A Combination of Acetate, Propionate, and Butyrate Increases Glucose Uptake in C2C12 Myotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
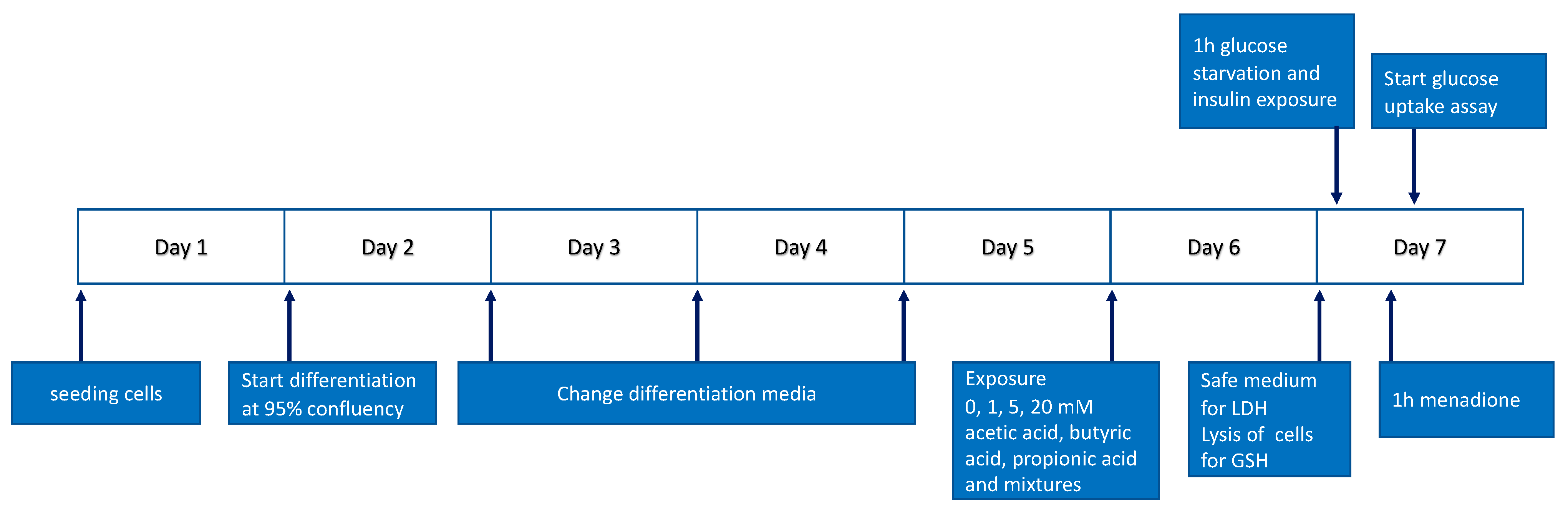
2.2. Cell Culture and Treatments
2.3. Cytotoxicity Measurement
2.4. Glucose Uptake Measurement
2.5. GSH Measurement
2.6. Statistical Analysis
3. Results
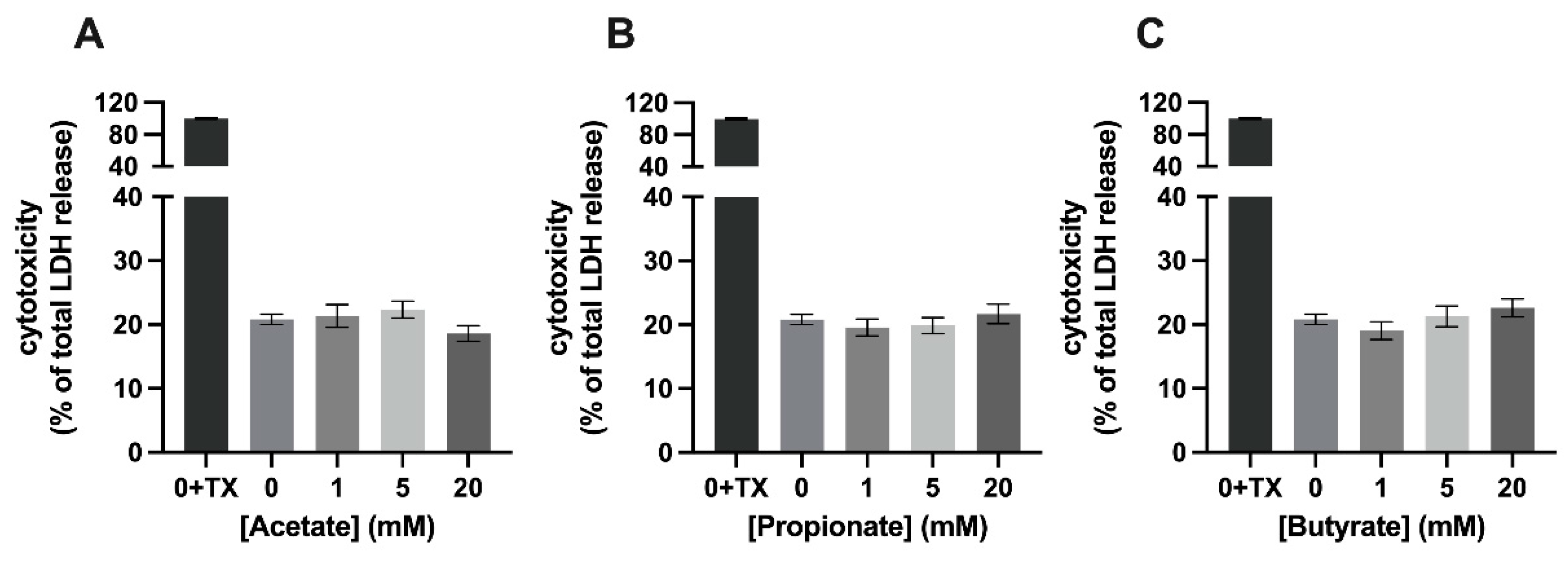
3.1. Mixture 1, a Combination of SCFAs, Is Cytotoxic to C2C12 Myotubes at a Concentration of 20 mM
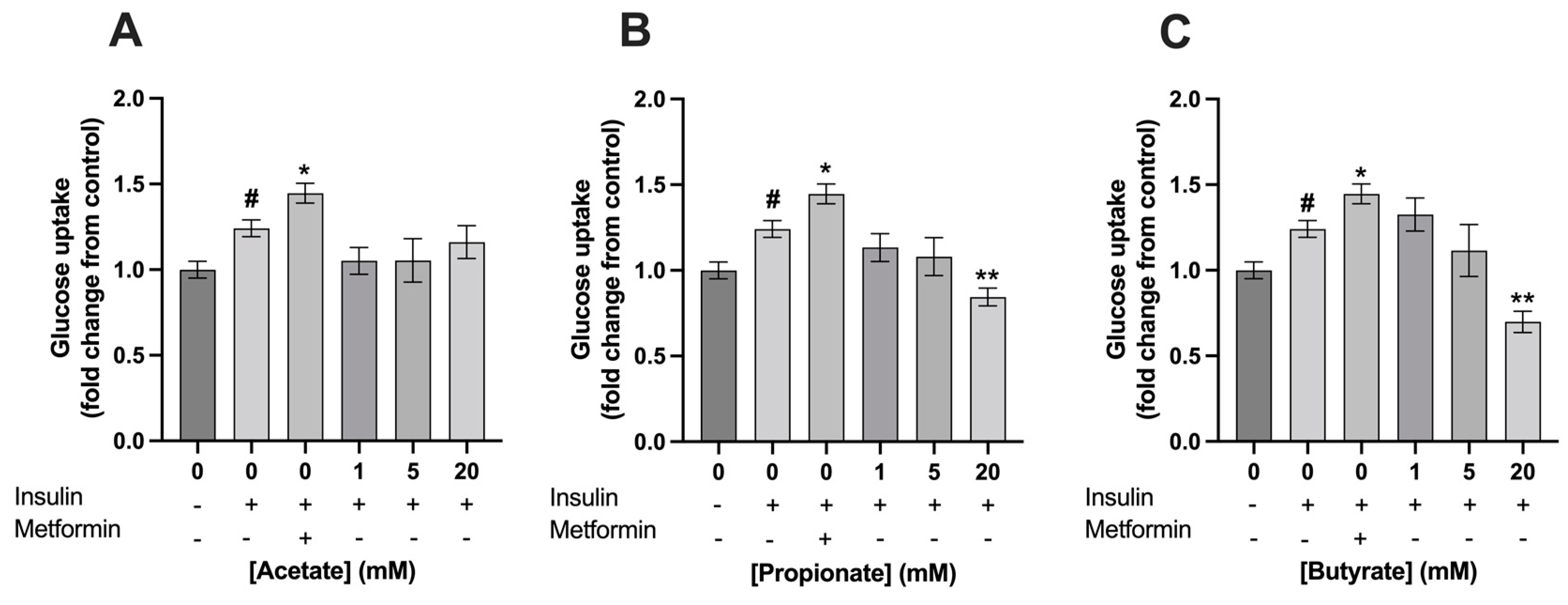
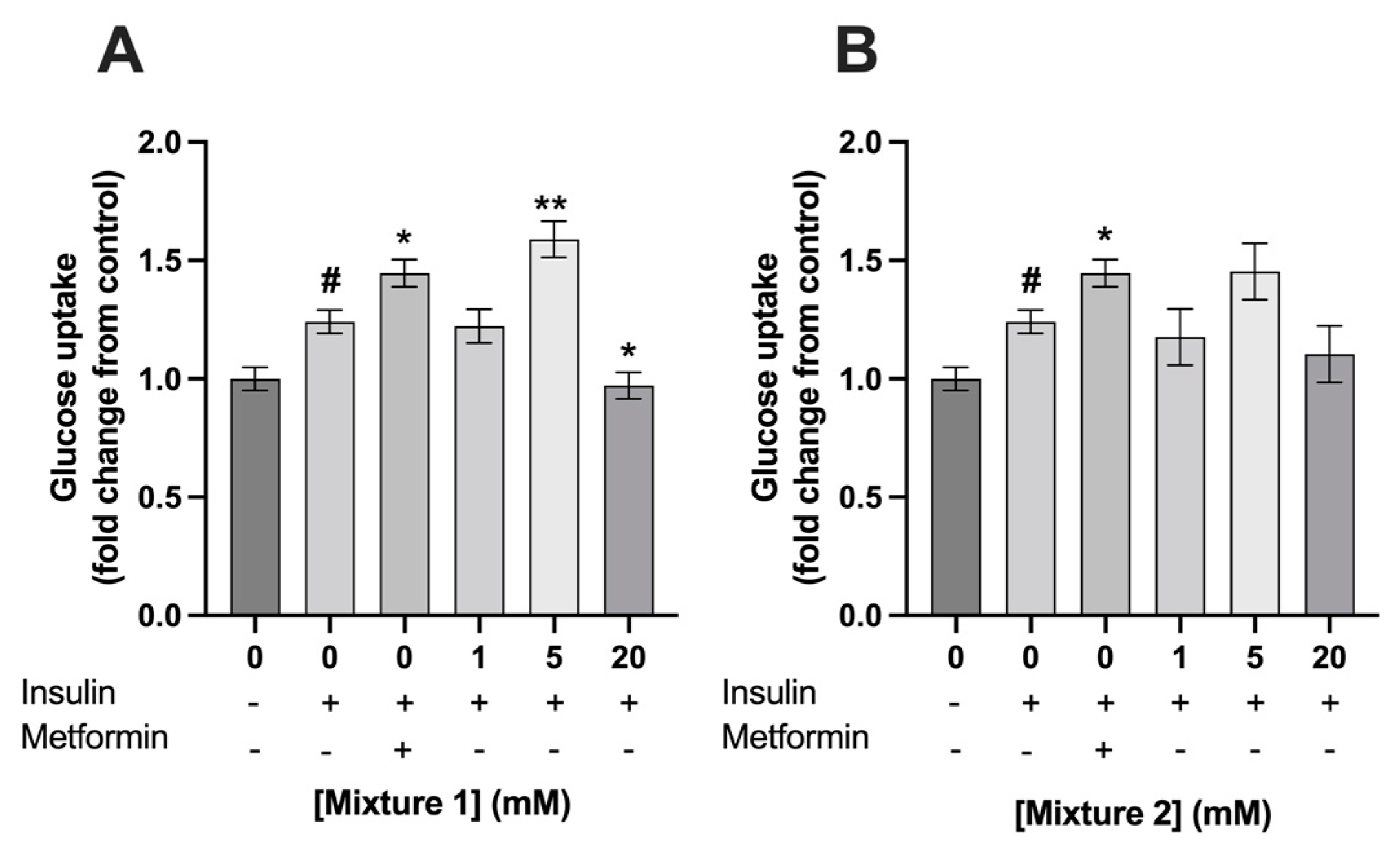
3.2. Exposure to 5 mM of Mixture 1 Increased Glucose Uptake in C2C12 Myotubes
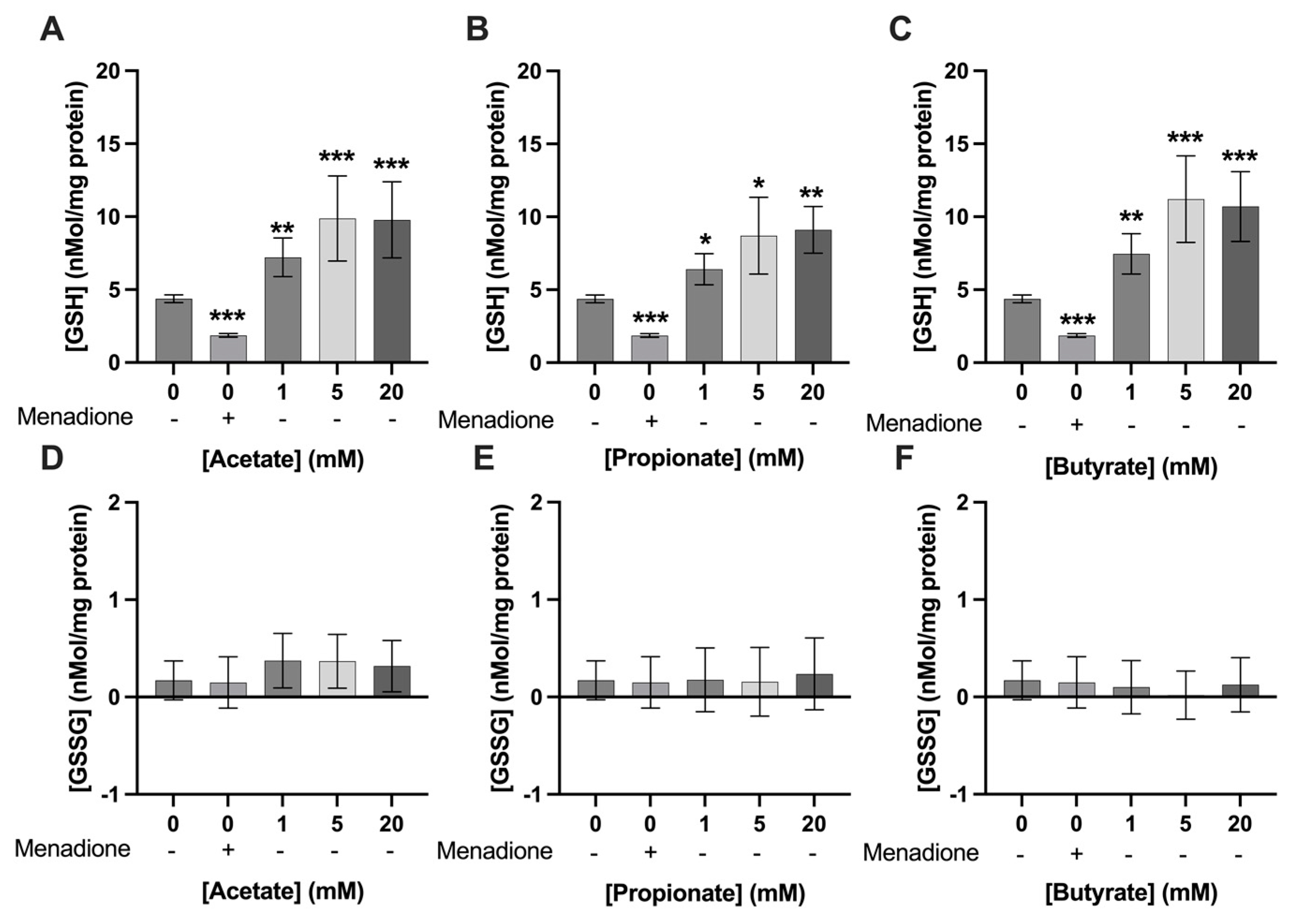
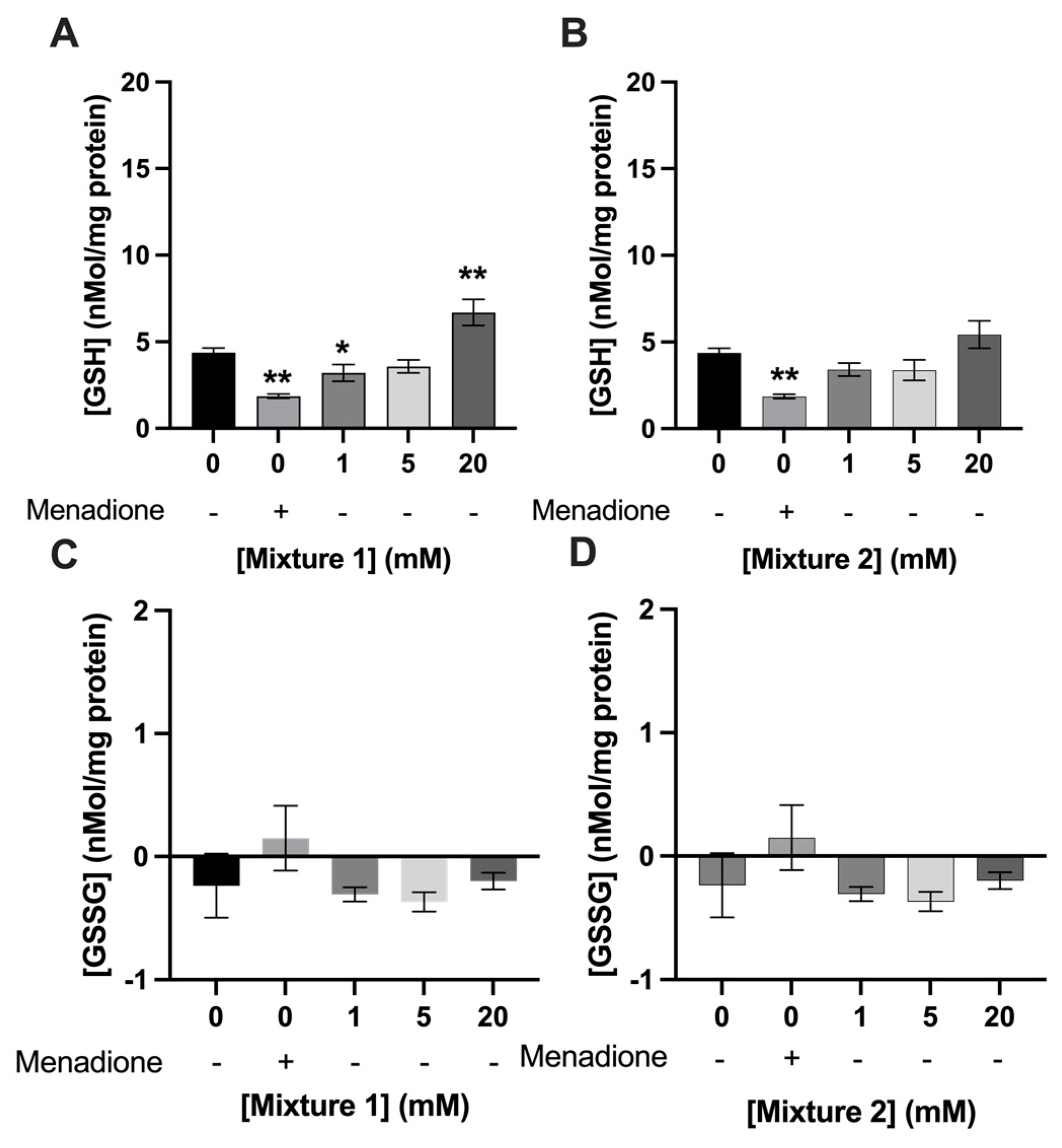
3.3. Exposure to SCFAs Increased GSH Levels in C2C12 Myotubes
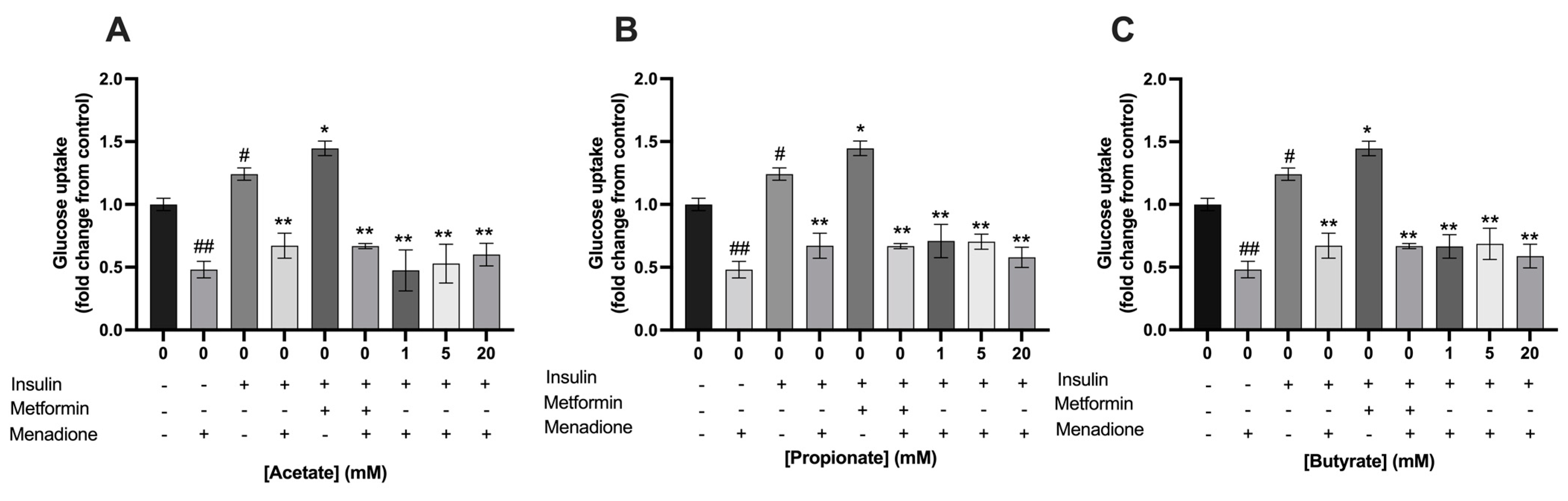
3.4. Single- and Mixtures of SCFAs Did Not Prevent the Menadione-Induced Decrease in Glucose Uptake in C2C12 Myotubes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Portincasa, P.; Bonfrate, L.; Vacca, M.; De Angelis, M.; Farella, I.; Lanza, E.; Khalil, M.; Wang, D.Q.-H.; Sperandio, M.; Di Ciaula, A. Gut Microbiota and Short Chain Fatty Acids: Implications in Glucose Homeostasis. Int. J. Mol. Sci. 2022, 23, 1105. [Google Scholar] [CrossRef] [PubMed]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-muscle axis exists and may affect skeletal muscle adaptation to training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef]
- Hernández, M.G.; Canfora, E.; Blaak, E. Faecal microbial metabolites of proteolytic and saccharolytic fermentation in relation to degree of insulin resistance in adult individuals. Benef. Microbes 2021, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Vinolo, M.; Rodrigues, H.; Nachbar, R.; Curi, R. Modulation of inflammatory and immune responses by short-chain fatty acids. In Diet, Immunity and Inflammation; Elsevier: Amsterdam, The Netherlands, 2013; pp. 435–458. [Google Scholar]
- Ktsoyan, Z.A.; Mkrtchyan, M.S.; Zakharyan, M.K.; Mnatsakanyan, A.A.; Arakelova, K.A.; Gevorgyan, Z.U.; Sedrakyan, A.M.; Hovhannisyan, A.I.; Arakelyan, A.A.; Aminov, R.I. Systemic concentrations of short chain fatty acids are elevated in salmonellosis and exacerbation of familial mediterranean fever. Front. Microbiol. 2016, 7, 776. [Google Scholar] [CrossRef] [PubMed]
- Ohira, H.; Tsutsui, W.; Fujioka, Y. Are short chain fatty acids in gut microbiota defensive players for inflammation and atherosclerosis? J. Atheroscler. Thromb. 2017, 24, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.; Murphy, K.G.; Frost, G.; Chambers, E.S. Short-chain fatty acids as potential regulators of skeletal muscle metabolism and function. Nat. Metab. 2020, 2, 840–848. [Google Scholar] [CrossRef]
- Giron, M.; Thomas, M.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Gut microbes and muscle function: Can probiotics make our muscles stronger? J. Cachexia Sarcopenia Muscle 2022, 13, 1460–1476. [Google Scholar] [CrossRef]
- He, J.; Zhang, P.; Shen, L.; Niu, L.; Tan, Y.; Chen, L.; Zhao, Y.; Bai, L.; Hao, X.; Li, X.; et al. Short-chain fatty acids and their association with signalling pathways in inflammation, glucose and lipid metabolism. Int. J. Mol. Sci. 2020, 21, 6356. [Google Scholar] [CrossRef]
- Tang, R.; Li, L. Modulation of short-chain fatty acids as potential therapy method for type 2 diabetes mellitus. Can. J. Infect. Dis. Med. Microbiol. 2021, 2021, 6632266. [Google Scholar]
- Rayasam, G.V.; Tulasi, V.K.; Davis, J.A.; Bansal, V.S. Fatty acid receptors as new therapeutic targets for diabetes. Expert Opin. Ther. Targets 2007, 11, 661–671. [Google Scholar] [CrossRef]
- Li, M.; Van Esch, B.C.; Henricks, P.A.; Folkerts, G.; Garssen, J. The anti-inflammatory effects of short chain fatty acids on lipopolysaccharide-or tumor necrosis factor α-stimulated endothelial cells via activation of GPR41/43 and inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef]
- Zong, H.; Ren, J.M.; Young, L.H.; Pypaert, M.; Mu, J.; Birnbaum, M.J.; Shulman, G.I. AMP kinase is required for mitochondrial biogenesis in skeletal muscle in response to chronic energy deprivation. Proc. Natl. Acad. Sci. USA 2002, 99, 15983–15987. [Google Scholar] [CrossRef] [PubMed]
- Katz, A. Modulation of glucose transport in skeletal muscle by reactive oxygen species. J. Appl. Physiol. 2007, 102, 1671–1676. [Google Scholar] [CrossRef] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Sthijns, M.M.; van Blitterswijk, C.A.; LaPointe, V.L. Redox regulation in regenerative medicine and tissue engineering: The paradox of oxygen. J. Tissue Eng. Regen. Med. 2018, 12, 2013–2020. [Google Scholar] [CrossRef]
- Yamamoto, M.; Kensler, T.W.; Motohashi, H. The KEAP1-NRF2 system: A thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol. Rev. 2018, 98, 1169–1203. [Google Scholar] [CrossRef]
- Mann, G.E.; Forman, H.J. Introduction to special issue on’Nrf2 regulated redox signaling and metabolism in physiology and medicine. Free. Radic. Biol. Med. 2015, 88, 91–92. [Google Scholar] [CrossRef]
- Harvey, C.J.; Thimmulappa, R.K.; Singh, A.; Blake, D.J.; Ling, G.; Wakabayashi, N.; Fujii, J.; Myers, A.; Biswal, S. Nrf2-regulated glutathione recycling independent of biosynthesis is critical for cell survival during oxidative stress. Free Radic. Biol. Med. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- González-Bosch, C.; Boorman, E.; Zunszain, P.A.; Mann, G.E. Short-chain fatty acids as modulators of redox signaling in health and disease. Redox Biol. 2021, 47, 102165. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Jia, Y.; Liu, X.; Zhang, H.; Li, T.; Huang, W.; Chen, X.; Wang, F.; Sun, W.; Wu, H. Sodium butyrate activates NRF2 to ameliorate diabetic nephropathy possibly via inhibition of HDAC. J. Endocrinol. 2017, 232, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Merz, K.E.; Thurmond, D.C. Role of skeletal muscle in insulin resistance and glucose uptake. Compr. Physiol. 2011, 10, 785–809. [Google Scholar]
- Valley, M.P.; Karassina, N.; Aoyama, N.; Carlson, C.; Cali, J.J.; Vidugiriene, J. A bioluminescent assay for measuring glucose uptake. Anal. Biochem. 2016, 505, 43–50. [Google Scholar] [CrossRef]
- Herman, R.; Kravos, N.A.; Jensterle, M.; Janež, A.; Dolžan, V. Metformin and insulin resistance: A review of the underlying mechanisms behind changes in GLUT4-mediated glucose transport. Int. J. Mol. Sci. 2022, 23, 1264. [Google Scholar] [CrossRef]
- Hardie, D. AMPK: A key regulator of energy balance in the single cell and the whole organism. Int. J. Obes. 2008, 32, S7–S12. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Jia, Y.; Pan, S.; Jia, L.; Li, H.; Han, Z.; Cai, D.; Zhao, R. Butyrate alleviates high fat diet-induced obesity through activation of adiponectin-mediated pathway and stimulation of mitochondrial function in the skeletal muscle of mice. Oncotarget 2016, 7, 56071. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.H.; Kim, J.H.; Kim, H.M.; Lee, E.S.; Shin, D.-H.; Kim, S.; Shin, M.; Kim, S.H.; Lee, J.H.; Kim, Y.J. Acetic acid enhances endurance capacity of exercise-trained mice by increasing skeletal muscle oxidative properties. Biosci.Biotechnol. Biochem. 2015, 79, 1535–1541. [Google Scholar] [CrossRef]
- González Hernández, M.A.; Blaak, E.E.; Hoebers, N.T.; Essers, Y.P.; Canfora, E.E.; Jocken, J.W. Acetate Does Not Affect Palmitate Oxidation and AMPK Phosphorylation in Human Primary Skeletal Muscle Cells. Front. Endocrinol. 2021, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, S.; Ren, J.; Lee, J.K.W.; Wang, R.; Chen, P. Role of histone deacetylases in skeletal muscle physiology and systemic energy homeostasis: Implications for metabolic diseases and therapy. Front. Physiol. 2020, 11, 949. [Google Scholar] [CrossRef]
- Takigawa-Imamura, H.; Sekine, T.; Murata, M.; Takayama, K.; Nakazawa, K.; Nakagawa, J. Stimulation of glucose uptake in muscle cells by prolonged treatment with scriptide, a histone deacetylase inhibitor. Biosci.Biotechnol. Biochem. 2003, 67, 1499–1506. [Google Scholar] [CrossRef]
- Layden, B.T.; Angueira, A.R.; Brodsky, M.; Durai, V.; Lowe Jr, W.L. Short chain fatty acids and their receptors: New metabolic targets. Transl. Res. 2013, 161, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Milligan, G.; Shimpukade, B.; Ulven, T.; Hudson, B.D. Complex pharmacology of free fatty acid receptors. Chem. Rev. 2017, 117, 67–110. [Google Scholar] [CrossRef] [PubMed]
- Kimura, I.; Ichimura, A.; Ohue-Kitano, R.; Igarashi, M. Free fatty acid receptors in health and disease. Physiol. Rev. 2019, 1, 171–210. [Google Scholar] [CrossRef]
- Yoshida, H.; Ishii, M.; Akagawa, M. Propionate suppresses hepatic gluconeogenesis via GPR43/AMPK signaling pathway. Arch. Biochem. Biophys. 2019, 672, 108057. [Google Scholar] [CrossRef] [PubMed]
- Maruta, H.; Yamashita, H. Acetic acid stimulates G-protein-coupled receptor GPR43 and induces intracellular calcium influx in L6 myotube cells. PLoS One 2020, 15, e0239428. [Google Scholar] [CrossRef] [PubMed]
- Lahiri, S.; Kim, H.; Garcia-Perez, I.; Reza, M.M.; Martin, K.A.; Kundu, P.; Cox, L.M.; Selkrig, J.; Posma, J.M.; Zhang, H.; et al. The gut microbiota influences skeletal muscle mass and function in mice. Sci. Transl. Med. 2019, 11, eaan5662. [Google Scholar] [CrossRef]
- Austin, S.; St-Pierre, J. PGC1α and mitochondrial metabolism–emerging concepts and relevance in ageing and neurodegenerative disorders. J. Cell Sci. 2012, 125, 4963–4971. [Google Scholar] [CrossRef]
- Gehlert, S.; Bloch, W.; Suhr, F. Ca2+-dependent regulations and signaling in skeletal muscle: From electro-mechanical coupling to adaptation. Int. J. Mol. Sci. 2015, 16, 1066–1095. [Google Scholar] [CrossRef] [PubMed]
- Mollica, M.P.; Mattace Raso, G.; Cavaliere, G.; Trinchese, G.; De Filippo, C.; Aceto, S.; Prisco, M.; Pirozzi, C.; Di Guida, F.; Lama, A.; et al. Butyrate regulates liver mitochondrial function, efficiency, and dynamics in insulin-resistant obese mice. Diabetes 2017, 66, 1405–1418. [Google Scholar] [CrossRef]
- Chung, L.-H.; Liu, S.-T.; Huang, S.-M.; Salter, D.M.; Lee, H.-S.; Hsu, Y.-J. High phosphate induces skeletal muscle atrophy and suppresses myogenic differentiation by increasing oxidative stress and activating Nrf2 signaling. Aging (Albany NY) 2020, 12, 21446. [Google Scholar] [CrossRef] [PubMed]


| Acetate | Propionate | Butyrate | |||||
|---|---|---|---|---|---|---|---|
| Concentration Total SCFA (mM) | Relative Amount (%) | Concentration (mM) | Relative Amount (%) | Concentration (mM) | Relative Amount (%) | Concentration (mM) | |
| 1 | 60 | 0.6 | 20 | 0.2 | 20 | 0.2 | |
| Mixture 1 | 5 | 60 | 3 | 20 | 1 | 20 | 1 |
| 20 | 60 | 12 | 20 | 4 | 20 | 4 | |
| 1 | 80 | 0.8 | 10 | 0.1 | 10 | 0.1 | |
| Mixture 2 | 5 | 80 | 4 | 10 | 0.5 | 10 | 0.5 |
| 20 | 80 | 16 | 10 | 2 | 10 | 2 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otten, B.M.J.; Sthijns, M.M.J.P.E.; Troost, F.J. A Combination of Acetate, Propionate, and Butyrate Increases Glucose Uptake in C2C12 Myotubes. Nutrients 2023, 15, 946. https://doi.org/10.3390/nu15040946
Otten BMJ, Sthijns MMJPE, Troost FJ. A Combination of Acetate, Propionate, and Butyrate Increases Glucose Uptake in C2C12 Myotubes. Nutrients. 2023; 15(4):946. https://doi.org/10.3390/nu15040946
Chicago/Turabian StyleOtten, Britt M. J., Mireille M. J. P. E. Sthijns, and Freddy J. Troost. 2023. "A Combination of Acetate, Propionate, and Butyrate Increases Glucose Uptake in C2C12 Myotubes" Nutrients 15, no. 4: 946. https://doi.org/10.3390/nu15040946
APA StyleOtten, B. M. J., Sthijns, M. M. J. P. E., & Troost, F. J. (2023). A Combination of Acetate, Propionate, and Butyrate Increases Glucose Uptake in C2C12 Myotubes. Nutrients, 15(4), 946. https://doi.org/10.3390/nu15040946






