Dietary Supplements Potentially Target Plasma Glutathione Levels to Improve Cardiometabolic Health in Patients with Diabetes Mellitus: A Systematic Review of Randomized Clinical Trials
Abstract
1. Introduction
2. Literature Search, Study Inclusion, and Quality Assessment
3. Results
3.1. Characteristic Features of the Included Studies
3.2. Evidence on the Effects of Dietary Supplements on Plasma Glutathione Levels and Cardiometabolic Health
3.2.1. Supplementation with CoQ10
3.2.2. Supplementation with Selenium
3.2.3. Supplementation with Melatonin and Curcumin
3.2.4. Supplementation with Omega-3 Fatty Acids and Vitamin E/D
3.2.5. Supplementation with Plant Extracts
4. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization (WHO). The Top Ten Leading Causes of Death. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 6 January 2023).
- International Diabetes Federation (IDF). IDF Diabetes Atlas 2021. Available online: https://diabetesatlas.org/atlas/tenth-edition/ (accessed on 5 January 2023).
- Fan, W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 8–16. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases. Diabetes and cardiovascular disease. The link between blood sugar, heart attacks and stroke. Mayo Clin. Womens Healthsource 2009, 13, 1–2. Available online: https://www.niddk.nih.gov/health-information/diabetes/overview/preventing-problems/heart-disease-stroke (accessed on 5 January 2023).
- Dwyer, J.T.; Coates, P.M.; Smith, M.J. Dietary Supplements: Regulatory Challenges and Research Resources. Nutrients 2018, 10, 41. [Google Scholar] [CrossRef]
- Crawford, C.; Boyd, C.; Avula, B.; Wang, Y.H.; Khan, I.A.; Deuster, P.A. A Public Health Issue: Dietary Supplements Promoted for Brain Health and Cognitive Performance. J. Altern. Complement. Med. 2020, 26, 265–272. [Google Scholar] [CrossRef]
- Rautiainen, S.; Manson, J.E.; Lichtenstein, A.H.; Sesso, H.D. Dietary supplements and disease prevention-a global overview. Nat. Rev. Endocrinol. 2016, 12, 407–420. [Google Scholar] [CrossRef]
- Shlisky, J.; Bloom, D.E.; Beaudreault, A.R.; Tucker, K.L.; Keller, H.H.; Freund-Levi, Y.; Fielding, R.A.; Cheng, F.W.; Jensen, G.L.; Wu, D.; et al. Nutritional Considerations for Healthy Aging and Reduction in Age-Related Chronic Disease. Adv. Nutr. 2017, 8, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef]
- Steven, S.; Frenis, K.; Oelze, M.; Kalinovic, S.; Kuntic, M.; Bayo Jimenez, M.T.; Vujacic-Mirski, K.; Helmstädter, J.; Kröller-Schön, S.; Münzel, T.; et al. Vascular Inflammation and Oxidative Stress: Major Triggers for Cardiovascular Disease. Oxidative Med. Cell. Longev. 2019, 2019, 7092151. [Google Scholar] [CrossRef]
- Webb, R.J.; Mazidi, M.; Lip, G.Y.H.; Kengne, A.P.; Banach, M.; Davies, I.G. The role of adiposity, diet and inflammation on the discordance between LDL-C and apolipoprotein B. Nutr. Metab. Cardiovasc. Dis. NMCD 2022, 32, 605–615. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.K.; Dhalla, N.S. Effectiveness of Some Vitamins in the Prevention of Cardiovascular Disease: A Narrative Review. Front. Physiol. 2021, 12, 729255. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Nkambule, B.B.; Nyambuya, T.M.; Ziqubu, K.; Mabhida, S.E.; Mxinwa, V.; Mokgalaboni, K.; Ndevahoma, F.; Hanser, S.; Mazibuko-Mbeje, S.E.; et al. Vitamin C intake potentially lowers total cholesterol to improve endothelial function in diabetic patients at increased risk of cardiovascular disease: A systematic review of randomized controlled trials. Front. Nutr. 2022, 9, 1011002. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- Silvagno, F.; Vernone, A.; Pescarmona, G.P. The Role of Glutathione in Protecting against the Severe Inflammatory Response Triggered by COVID-19. Antioxidants 2020, 9, 624. [Google Scholar] [CrossRef]
- Shimizu, H.; Kiyohara, Y.; Kato, I.; Kitazono, T.; Tanizaki, Y.; Kubo, M.; Ueno, H.; Ibayashi, S.; Fujishima, M.; Iida, M. Relationship between plasma glutathione levels and cardiovascular disease in a defined population: The Hisayama study. Stroke 2004, 35, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Oxidative enzymopathies and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vilchis-Landeros, M.M.; Vázquez-Meza, H. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef]
- Dludla, P.V.; Muller, C.J.; Joubert, E.; Louw, J.; Essop, M.F.; Gabuza, K.B.; Ghoor, S.; Huisamen, B.; Johnson, R. Aspalathin Protects the Heart against Hyperglycemia-Induced Oxidative Damage by Up-Regulating Nrf2 Expression. Molecules 2017, 22, 129. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Muller, C.J.; Louw, J.; Joubert, E.; Salie, R.; Opoku, A.R.; Johnson, R. The cardioprotective effect of an aqueous extract of fermented rooibos (Aspalathus linearis) on cultured cardiomyocytes derived from diabetic rats. Phytomed. Int. J. Phytother. Phytopharm. 2014, 21, 595–601. [Google Scholar] [CrossRef]
- Dludla, P.V.J.R.; Mazibuko-Mbeje, S.E.; Muller, C.J.F.; Louw, J.; Joubert, E.; Orlando, P.; Silvestri, S.; Chellan, N.; Nkmabule, B.; Essop, M.F.; et al. Fermented rooibos extract attenuates hyperglycemia-induced myocardial oxidative damage by improving mitochondrial energetics and intracellular antioxidant capacity. S. Afr. J. Bot. 2020, 131, 143–150. [Google Scholar] [CrossRef]
- Ceriello, A.; Giugliano, D.; Quatraro, A.; Lefebvre, P.J. Anti-oxidants show an anti-hypertensive effect in diabetic and hypertensive subjects. Clin. Sci. 1991, 81, 739–742. [Google Scholar] [CrossRef]
- Knezević, V.; Mujović, V.M.; Milosević, A. Effect of vitamin E on erythrocyte enzymes and total antioxidant status in diabetic patients with ischemic heart disease. Srp. Arh. Za Celok. Lek. 2000, 128, 241–246. [Google Scholar]
- Qian, Q.; Qian, S.; Fan, P.; Huo, D.; Wang, S. Effect of Salvia miltiorrhiza hydrophilic extract on antioxidant enzymes in diabetic patients with chronic heart disease: A randomized controlled trial. Phytother. Res. PTR 2012, 26, 60–66. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, I.; Taub, P.R.; Ciaraldi, T.P.; Nogueira, L.; Coe, T.; Perkins, G.; Hogan, M.; Maisel, A.S.; Henry, R.R.; Ceballos, G.; et al. (-)-Epicatechin rich cocoa mediated modulation of oxidative stress regulators in skeletal muscle of heart failure and type 2 diabetes patients. Int. J. Cardiol. 2013, 168, 3982–3990. [Google Scholar] [CrossRef]
- Huguenin, G.V.; Oliveira, G.M.; Moreira, A.S.; Saint’Pierre, T.D.; Gonçalves, R.A.; Pinheiro-Mulder, A.R.; Teodoro, A.J.; Luiz, R.R.; Rosa, G. Improvement of antioxidant status after Brazil nut intake in hypertensive and dyslipidemic subjects. Nutr. J. 2015, 14, 54. [Google Scholar] [CrossRef]
- Raygan, F.; Rezavandi, Z.; Dadkhah Tehrani, S.; Farrokhian, A.; Asemi, Z. The effects of coenzyme Q10 administration on glucose homeostasis parameters, lipid profiles, biomarkers of inflammation and oxidative stress in patients with metabolic syndrome. Eur. J. Nutr. 2016, 55, 2357–2364. [Google Scholar] [CrossRef]
- Raygan, F.; Behnejad, M.; Ostadmohammadi, V.; Bahmani, F.; Mansournia, M.A.; Karamali, F.; Asemi, Z. Selenium supplementation lowers insulin resistance and markers of cardio-metabolic risk in patients with congestive heart failure: A randomised, double-blind, placebo-controlled trial. Br. J. Nutr. 2018, 120, 33–40. [Google Scholar] [CrossRef]
- Raygan, F.; Behnejad, M.; Ostadmohammadi, V.; Bahmani, F.; Mansournia, M.A.; Karamali, F.; Asemi, Z. Selenium supplementation lowers insulin resistance and markers of cardio-metabolic risk in patients with congestive heart failure: A randomised, double-blind, placebo-controlled trial-Expression of concern. Br. J. Nutr. 2022, 127, 157. [Google Scholar] [CrossRef]
- Raygan, F.; Taghizadeh, M.; Mirhosseini, N.; Akbari, E.; Bahmani, F.; Memarzadeh, M.R.; Sharifi, N.; Jafarnejad, S.; Banikazemi, Z.; Asemi, Z. A comparison between the effects of flaxseed oil and fish oil supplementation on cardiovascular health in type 2 diabetic patients with coronary heart disease: A randomized, double-blinded, placebo-controlled trial. Phytother. Res. PTR 2019, 33, 1943–1951. [Google Scholar] [CrossRef]
- Sekhar, R.V.; McKay, S.V.; Patel, S.G.; Guthikonda, A.P.; Reddy, V.T.; Balasubramanyam, A.; Jahoor, F. Glutathione synthesis is diminished in patients with uncontrolled diabetes and restored by dietary supplementation with cysteine and glycine. Diabetes Care 2011, 34, 162–167. [Google Scholar] [CrossRef]
- Flagg, E.W.; Coates, R.J.; Eley, J.W.; Jones, D.P.; Gunter, E.W.; Byers, T.E.; Block, G.S.; Greenberg, R.S. Dietary glutathione intake in humans and the relationship between intake and plasma total glutathione level. Nutr. Cancer 1994, 21, 33–46. [Google Scholar] [CrossRef]
- Pimson, C.; Chatuphonprasert, W.; Jarukamjorn, K. Improvement of antioxidant balance in diabetes mellitus type 1 mice by glutathione supplement. Pak. J. Pharm. Sci. 2014, 27, 1731–1737. [Google Scholar]
- Dludla, P.V.; Dias, S.C.; Obonye, N.; Johnson, R.; Louw, J.; Nkambule, B.B. A Systematic Review on the Protective Effect of N-Acetyl Cysteine Against Diabetes-Associated Cardiovascular Complications. Am. J. Cardiovasc. Drugs Drugs Devices Other Interv. 2018, 18, 283–298. [Google Scholar] [CrossRef]
- Mason, S.A.; Trewin, A.J.; Parker, L.; Wadley, G.D. Antioxidant supplements and endurance exercise: Current evidence and mechanistic insights. Redox Biol. 2020, 35, 101471. [Google Scholar] [CrossRef]
- Mandal, P.K.; Shukla, D.; Tripathi, M.; Ersland, L. Cognitive Improvement with Glutathione Supplement in Alzheimer’s Disease: A Way Forward. J. Alzheimers Dis. JAD 2019, 68, 531–535. [Google Scholar] [CrossRef]
- Kerksick, C.; Willoughby, D. The antioxidant role of glutathione and N-acetyl-cysteine supplements and exercise-induced oxidative stress. J. Int. Soc. Sport. Nutr. 2005, 2, 38–44. [Google Scholar] [CrossRef]
- Zozina, V.I.; Covantev, S.; Goroshko, O.A.; Krasnykh, L.M.; Kukes, V.G. Coenzyme Q10 in Cardiovascular and Metabolic Diseases: Current State of the Problem. Curr. Cardiol. Rev. 2018, 14, 164–174. [Google Scholar] [CrossRef]
- Rabanal-Ruiz, Y.; Llanos-González, E.; Alcain, F.J. The Use of Coenzyme Q10 in Cardiovascular Diseases. Antioxidants 2021, 10, 755. [Google Scholar] [CrossRef]
- Sabbatinelli, J.; Orlando, P.; Galeazzi, R.; Silvestri, S.; Cirilli, I.; Marcheggiani, F.; Dludla, P.V.; Giuliani, A.; Bonfigli, A.R.; Mazzanti, L.; et al. Ubiquinol Ameliorates Endothelial Dysfunction in Subjects with Mild-to-Moderate Dyslipidemia: A Randomized Clinical Trial. Nutrients 2020, 12, 1098. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nyambuya, T.M.; Orlando, P.; Silvestri, S.; Mxinwa, V.; Mokgalaboni, K.; Nkambule, B.B.; Louw, J.; Muller, C.J.F.; Tiano, L. The impact of coenzyme Q(10) on metabolic and cardiovascular disease profiles in diabetic patients: A systematic review and meta-analysis of randomized controlled trials. Endocrinol. Diabetes Metab. 2020, 3, e00118. [Google Scholar] [CrossRef] [PubMed]
- Dludla, P.V.; Orlando, P.; Silvestri, S.; Marcheggiani, F.; Cirilli, I.; Nyambuya, T.M.; Mxinwa, V.; Mokgalaboni, K.; Nkambule, B.B.; Johnson, R.; et al. Coenzyme Q(10) Supplementation Improves Adipokine Levels and Alleviates Inflammation and Lipid Peroxidation in Conditions of Metabolic Syndrome: A Meta-Analysis of Randomized Controlled Trials. Int. J. Mol. Sci. 2020, 21, 3247. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Liang, Y.; Dai, S.; Hou, S.; Liu, Z.; Liu, M.; Dong, X.; Zhan, Y.; Tian, Z.; Yang, Y. Dose-Response Effect of Coenzyme Q10 Supplementation on Blood Pressure among Patients with Cardiometabolic Disorders: A Grading of Recommendations Assessment, Development, and Evaluation (GRADE)-Assessed Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 2180–2194. [Google Scholar] [PubMed]
- Mantle, D.; Dybring, A. Bioavailability of Coenzyme Q(10): An Overview of the Absorption Process and Subsequent Metabolism. Antioxidants 2020, 9, 386. [Google Scholar] [CrossRef] [PubMed]
- Pravst, I.; Rodríguez Aguilera, J.C.; Cortes Rodriguez, A.B.; Jazbar, J.; Locatelli, I.; Hristov, H.; Žmitek, K. Comparative Bioavailability of Different Coenzyme Q10 Formulations in Healthy Elderly Individuals. Nutrients 2020, 12, 784. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Asemi, Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 1594–1598. [Google Scholar] [CrossRef]
- Tinggi, U. Selenium: Its role as antioxidant in human health. Environ. Health Prev. Med. 2008, 13, 102–108. [Google Scholar] [CrossRef]
- Joseph, J. Selenium and cardiometabolic health: Inconclusive yet intriguing evidence. Am. J. Med. Sci. 2013, 346, 216–220. [Google Scholar] [CrossRef]
- Ferreira, R.L.U.; Sena-Evangelista, K.C.M.; de Azevedo, E.P.; Pinheiro, F.I.; Cobucci, R.N.; Pedrosa, L.F.C. Selenium in Human Health and Gut Microflora: Bioavailability of Selenocompounds and Relationship with Diseases. Front. Nutr. 2021, 8, 685317. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J.; Collings, R.; Hurst, R. Selenium bioavailability: Current knowledge and future research requirements. Am. J. Clin. Nutr. 2010, 91, 1484s–1491s. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Braat, S.; Graham, R.M. Selenium Status Is Associated with Insulin Resistance Markers in Adults: Findings From the 2013 to 2018 National Health and Nutrition Examination Survey (NHANES). Front. Nutr. 2021, 8, 696024. [Google Scholar] [CrossRef]
- Karalis, D.T. The Beneficiary Role of Selenium in Type II Diabetes: A Longitudinal Study. Cureus 2019, 11, e6443. [Google Scholar] [CrossRef] [PubMed]
- Bazyar, H.; Zare Javid, A.; Bavi Behbahani, H.; Moradi, F.; Moradi Poode, B.; Amiri, P. Consumption of melatonin supplement improves cardiovascular disease risk factors and anthropometric indices in type 2 diabetes mellitus patients: A double-blind, randomized, placebo-controlled trial. Trials 2021, 22, 231. [Google Scholar] [CrossRef]
- Raygan, F.; Ostadmohammadi, V.; Bahmani, F.; Reiter, R.J.; Asemi, Z. Melatonin administration lowers biomarkers of oxidative stress and cardio-metabolic risk in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2019, 38, 191–196. [Google Scholar] [CrossRef]
- Tobeiha, M.; Jafari, A.; Fadaei, S.; Mirazimi, S.M.A.; Dashti, F.; Amiri, A.; Khan, H.; Asemi, Z.; Reiter, R.J.; Hamblin, M.R.; et al. Evidence for the Benefits of Melatonin in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 9, 888319. [Google Scholar] [CrossRef] [PubMed]
- Karaaslan, C.; Suzen, S. Antioxidant properties of melatonin and its potential action in diseases. Curr. Top. Med. Chem. 2015, 15, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Scheer, F.A.; Van Montfrans, G.A.; van Someren, E.J.; Mairuhu, G.; Buijs, R.M. Daily nighttime melatonin reduces blood pressure in male patients with essential hypertension. Hypertension 2004, 43, 192–197. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Mobini, M.; Raygan, F.; Aghadavod, E.; Ostadmohammadi, V.; Amirani, E.; Mansournia, M.A.; Asemi, Z. Curcumin administration and the effects on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes and coronary heart disease. Clin. Nutr. ESPEN 2020, 40, 77–82. [Google Scholar] [CrossRef]
- Kim, Y.; Clifton, P. Curcumin, Cardiometabolic Health and Dementia. Int. J. Environ. Res. Public Health 2018, 15, 2093. [Google Scholar] [CrossRef]
- Mokgalaboni, K.; Ntamo, Y.; Ziqubu, K.; Nyambuya, T.M.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Gabuza, K.B.; Chellan, N.; Tiano, L.; Dludla, P.V. Curcumin supplementation improves biomarkers of oxidative stress and inflammation in conditions of obesity, type 2 diabetes and NAFLD: Updating the status of clinical evidence. Food Funct. 2021, 12, 12235–12249. [Google Scholar] [CrossRef]
- Lopresti, A.L. The Problem of Curcumin and Its Bioavailability: Could Its Gastrointestinal Influence Contribute to Its Overall Health-Enhancing Effects? Adv. Nutr. 2018, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- DeMuro, R.L.; Nafziger, A.N.; Blask, D.E.; Menhinick, A.M.; Bertino, J.S., Jr. The absolute bioavailability of oral melatonin. J. Clin. Pharmacol. 2000, 40, 781–784. [Google Scholar] [CrossRef]
- Shakoor, H.; Feehan, J.; Al Dhaheri, A.S.; Ali, H.I.; Platat, C.; Ismail, L.C.; Apostolopoulos, V.; Stojanovska, L. Immune-boosting role of vitamins D, C, E, zinc, selenium and omega-3 fatty acids: Could they help against COVID-19? Maturitas 2021, 143, 1–9. [Google Scholar] [CrossRef]
- Bourre, J.M.; Galea, F. An important source of omega-3 fatty acids, vitamins D and E, carotenoids, iodine and selenium: A new natural multi-enriched egg. J. Nutr. Health Aging 2006, 10, 371–376. [Google Scholar] [PubMed]
- Mone, P.; Varzideh, F.; Kansakar, U.; Infante, C.; Lombardi, A.; de Donato, A.; Frullone, S.; Santulli, G. Omega-3 fatty acids coordinate glucose and lipid metabolism in diabetic patients. Lipids Health Dis. 2022, 21, 31. [Google Scholar] [CrossRef]
- Liu, H.; Wang, F.; Liu, X.; Xie, Y.; Xia, H.; Wang, S.; Sun, G. Effects of marine-derived and plant-derived omega-3 polyunsaturated fatty acids on erythrocyte fatty acid composition in type 2 diabetic patients. Lipids Health Dis. 2022, 21, 20. [Google Scholar] [CrossRef] [PubMed]
- Itsiopoulos, C.; Marx, W.; Mayr, H.L.; Tatucu-Babet, O.A.; Dash, S.R.; George, E.S.; Trakman, G.L.; Kelly, J.T.; Thomas, C.J.; Brazionis, L. The role of omega-3 polyunsaturated fatty acid supplementation in the management of type 2 diabetes mellitus: A narrative review. J. Nutr. Intermed. Metab. 2018, 14, 42–51. [Google Scholar] [CrossRef]
- Patten, A.R.; Brocardo, P.S.; Christie, B.R. Omega-3 supplementation can restore glutathione levels and prevent oxidative damage caused by prenatal ethanol exposure. J. Nutr. Biochem. 2013, 24, 760–769. [Google Scholar] [CrossRef]
- de la Guía-Galipienso, F.; Martínez-Ferran, M.; Vallecillo, N.; Lavie, C.J.; Sanchis-Gomar, F.; Pareja-Galeano, H. Vitamin D and cardiovascular health. Clin. Nutr. 2021, 40, 2946–2957. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Wright, G.; Bryant, H.; Wiggins, L.A.; Dal Zotto, V.L.; Schuler, M.; Malozzi, C.; Cohen, M.V.; Gassman, N.R. Cytoprotective Effect of Vitamin D on Doxorubicin-Induced Cardiac Toxicity in Triple Negative Breast Cancer. Int. J. Mol. Sci. 2021, 22, 7439. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.A.; Perrelli, A.; Ragni, A.; Retta, F.; De Silva, T.M.; Sobey, C.G.; Retta, S.F. Vitamin D Deficiency and the Risk of Cerebrovascular Disease. Antioxidants 2020, 9, 327. [Google Scholar] [CrossRef] [PubMed]
- Hoseini, R.; Rahim, H.A.; Ahmed, J.K. Concurrent alteration in inflammatory biomarker gene expression and oxidative stress: How aerobic training and vitamin D improve T2DM. BMC Complement. Med. Ther. 2022, 22, 165. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The role of vitamin e in human health and some diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157–e165. [Google Scholar]
- Barbagallo, M.; Dominguez, L.J.; Tagliamonte, M.R.; Resnick, L.M.; Paolisso, G. Effects of vitamin E and glutathione on glucose metabolism: Role of magnesium. Hypertension 1999, 34, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Maurya, V.K.; Aggarwal, M. Factors influencing the absorption of vitamin D in GIT: An overview. J. Food Sci. Technol. 2017, 54, 3753–3765. [Google Scholar] [CrossRef]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of vitamin E in humans: An update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Food and plant bioactives for reducing cardiometabolic disease risk: An evidence based approach. Food Funct. 2017, 8, 2076–2088. [Google Scholar] [CrossRef]
- Hathcock, J. Dietary supplements: How they are used and regulated. J. Nutr. 2001, 131, 1114s–1117s. [Google Scholar] [CrossRef] [PubMed]
- Placentino, U.; Sogari, G.; Viscecchia, R.; De Devitiis, B.; Monacis, L. The New Challenge of Sports Nutrition: Accepting Insect Food as Dietary Supplements in Professional Athletes. Foods 2021, 10, 1117. [Google Scholar] [CrossRef] [PubMed]
- Medina Pérez, O.M.; Flórez-Vargas, O.; Rincón Cruz, G.; Rondón González, F.; Rocha Muñoz, L.; Sánchez Rodríguez, L.H. Glutathione-related genetic polymorphisms are associated with mercury retention and nephrotoxicity in gold-mining settings of a Colombian population. Sci. Rep. 2021, 11, 8716. [Google Scholar] [CrossRef] [PubMed]
- Safarinejad, M.R.; Shafiei, N.; Safarinejad, S.H. Glutathione S-transferase gene polymorphisms (GSTM1, GSTT1, GSTP1) and prostate cancer: A case-control study in Tehran, Iran. Prostate Cancer Prostatic Dis. 2011, 14, 105–113. [Google Scholar] [CrossRef]
- Etemad, A.; Vasudevan, R.; Aziz, A.F.; Yusof, A.K.; Khazaei, S.; Fawzi, N.; Jamalpour, S.; Arkani, M.; Mohammad, N.A.; Ismail, P. Analysis of selected glutathione S-transferase gene polymorphisms in Malaysian type 2 diabetes mellitus patients with and without cardiovascular disease. Genet. Mol. Res. GMR 2016, 15, 1–9. [Google Scholar] [CrossRef]
- Azarova, I.; Klyosova, E.; Polonikov, A. The Link between Type 2 Diabetes Mellitus and the Polymorphisms of Glutathione-Metabolizing Genes Suggests a New Hypothesis Explaining Disease Initiation and Progression. Life 2021, 11, 886. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]


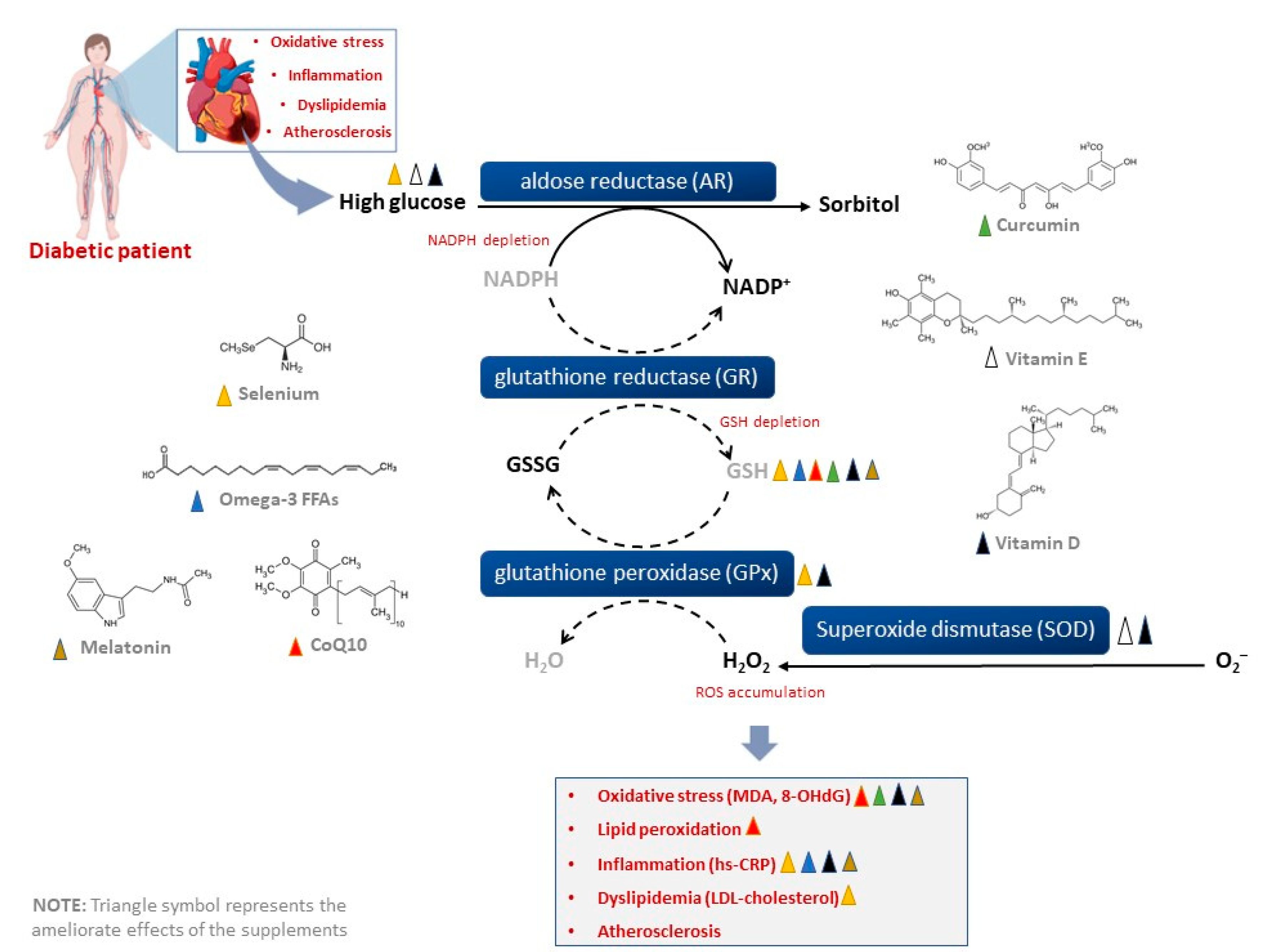
| Author, Year | Country | Patients, Including Number and Average Age | Supplements, Dose and Intervention Period | Main Findings |
|---|---|---|---|---|
| Ceriello et al., 1991 [20] | Italy | Hypertensive and non-hypertensive diabetic patients (n = 20), with an age between 28 and 45 years | Received vitamin C, thiopronine and glutathione (GSH) at doses, reaching 6 and 12 mmol. Evaluation of blood pressure and heart rate was at 10/15 min intervals during a 1/2 h basal periods after intake of antioxidants | GSH and thiopronine displayed hypotensive effects at a dose of 12 mmol. However, all antioxidants had no effect on blood pressure in healthy normal subjects |
| Knezević et al., 2000 [21] | Serbia | Patients with ischemic heart disease (n = 51), and some with diabetes mellitus (18%), age not reported | Received tocopherol (vitamin E) acetate at 450 mg for 3 months | Treatment improved blood glucose control and superoxide dismutase (SOD) levels in patients with diabetes. However, increased total antioxidant capacity in patients without diabetes. No effect was seen with glutathione-peroxidase (Gpx) |
| Qian et al., 2012 [22] | China | Diabetic patients with chronic heart disease (n = 24), with an average age of 60 years | Received a tablet of Salvia miltiorrhiza hydrophilic extract at 5 g twice per day for 60 days in addition to their existing hypoglycemic therapy | Treatment did not affect lipid profiles but reduced markers of oxidative stress, including the levels of malondialdehyde (MDA). This was accompanied by increased serum GSH level, SOD, paraoxonase and GSH reductase |
| Ramirez-Sanchez et al., 2013 [23] | Mexico | Patients with stable New York Heart Association stages II and III HF and T2D (n = 5), with an age between 47 and 70 years | Received Hershey’s extra dark 60% Cacao chocolate and cocoa beverages containing 18 g of natural cocoa powder (twice daily) for 3 months, with a total of 100 mg (-)-epicatechin | Treatment promoted recovery in GSH levels and reduced nitrotyrosilation and carbonylation of proteins within the skeletal muscle of patients, as well as transcriptional factors related to the enhancement of SOD2 and catalase protein expression |
| Huguenin et al., 2015 [24] | Brazil | Hypertensive and dyslipidemic subjects (n = 52), with an average age of 62 years | Received a diet combined with granulated Brazil nut at 13 g per day (≈227.5 μg/day of selenium) for 3 months with 1 month washout interval | Treatment improved lipid profile by reducing low-density lipoprotein (LDL) levels and this was inversely linked with Gpx3 concentrations. However, did not affect the oxidative stress marker, 8-epi-prostaglandin F2alpha (8-epi PGF2α) |
| Raygan et al., 2016 [25] | Iran | Overweight or obese patients with T2D and coronary heart disease (n = 30), with an age between 40 and 85 years | Received 100 mg coenzyme Q10 (CoQ10) supplementation for 2 months | Treatment improved metabolic profiles, including serum insulin levels and Homeostatic Model Assessment for Insulin Resistance (HOMA-IR). Plasma total antioxidant capacity was neutralized after adjusting for age. However, GSH levels were increased, and MDA was reduced. However, treatment did not affect fasting plasma glucose, lipid concentrations and inflammatory markers |
| Raygan et al., 2018 [26,27] | Iran | Patients with congestive heart failure and impaired fasting plasma glucose (n = 26), with an average age between 45 and 85 years | Received selenium at 200 µg/d for 3 months | Treatment improved insulin metabolism and reduced cardiometabolic risk by decreasing LDL-cholesterol and hs-CRP, while increasing plasma total antioxidant capacity and total GSH levels |
| Raygan et al., 2019 [28] | Iran | Patients with T2D and coronary heart disease (n = 30), with an average age of 65 years | Received omega-3 fatty acids from fish oil at 1000 mg or omega-3 fatty acids from flaxseed oil at 1000 mg twice a day for 3 months | Treatment improved metabolic profile, including decreasing insulin and hs-CPR levels, while increasing the total antioxidant capacity, including GSH levels |
| Raygan et al., 2019 [29] | Iran | Patients with T2D and coronary heart disease (n = 27), with an average age of 65 years | Received selenium at 200 μg/day plus 8 × 109 CFU/day probiotic for 3 months | Treatment improved metabolic profiles such as fasting plasma glucose, and serum insulin levels. In fact, co-supplementation improved lipid profiles by reducing triglycerides, total cholesterol, and hs-CRP, while enhancing levels of nitric oxide, total antioxidant capacity and total GSH |
| Raygan et al., 2019 [30] | Iran | Patients with T2D and coronary heart disease (n = 27), with an average age of 65 years | Received melatonin capsules at 10 mg (2 melatonin capsules, 5 mg each) once a day for 3 months | Treatment improved metabolic and lipid profiles by decreasing fasting plasma glucose, HOMA-IR, and total cholesterol. In addition, reduced blood pressure and markers of inflammation and oxidative stress like MDA, hs-CRP and increasing plasma GSH levels |
| Shafabakhsh et al., 2020 [31] | Iran | Patients with T2D and coronary heart disease (n = 27), with an average age between 45 and 85 years | Received curcumin at 1000 mg/day for 3 months | Treatment improved sleep quality, by reducing Pittsburgh Sleep Quality Index. This was accompanied by reduced markers of oxidative stress like MDA. Furthermore, total antioxidant capacity and GSH levels were also increased. In addition, the expression of peroxisome proliferator-activated receptor gamma from mononuclear cells from peripheral blood was increased |
| Hoseini et al., 2022 [32] | Iran | Patients with T2D (n = 40), with an average age between 35 and 50 years | Received vitamin D at 50,000 IU for 2 months, with aerobic training program executed for 20–40 min/day, at 60–75% of heart rate maximum, for 3 days/week | Improved metabolic profile like fasting plasma glucose, insulin, and HOMA-IR; and reduced markers of oxidative stress (MDA and 8-OHdG). Markers of inflammation were also reduced, including hs-CRP, and gene expression levels of tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), mitogen-activated protein kinases 1, nuclear factor kappa B (NF-κB) 1. This was consistent with elevated levels of total GSH, Gpx, SOD, catalase, and total antioxidant capacity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dludla, P.V.; Ziqubu, K.; Mabhida, S.E.; Mazibuko-Mbeje, S.E.; Hanser, S.; Nkambule, B.B.; Basson, A.K.; Pheiffer, C.; Tiano, L.; Kengne, A.P. Dietary Supplements Potentially Target Plasma Glutathione Levels to Improve Cardiometabolic Health in Patients with Diabetes Mellitus: A Systematic Review of Randomized Clinical Trials. Nutrients 2023, 15, 944. https://doi.org/10.3390/nu15040944
Dludla PV, Ziqubu K, Mabhida SE, Mazibuko-Mbeje SE, Hanser S, Nkambule BB, Basson AK, Pheiffer C, Tiano L, Kengne AP. Dietary Supplements Potentially Target Plasma Glutathione Levels to Improve Cardiometabolic Health in Patients with Diabetes Mellitus: A Systematic Review of Randomized Clinical Trials. Nutrients. 2023; 15(4):944. https://doi.org/10.3390/nu15040944
Chicago/Turabian StyleDludla, Phiwayinkosi V., Khanyisani Ziqubu, Sihle E. Mabhida, Sithandiwe E. Mazibuko-Mbeje, Sidney Hanser, Bongani B. Nkambule, Albertus K. Basson, Carmen Pheiffer, Luca Tiano, and André P. Kengne. 2023. "Dietary Supplements Potentially Target Plasma Glutathione Levels to Improve Cardiometabolic Health in Patients with Diabetes Mellitus: A Systematic Review of Randomized Clinical Trials" Nutrients 15, no. 4: 944. https://doi.org/10.3390/nu15040944
APA StyleDludla, P. V., Ziqubu, K., Mabhida, S. E., Mazibuko-Mbeje, S. E., Hanser, S., Nkambule, B. B., Basson, A. K., Pheiffer, C., Tiano, L., & Kengne, A. P. (2023). Dietary Supplements Potentially Target Plasma Glutathione Levels to Improve Cardiometabolic Health in Patients with Diabetes Mellitus: A Systematic Review of Randomized Clinical Trials. Nutrients, 15(4), 944. https://doi.org/10.3390/nu15040944








