamin D and calcium supplements during previous 6 months; history of diabetes, renal failure, kidney stones, gastrointestinal diseases |
| [35] | 62 (missing data) | 41.5 ± 1.8 years | Inclusion: 18–65 years; diagnosis of major depressive disorder based on DSM-V criteria | Exclusion: abnormal PTH level; renal or hepatic impairment |
| [36] | 106 (78) | 46.3 ± 9.7 years (vitamin D group) 43.3 ± 13.7 years (control group) | Inclusion: 18–60 years; diagnosis of major depressive disorders according to DSM-V; Han Chinese ethnicity; serum 25(OH) D ≤ 75 nmol/L | Exclusion: other concurrent psychiatric disorders defined in DSM-V; substance use disorders; current severe physical conditions; pregnancy; lactation |
| Ref. | Vitamin D Supplementation Dose Regimen | Vitamin D Supplementation Duration | Psychological Measure of Depression | Summary of Conclusions * |
|---|---|---|---|---|
| [29] | 1500 IU of vitamin D3 daily | 8 weeks | 24-item Hamilton Depression Rating Scale (HDRS) 21-item Beck Depression Inventory (BDI) | Confirming |
| [30] | 1500 IU of vitamin D3 daily | 12 weeks | Montgomery-Åsberg Depression Rating Scale (MADRS) | Not confirming |
| [31] | 2800 IU of vitamin D3 daily | 12 weeks | Hamilton Depression Rating Scale-17 (HAMD-17) Major Depression Inventory (MDI) World Health Organization-Five Well-Being Index (WHO-5) | Not confirming |
| [32] | 50,000 IU of vitamin D3 weekly | 8 weeks | Geriatric Depression Scale-15 (GDS-15) | Confirming |
| [33] | 50,000 IU of vitamin D3 biweekly | 8 weeks | Beck Depression Inventory-II (BDI-II) | Confirming |
| [34] | 50,000 IU of vitamin D3 biweekly | 8 weeks | Iranian Edinburgh Postnatal Depression Scale (EPDS) | Confirming |
| [35] | 50,000 IU of vitamin D3 weekly | 12 weeks | Beck Depression Inventory (BDI) | Confirming |
| [36] | 1600 IU of vitamin D3 daily | 6 months | Hamilton Depression Rating Scale-17 (HAMD-17) | Not confirming |
| Ref. | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Overall Bias |
|---|---|---|---|---|---|---|
| [29] |  |  |  |  |  |  |
| [30] |  |  |  |  |  |  |
| [31] |  |  |  |  |  |  |
| [32] |  |  |  |  |  |  |
| [33] |  |  |  |  |  |  |
| [34] |  |  |  |  |  |  |
| [35] |  |  |  |  |  |  |
| [36] |  |  |  |  |  |  |
 —Low risk of bias;
—Low risk of bias;  —Some concerns associated with risk of bias
—Some concerns associated with risk of bias  —High risk of bias; Domains: 1—arising from the randomization process; 2—deviations from the intended interventions; 3—missing outcome data; 4—measurement of the outcome; 5—selection of the reported result.
—High risk of bias; Domains: 1—arising from the randomization process; 2—deviations from the intended interventions; 3—missing outcome data; 4—measurement of the outcome; 5—selection of the reported result.Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzek, D.; Kołota, A.; Lachowicz, K.; Skolmowska, D.; Stachoń, M.; Głąbska, D. Effect of Vitamin D Supplementation on Depression in Adults: A Systematic Review of Randomized Controlled Trials (RCTs). Nutrients 2023, 15, 951. https://doi.org/10.3390/nu15040951
Guzek D, Kołota A, Lachowicz K, Skolmowska D, Stachoń M, Głąbska D. Effect of Vitamin D Supplementation on Depression in Adults: A Systematic Review of Randomized Controlled Trials (RCTs). Nutrients. 2023; 15(4):951. https://doi.org/10.3390/nu15040951
Chicago/Turabian StyleGuzek, Dominika, Aleksandra Kołota, Katarzyna Lachowicz, Dominika Skolmowska, Małgorzata Stachoń, and Dominika Głąbska. 2023. "Effect of Vitamin D Supplementation on Depression in Adults: A Systematic Review of Randomized Controlled Trials (RCTs)" Nutrients 15, no. 4: 951. https://doi.org/10.3390/nu15040951
APA StyleGuzek, D., Kołota, A., Lachowicz, K., Skolmowska, D., Stachoń, M., & Głąbska, D. (2023). Effect of Vitamin D Supplementation on Depression in Adults: A Systematic Review of Randomized Controlled Trials (RCTs). Nutrients, 15(4), 951. https://doi.org/10.3390/nu15040951









