Effect of Potassium Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis of Intervention Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Study Selection and Data Extraction
2.3. Risk of Bias
2.4. Grading of Evidence
2.5. Statistical Analysis
3. Results
3.1. Effect of Potassium Supplementation on Endothelial Function
3.1.1. Publication Bias
3.1.2. Additional Analyses
3.1.3. Quality of Body of Evidence
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aburto, N.J.; Hanson, S.; Gutierrez, H.; Hooper, L.; Elliott, P.; Cappuccio, F.P. Effect of increased potassium intake on cardiovascu-lar risk factors and disease: Systematic review and meta-analyses. BMJ 2013, 346, f1378. [Google Scholar] [PubMed]
- Filippini, T.; Naska, A.; Kasdagli, M.; Torres, D.; Lopes, C.; Carvalho, C.; Moreira, P.; Malavolti, M.; Orsini, N.; Whelton, P.K.; et al. Potassium Intake and Blood Pressure: A Dose-Response Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2020, 9, e015719. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Barba, G.; Cappuccio, F.P.; Strazzullo, P. Potassium Intake, Stroke, and Cardiovascular Disease: A Meta-Analysis of Prospective Studies. J. Am. Coll. Cardiol. 2011, 57, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Iannotta, C.; Sabino, P.; Ippolito, R. Potassium-rich diet and risk of stroke: Updated meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 585–587. [Google Scholar] [CrossRef]
- D’Elia, L.; Masulli, M.; Cappuccio, F.P.; Zarrella, A.F.; Strazzullo, P.; Galletti, F. Dietary Potassium Intake and Risk of Diabetes: A Systematic Review and Meta-Analysis of Prospective Studies. Nutrients 2022, 14, 4785. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Potassium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012; pp. 1–52. [Google Scholar]
- Hunt, B.D.; Cappuccio, F.P. Potassium intake and stroke risk: A review of the evidence and practical considerations for achieving a minimum target. Stroke 2014, 45, 1519–1522. [Google Scholar] [CrossRef]
- Sacks, F.M.; Svetkey, L.P.; Vollmer, W.M.; Appel, L.J.; Bray, G.A.; Harsha, D.; Obarzanek, E.; Conlin, P.R.; Miller, E.R.; Simons-Morton, D.G.; et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N. Engl. J. Med. 2001, 344, 3–10. [Google Scholar]
- Neal, B.; Wu, Y.; Feng, X.; Zhang, R.; Zhang, Y.; Shi, J.; Zhang, J.; Tian, M.; Huang, L.; Li, Z.; et al. Effect of Salt Substitution on Cardiovascular Events and Death. N. Engl. J. Med. 2021, 385, 1067–1077. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; Buchanan, L.A.; Ji, C.; Siani, A.; Miller, M.A. Systematic review and meta-analysis of randomised controlled clini-cal trials on the effects of potassium supplementation on serum potassium and creatinine. BMJ Open 2016, 6, e011716. [Google Scholar]
- Zhou, M.-S.; Kosaka, H.; Yoneyama, H. Potassium augments vascular relaxation mediated by nitric oxide in the carotid arteries of hypertensive Dahl rats. Am. J. Hypertens. 2000, 13, 666–672. [Google Scholar] [CrossRef]
- Kido, M.; Ando, K.; Onozato, M.L.; Tojo, A.; Yoshikawa, M.; Ogita, T.; Fujita, T. Protective Effect of Dietary Potassium Against Vascular Injury in Salt-Sensitive Hypertension. Hypertension 2008, 51, 225–231. [Google Scholar] [CrossRef]
- McCabe, R.D.; Bakarich, M.A.; Srivastava, K.; Young, D.B. Potassium inhibits free radical formation. Hypertension 1994, 24, 77–82. [Google Scholar] [CrossRef] [PubMed]
- McCabe, R.D.; Young, D.B. Potassium Inhibits Cultured Vascular Smooth Muscle Cell Proliferation. Am. J. Hypertens. 1994, 7, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Oberleithner, H.; Callies, C.; Kusche-Vihrog, K.; Schillers, H.; Shahin, V.; Riethmuller, C.; Macgregor, G.A.; de Wardener, H.E. Potassium softens vascular endothelium and increases nitric oxide release. Proc. Natl. Acad. Sci. USA 2009, 106, 2829–2834. [Google Scholar] [CrossRef]
- Thijssen, D.H.J.; Bruno, R.M.; Van Mil, A.C.C.M.; Holder, S.M.; Faita, F.; Greyling, A.; Zock, P.L.; Taddei, S.; Deanfield, J.E.; Luscher, T.; et al. Expert consensus and evidence-based recommendations for the assessment of flow-mediated dilation in humans. Eur. Heart J. 2019, 40, 2534–2547. [Google Scholar] [CrossRef]
- Hu, J.; Jiang, X.; Li, N.; Yu, X.; Perkovic, V.; Chen, B.; Zhao, L.; Neal, B.; Wu, Y. Effects of salt substitute on pulse wave analysis among individuals at high cardiovascular risk in rural China: A randomized controlled trial. Hypertens. Res. 2009, 32, 282–288. [Google Scholar] [CrossRef]
- Berry, S.E.; Mulla, U.Z.; Chowienczyk, P.; Sanders, T. Increased potassium intake from fruit and vegetables or supplements does not lower blood pressure or improve vascular function in UK men and women with early hypertension: A randomised controlled trial. Br. J. Nutr. 2010, 104, 1839–1847. [Google Scholar] [CrossRef]
- Blanch, N.; Clifton, P.; Petersen, K.; Willoughby, S.; Keogh, J. Effect of high potassium diet on endothelial function. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 983–989. [Google Scholar] [CrossRef]
- Gijsbers, L.; Dower, J.I.; Schalkwijk, C.G.; Kusters, Y.H.A.M.; Bakker, S.J.L.; Hollman, P.C.H.; Geleijnse, J.M. Effects of sodium and potassium supplementation on endothelial function: A fully controlled dietary intervention study. Br. J. Nutr. 2015, 114, 1419–1426. [Google Scholar] [CrossRef]
- He, F.J.; Marciniak, M.; Carney, C.; Markandu, N.D.; Anand, V.; Fraser, W.D.; Dalton, R.N.; Kaski, J.C.; MacGregor, G.A. Effects of Potassium Chloride and Potassium Bicarbonate on Endothelial Function, Cardiovascular Risk Factors, and Bone Turnover in Mild Hypertensives. Hypertension 2010, 55, 681–688. [Google Scholar] [CrossRef]
- Smiljanec, K.; Mbakwe, A.; Gonzalez, M.R.; Farquhar, W.B.; Lennon, S.L. Dietary Potassium Attenuates the Effects of Dietary Sodium on Vascular Function in Salt-Resistant Adults. Nutrients 2020, 12, 1206. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Wu, B.; Luo, Y.; Peng, L.; Chen, Y.; Zhu, J.; Peng, C.; Li, S.; Liu, J. Effect of potassium supplementation on vascular function: A meta-analysis of randomized controlled trials. Int. J. Cardiol. 2016, 228, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3 (Updated February 2022); John Wiley & Sons: Hoboken, NJ, USA, 2019; Available online: www.training.cochrane.org/handbook (accessed on 7 December 2022).
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. GRADE Handbook. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Updated October 2013. Available online: https://gdt.gradepro.org/app/handbook/handbook.html (accessed on 7 December 2020).
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.-Z.; Wang, P.; Guan, S.-Y.; Li, H.-M.; Leng, R.-X.; Pan, H.-F.; Ye, D.-Q. Decreased flow-mediated dilatation in patients with rheumatoid arthritis: A meta-analysis. Postgrad. Med. J. 2016, 93, 260–265. [Google Scholar] [CrossRef]
- Grimm, R.H., Jr.; Neaton, J.D.; Elmer, P.J.; Svendsen, K.H.; Levin, J.; Segal, M.; Holland, L.; Witte, L.J.; Clearman, D.R.; Kofron, P.; et al. The Influence of Oral Potassium Chloride on Blood Pressure in Hypertensive Men on a Low-Sodium Diet. N. Engl. J. Med. 1990, 322, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Young, D.B. Role of Potassium in Preventive Cardiovascular Medicine; Kluwer Academic Publishers: Norwell, MA, USA, 2001. [Google Scholar] [CrossRef]
- Ishimitsu, T.; Tobian, L.; Sugimoto, K.; Everson, T. High Potassium Diets Reduce Vascular and Plasma Lipid Peroxides in Stroke-Prone Spontaneously Hypertensive Rats. Clin. Exp. Hypertens. 1996, 18, 659–673. [Google Scholar] [CrossRef]
- Ishimitsu, T.; Tobian, L. High potassium diets reduce endothelial permeability in stroke-prone spontaneously hypertensive rats. Clin. Exp. Pharmacol. Physiol. 1996, 23, 241–245. [Google Scholar] [CrossRef]
- Ishimitsu, T.; Tobian, L.; Sugimoto, K.-I.; Lange, J.M. High Potassium Diets Reduce Macrophage Adherence to the Vascular Wall in Stroke-Prone Spontaneously Hypertensive Rats. J. Vasc. Res. 1995, 32, 406–412. [Google Scholar] [CrossRef]
- Rigsby, C.S.; Pollock, D.M.; Dorrance, A.M. Dietary potassium supplementation improves vascular structure and ameliorates the damage caused by cerebral ischemia in normotensive rats. Nutr. Metab. 2008, 5, 3. [Google Scholar] [CrossRef]
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Shannon, O.M.; Mendes, I.; Köchl, C.; Mazidi, M.; Ashor, A.W.; Rubele, S.; Minihane, A.-M.; Mathers, J.C.; Siervo, M. Mediterranean Diet Increases Endothelial Function in Adults: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Nutr. 2020, 150, 1151–1159. [Google Scholar] [CrossRef]
- Lehnhardt, A.; Kemper, M.J. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr. Nephrol. 2010, 26, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Weaver, C.M. Rise in Potassium Deficiency in the US Population Linked to Agriculture Practices and Dietary Potassium Deficits. J. Agric. Food Chem. 2020, 68, 11121–11127. [Google Scholar] [CrossRef]
- Sun, H.; Weaver, C.M. Rising Trend of Hypokalemia Prevalence in the US Population and Possible Food Causes. J. Am. Coll. Nutr. 2020, 40, 273–279. [Google Scholar] [CrossRef]
- Lopez, C.N.; Martinez-Gonzalez, M.A.; Sanchez-Villegas, A.; Alonso, A.; Pimenta, A.M.; Bes-Rastrollo, M. Costs of Mediterranean and western dietary pattern in a Spanish cohort and their relationship with prospective weight change. J. Epidemiol. Community Health 2009, 63, 920–927. [Google Scholar]
- Falk, R.S.; Heir, T.; Robsahm, T.E.; Tretli, S.; Sandvik, L.; Erikssen, J.E.; Paulsen, J.E. Fasting Serum Levels of Potassium and Sodium in Relation to Long-Term Risk of Cancer in Healthy Men. Clin. Epidemiol. 2020, 12, 1–8. [Google Scholar] [CrossRef]
- Falk, R.S.; Robsahm, T.E.; Paulsen, J.E.; Stocks, T.; Drake, I.; Heir, T. Fasting serum potassium and long-term mortality in healthy men. BMC Public Health 2021, 21, 711. [Google Scholar] [CrossRef]
- D’Elia, L.; Brajović, M.; Klisic, A.; Breda, J.; Jewell, J.; Cadjenović, V.; Cappuccio, F.P. Sodium and Potassium Intake, Knowledge At-titudes and Behaviour Towards Salt Consumption Amongst Adults in Podgorica, Montenegro. Nutrients 2019, 11, 160. [Google Scholar] [PubMed]
- Al-Mawali, A.; D’Elia, L.; Jayapal, S.K.; Morsi, M.; Al-Shekaili, W.N.; Pinto, A.D.; Al-Kharusi, H.; Al-Balushi, Z.; Idikula, J.; Al-Harrasi, A.; et al. National survey to estimate sodium and potassium intake and knowledge attitudes and behaviours towards salt consumption of adults in the Sultanate of Oman. BMJ Open 2020, 10, e037012. [Google Scholar] [CrossRef] [PubMed]
- D’Elia, L.; Obreja, G.; Ciobanu, A.; Breda, J.; Jewell, J.; Cappuccio, F.P. Sodium, Potassium and Iodine Intake, in a National Adult Population Sample of the Republic of Moldova. Nutrients 2019, 11, 2896. [Google Scholar] [CrossRef] [PubMed]
- Kumssa, D.; Joy, E.; Broadley, M. Global Trends (1961–2017) in Human Dietary Potassium Supplies. Nutrients 2021, 13, 1369. [Google Scholar] [CrossRef]
- World Health Organization. Guideline: Sodium Intake for Adults and Children; World Health Organization (WHO): Geneva, Switzerland, 2012. [Google Scholar]
- D’Elia, L.; Manfredi, M.; Strazzullo, P.; Galletti, F.; MINISAL-SIIA Study Group. Validation of an easy questionnaire on the assessment of salt habit: The MINISAL-SIIA Study Program. Eur. J. Clin. Nutr. 2018, 73, 793–800. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- D’Elia, L.; Dinu, M.; Sofi, F.; Volpe, M.; Strazzullo, P.; SINU Working Group, Endorsed by SIPREC. 100% Fruit juice intake and cardiovascular risk: A systematic review and meta-analysis of prospective and randomised controlled studies. Eur. J. Nutr. 2021, 60, 2449–2467. [Google Scholar] [CrossRef]
- D’Elia, L.; La Fata, E.; Galletti, F.; Scalfi, L.; Strazzullo, P. Coffee consumption and risk of hypertension: A dose-response me-ta-analysis of prospective studies. Eur. J. Nutr. 2019, 58, 271–280. [Google Scholar] [CrossRef]
- Weaver, C.M.; Stone, M.S.; Lobene, A.J.; Cladis, D.P.; Hodges, J.K. What Is the Evidence Base for a Potassium Requirement? Nutr. Today 2018, 53, 184–195. [Google Scholar] [CrossRef]
- National Academies of Sciences, Engineering, and Medicine. Dietary Reference Intakes for Sodium and Potassium; The National Academies Press: Washington, DC, USA, 2019. [Google Scholar] [CrossRef]
- Craiem, D.; Chironi, G.; Gariepy, J.; Miranda-Lacet, J.; Levenson, J.; Simon, A. New monitoring software for larger clinical application of brachial artery flow-mediated vasodilatation measurements. J. Hypertens. 2007, 25, 133–140. [Google Scholar] [CrossRef]
- Witte, D.R.; van der Graaf, Y.; Grobbee, D.E.; Bots, M.L.; Group, S.S. Measurement of flow-mediated dilatation of the brachial artery is affected by local elastic vessel wall properties in high-risk patients. Atherosclerosis 2005, 182, 323–330. [Google Scholar] [PubMed]
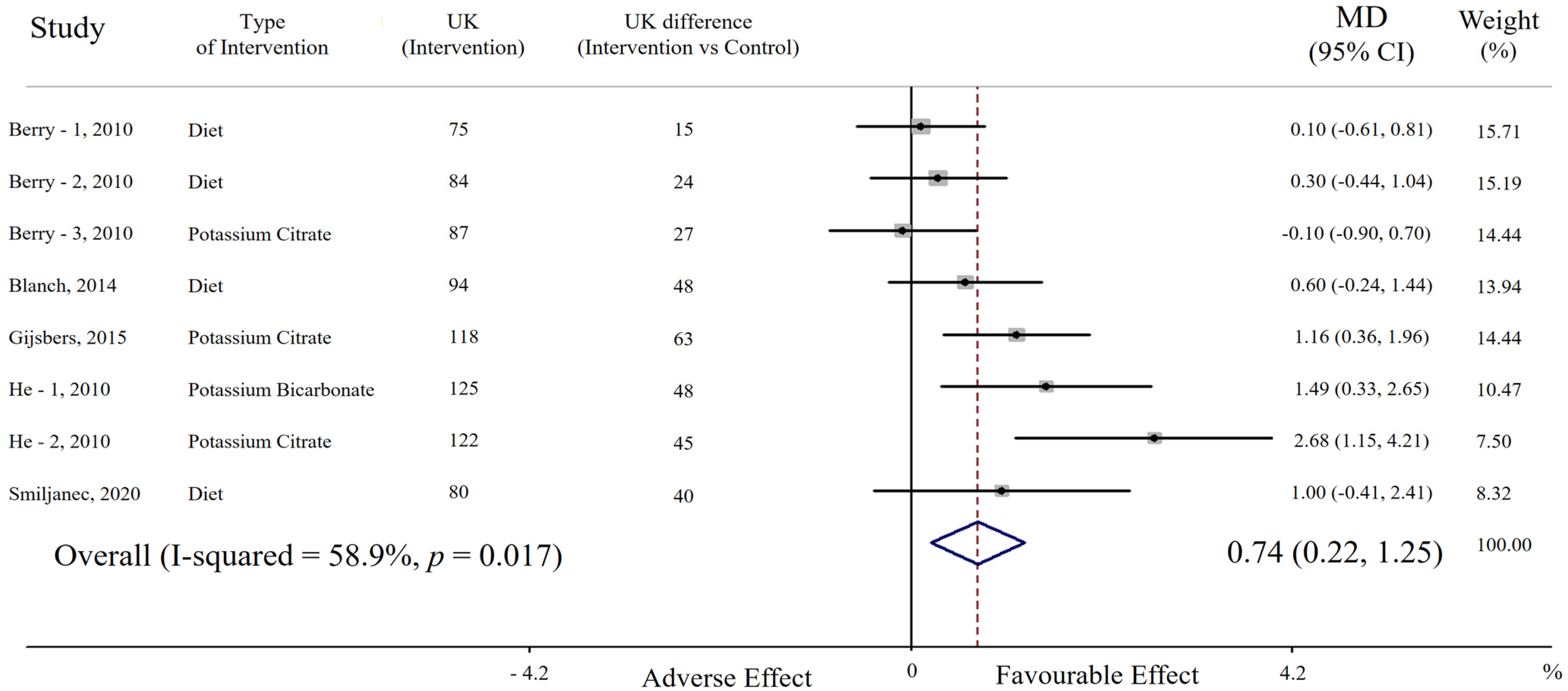
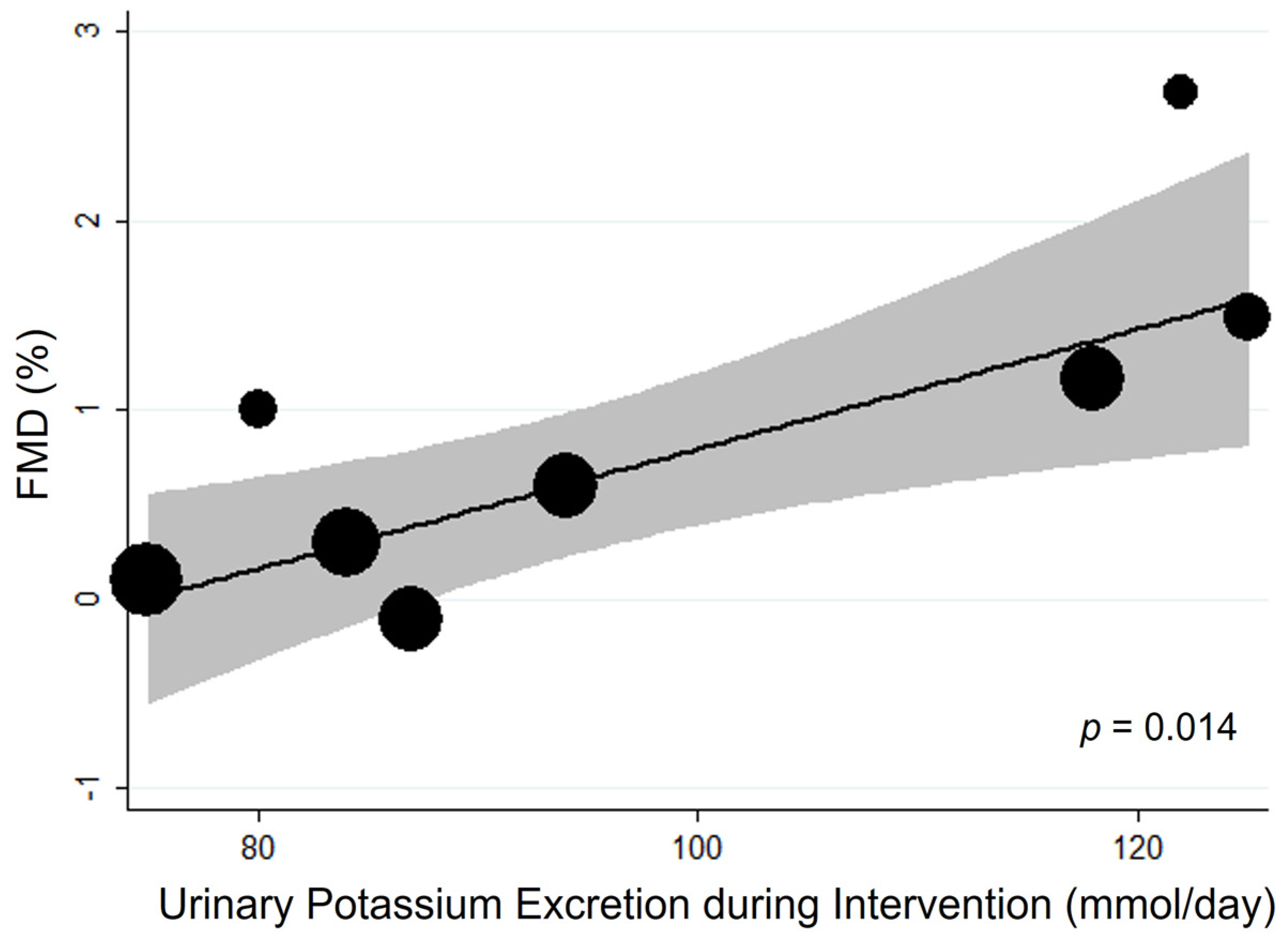
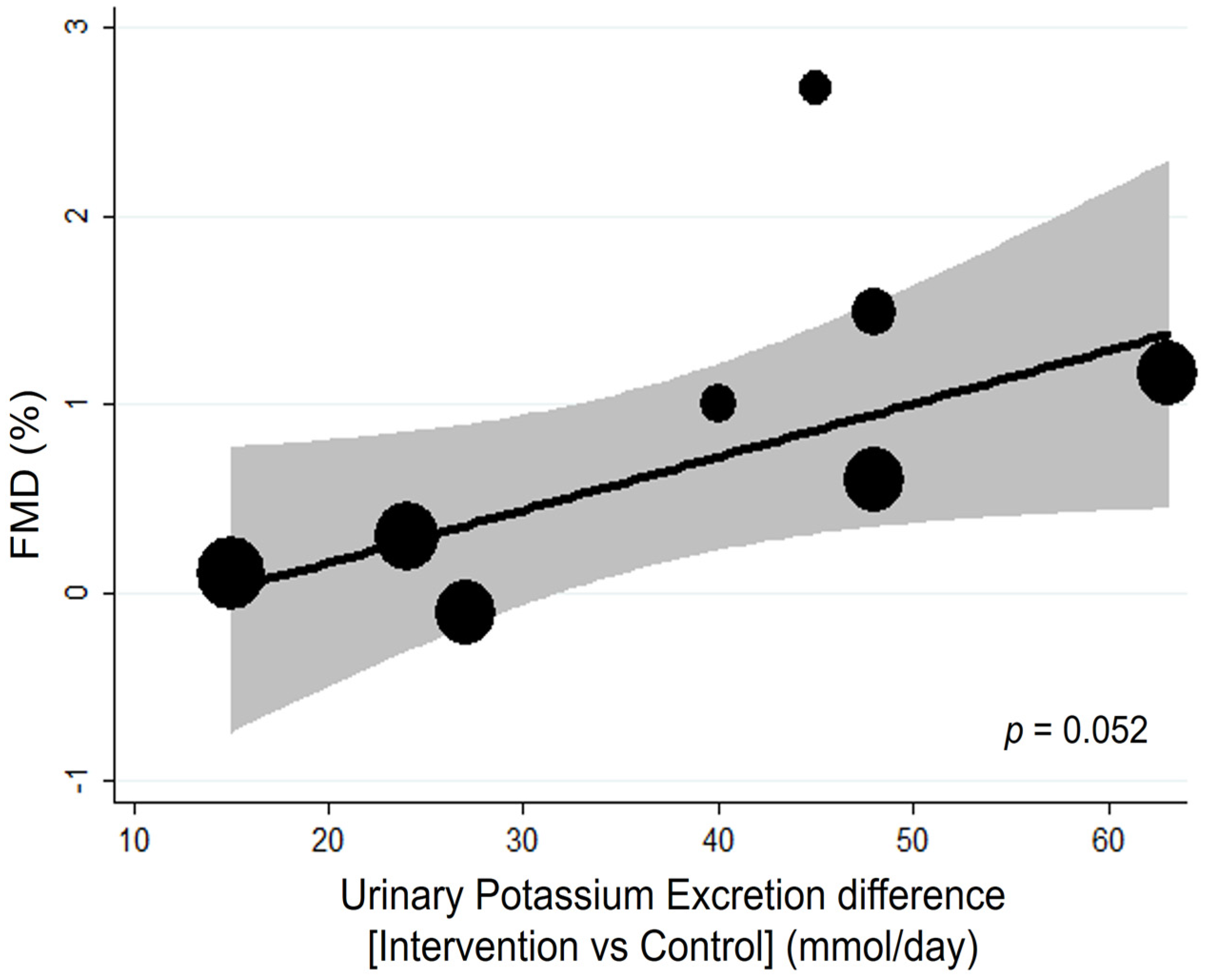
| First Author, Year [Ref] | Country | Cohort (n. of Participant) [Ethnicity] | Selected Features of the Study Participants | Intervention | Control | Duration | Age (Year) (Range) | BMI (kg/m2) | High vs. Low Potassium Comparison (mmol/24 h) * | Sodium Intake at High and Low Potassium Intake (mmol/24 h) * |
|---|---|---|---|---|---|---|---|---|---|---|
| Berry, 2010 [18] | UK | 48 (23 M, 25 W) [White 29, Black 10, Asian 9] | Diastolic BP > 80 and <100 mmHg | Potassium citrate cp—40 mmol/day) | Placebo cp | 6 weeks | 45.1 (22–65) | 28.4 | 87 vs. 60 | 113/106 |
| Potassium Diet—20 mmol/day | 75 vs. 60 | 116/106 | ||||||||
| Potassium Diet—40 mmol/day | 84 vs. 60 | 124/106 | ||||||||
| Blanch, 2014 [19] | Australia | 35 (9 M, 26 W) | Healthy participants | Potassium Diet—150 mmol/day | Usual potassium diet | 6 days | 31 (18–70) | 21.7 | 94 vs. 46 | 92/103 |
| Gijsbers, 2015 [20] | The Netherlands | 36 (24 M, 12 W) [White] | No smoking, Systolic BP 130–159 mm Hg, no CVD, no diabetes, no treatment. | Potassium chloride cp—72 mmol/day | Placebo cp | 4 weeks | 65.8 (40–80) | 27.2 | 118 vs. 55 | 96/105 |
| He, 2009 [21] | UK | 42 (30 M, 12 W) [White 29, Black 10, Asian 3] | Systolic BP 140–170 mmHg, Diastolic BP 90–105 mmHg, no treatment. | Potassium chloride cp—64 mmol/day | Placebo cp | 4 weeks | 51 (18–75) | 29.7 | 122 vs. 77 | 134/127 |
| Potassium bicarbonate cp—64 mmol/day | 125 vs. 77 | 129/127 | ||||||||
| Smiljanec, 2020 [22] | USA | 33 (16 M, 17 W) | Salt-resistant Healthy participants | Diet—120 mmol/day of potassium | Diet—65 mmol/day of potassium | 1 week | 27 (22–45) | 24 | 80 vs. 45 | 220/240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Elia, L.; Cappuccio, F.P.; Masulli, M.; La Fata, E.; Rendina, D.; Galletti, F. Effect of Potassium Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients 2023, 15, 853. https://doi.org/10.3390/nu15040853
D’Elia L, Cappuccio FP, Masulli M, La Fata E, Rendina D, Galletti F. Effect of Potassium Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients. 2023; 15(4):853. https://doi.org/10.3390/nu15040853
Chicago/Turabian StyleD’Elia, Lanfranco, Francesco P. Cappuccio, Maria Masulli, Ersilia La Fata, Domenico Rendina, and Ferruccio Galletti. 2023. "Effect of Potassium Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis of Intervention Studies" Nutrients 15, no. 4: 853. https://doi.org/10.3390/nu15040853
APA StyleD’Elia, L., Cappuccio, F. P., Masulli, M., La Fata, E., Rendina, D., & Galletti, F. (2023). Effect of Potassium Supplementation on Endothelial Function: A Systematic Review and Meta-Analysis of Intervention Studies. Nutrients, 15(4), 853. https://doi.org/10.3390/nu15040853







