Long-Term Effects and Potential Impact of Early Nutrition with Breast Milk or Infant Formula on Glucose Homeostasis Control in Healthy Children at 6 Years Old: A Follow-Up from the COGNIS Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics, Informed Consent, and Permissions
2.2. Study Design and Subjects
2.3. Demographical and Clinical Baseline Characteristics
2.4. Anthropometric Measures
2.4.1. Growth Velocity and Catch-Up
2.4.2. Body Fat Mass (BFM)
- a.
- BFM percentage calculated with the triceps and subscapular skinfolds using the Slaughter’s equations [28], as follows:
- b.
- BFM percentage calculated by Slaughter’s equations was corroborated by Tanita Body Composition Analyzer BC-418MA® (Biologica TM S.L., Barcelona, Spain), which indirectly measures total body water, fat mass, and fat-free mass using a high-frequency current (50 kHz, 90 µA) via 8-electrode. This method is based on the principle that body water conductivity changes in different body compartments [30,31]. Once BFM was obtained, children were classified by sex and age using percentile values according to the McCarthy’s tables (2006) [32], in the following groups: thinness (≤P2), NW (>P2 and <P85), or EW (≥P85 overweight and ≥P95 obese). The latter group included both overweight and obese children.
2.5. Continuous Glucose Monitoring (CGM)
2.6. Dietary Intake
2.7. Statistical Analysis
3. Results
3.1. Baseline Characteristics of the Six-Year-Old Children Participating in the COGNIS Study and Their Parents
3.2. Multiscale Sample Entropy (MSE) Analysis in Six-Year-Old Children
3.2.1. MSE Analysis Considering Study Groups at 6 Years Old
3.2.2. MSE Analysis in Children according to Catch-Up and Growth Velocity during the First Months of Life
3.2.3. MSE Analysis in Six-Year-Old Children Considering Study Groups according to Their BFM Calculated Using the Slaughter’s Equations
3.2.4. MSE Analysis in Six-Year-Old Children without Considering Study Groups according to Their BFM Calculated Using the Slaughter’s Equations
3.2.5. MSE Analysis in Six-Year-Old Children Considering Study Groups according to Their BFM Measured by Bioelectrical Impedance (TANITA®)
3.2.6. Children’s MSE Analysis according to Their BFM Measured with TANITA®
3.3. Dietary Intake Analysis in COGNIS Children at 6 Years Old
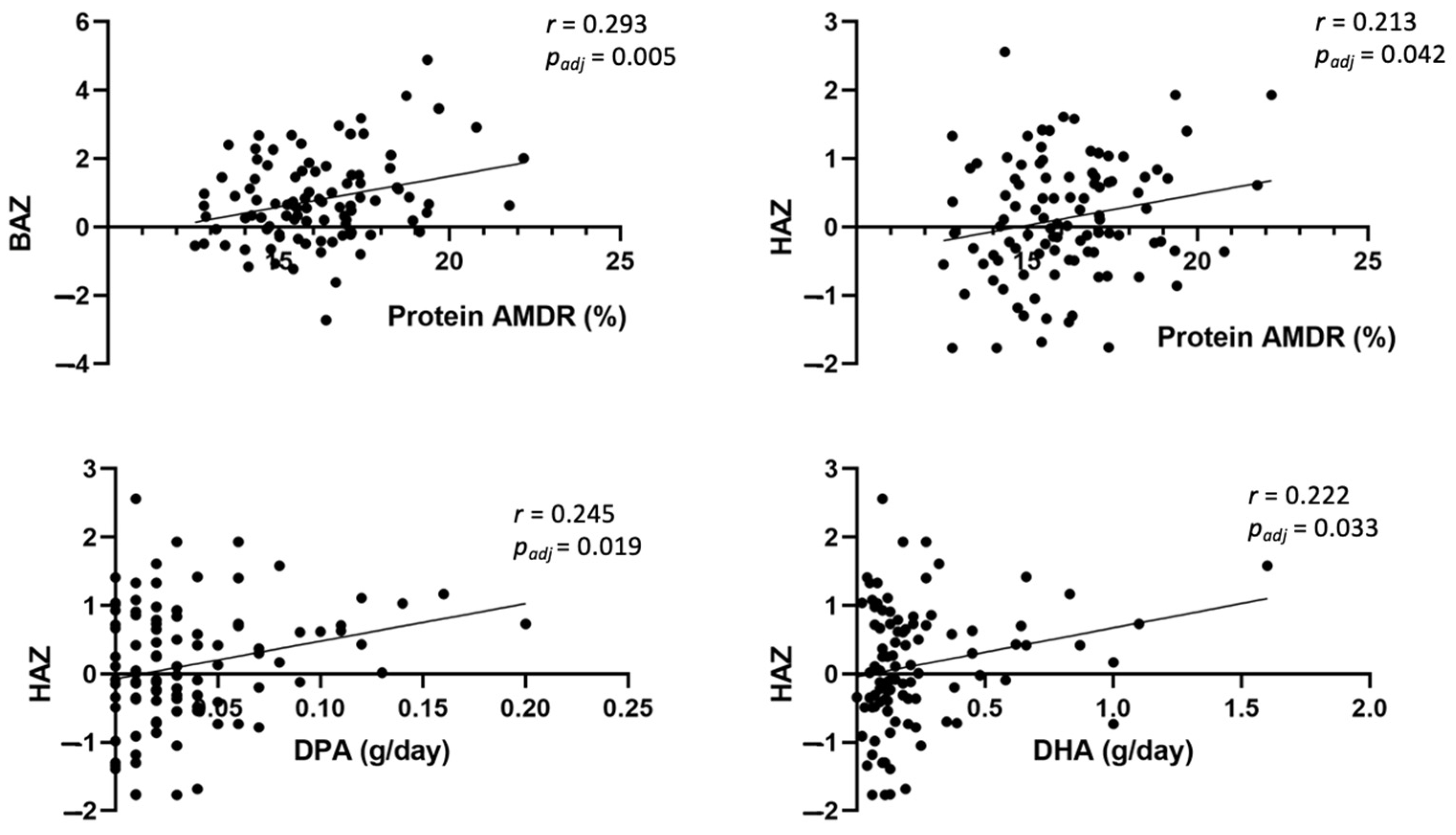
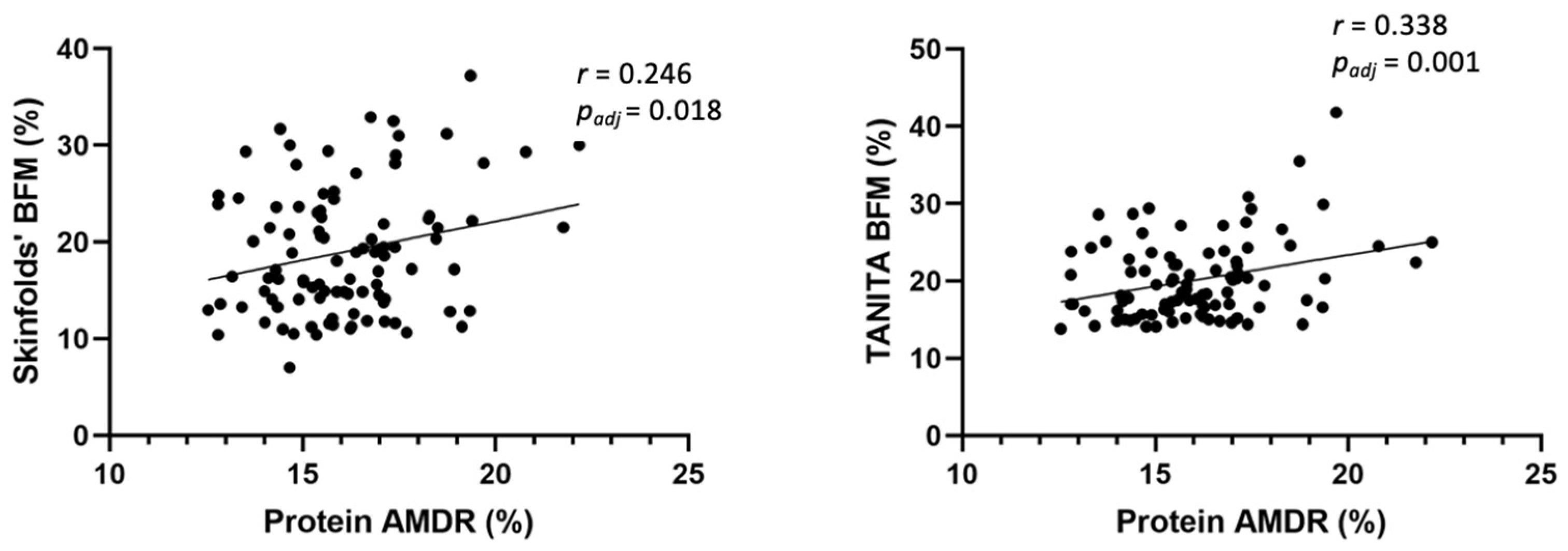
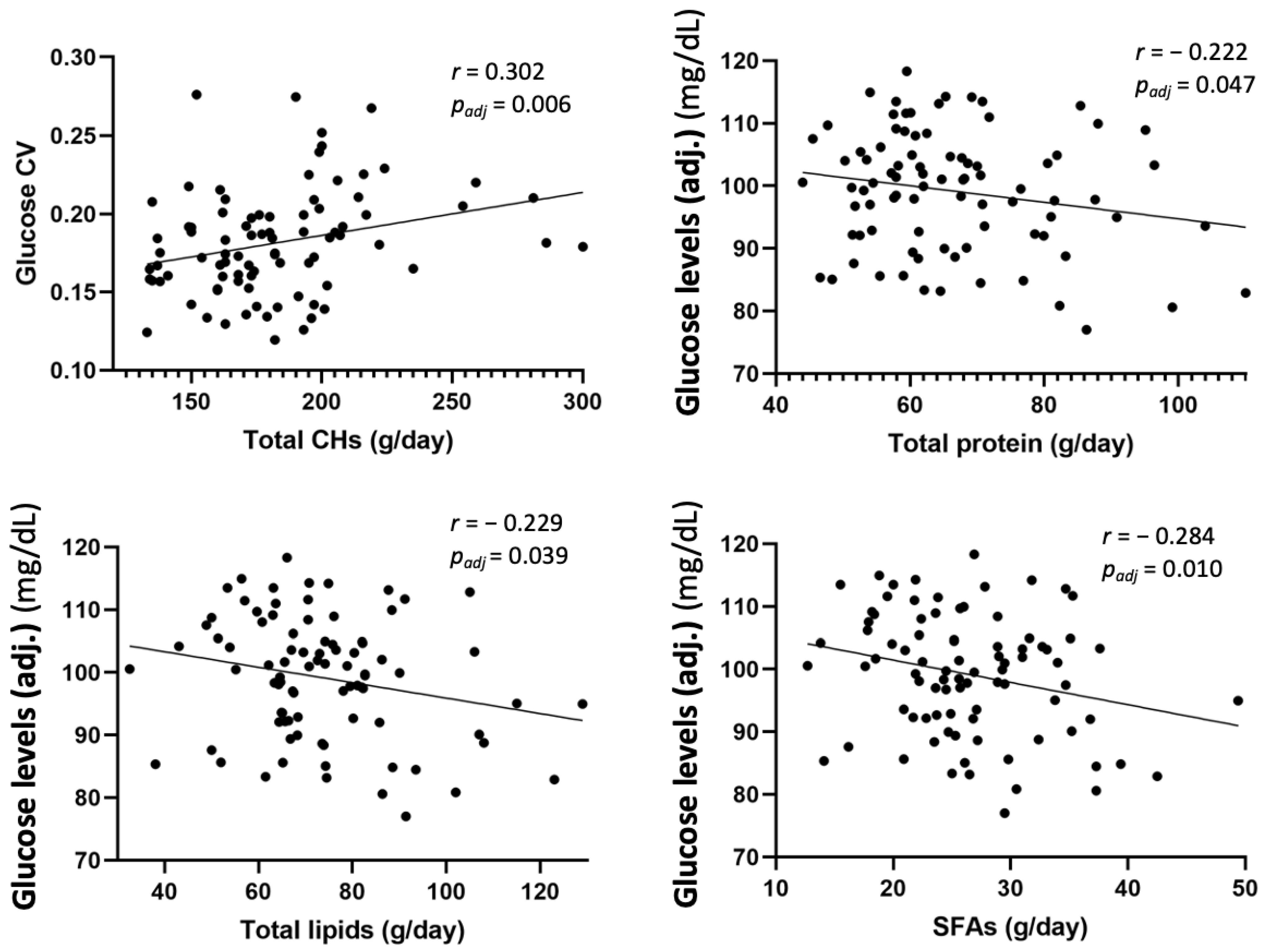
3.4. Association between Anthropometric Measures, Glucose Data, and Dietary Intake at 6 Years Old
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ellsworth, L.; Harman, E.; Padmanabhan, V.; Gregg, B. Lactational programming of glucose homeostasis: A window of opportunity. Reproduction 2018, 156, R23–R42. [Google Scholar] [CrossRef]
- Arner, P. Fat Tissue Growth and Development in Humans. Nestle Nutr. Inst. Workshop Ser. 2018, 89, 37–45. [Google Scholar] [PubMed]
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 19 October 2022).
- Spain. World Obesity Federation Global Obesity Observatory. Available online: https://data.worldobesity.org/country/spain-199/#data_prevalence (accessed on 19 October 2022).
- de Assunção, S.N.F.; Boa Sorte, N.C.A.; de Aragão Dantas Alves, C.; Mendes, P.S.A.; Alves, C.R.B.; Silva, L.R. Glucose alteration and insulin resistance in asymptomatic obese children and adolescents. J. De Pediatr. 2018, 94, 268–272. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Balland, E.; Cowley, M.A. Hypothalamic Insulin Resistance in Obesity: Effects on Glucose Homeostasis. Neuroendocrinology 2017, 104, 364–381. [Google Scholar] [CrossRef]
- Noordam, R.; Huurman, N.C.; Wijsman, C.A.; Akintola, A.A.; Jansen, S.W.M.; Stassen, S.; Beekman, M.; van de Rest, O.; Slagboom, P.E.; Mooijaart, S.P.; et al. High Adiposity Is Associated with Higher Nocturnal and Diurnal Glycaemia, but Not with Glycemic Variability in Older Individuals Without Diabetes. Front. Endocrinol. 2018, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- FMD. Variabilidad glucémica. Federación Mexicana de Diabetes, A.C. 2016. Available online: https://fmdiabetes.org/variabilidad-glucemica/ (accessed on 19 October 2022).
- American Diabetes Association Standards of Medical Care in Diabetes-2022 Abridged for Primary Care Providers. Clin. Diabetes 2022, 40, 10–38. [CrossRef]
- American Diabetes Association Standards of Medical Care in Diabetes-2017 Abridged for Primary Care Providers. Clin. Diabetes 2017, 35, 5–26. [CrossRef]
- Rodbard, D. Glucose Variability: A Review of Clinical Applications and Research Developments. Diabetes Technol. Ther. 2018, 20, S2-5–S2-15. [Google Scholar] [CrossRef]
- Akintola, A.A.; Noordam, R.; Jansen, S.W.; de Craen, A.J.; Ballieux, B.E.; Cobbaert, C.M.; Mooijaart, S.P.; Pijl, H.; Westendorp, R.G.; van Heemst, D. Accuracy of Continuous Glucose Monitoring Measurements in Normo-Glycemic Individuals. PLoS ONE 2015, 10, e0139973. [Google Scholar] [CrossRef]
- Castillo, E.D.; Nieto-Ruíz, A.; Escudero-Marín, M.; Campoy, C. Influence of Glycaemic Control on Cognitive Function in Diabetic Children and Adolescents. In Diabetes Food Plan; Waisundara, V., Ed.; InTech: London, UK, 2018; Available online: http://www.intechopen.com/books/diabetes-food-plan/influence-of-glycaemic-control-on-cognitive-function-in-diabetic-children-and-adolescents (accessed on 18 May 2020).
- Moreno Villares, J.M. Los mil primeros días de vida y la prevención de la enfermedad en el adulto. Nutr. Hosp. 2016, 33 (Suppl. 4), 8–11. Available online: http://revista.nutricionhospitalaria.net/index.php/nh/article/view/337 (accessed on 18 May 2020). [CrossRef]
- Marousez, L.; Lesage, J.; Eberlé, D. Epigenetics: Linking Early Postnatal Nutrition to Obesity Programming? Nutrients 2019, 11, 2966. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.; Sauer, P.J.; Boehm, G. Can we define an infant’s need from the composition of human milk? Am. J. Clin. Nutr. 2013, 98, 521S–528S. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.R.; Ling, P.-R.; Blackburn, G.L. Review of Infant Feeding: Key Features of Breast Milk and Infant Formula. Nutrients 2016, 8, E279. [Google Scholar] [CrossRef] [PubMed]
- Mazzocchi, A.; D’Oria, V.; De Cosmi, V.; Bettocchi, S.; Milani, G.P.; Silano, M.; Agostoni, C. The Role of Lipids in Human Milk and Infant Formulae. Nutrients 2018, 10, E567. [Google Scholar] [CrossRef] [PubMed]
- Bender, N.; Portmann, M.; Heg, Z.; Hofmann, K.; Zwahlen, M.; Egger, M. Fish or n3-PUFA intake and body composition: A systematic review and meta-analysis. Obes. Rev. 2014, 15, 657–665. [Google Scholar] [CrossRef]
- World Medical Association Inc. Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. J. Indian Med. Assoc. 2009, 107, 403–405. [Google Scholar]
- Association, W.M. World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar]
- Nieto-Ruiz, A.; García-Santos, J.A.; Bermúdez, M.G.; Herrmann, F.; Diéguez, E.; Sepúlveda-Valbuena, N.; García, S.; Miranda, M.T.; De-Castellar, R.; Rodríguez-Palmero, M.; et al. Cortical Visual Evoked Potentials and Growth in Infants Fed with Bioactive Compounds-Enriched Infant Formula: Results from COGNIS Randomized Clinical Trial. Nutrients 2019, 11, 2456. [Google Scholar] [CrossRef]
- Nieto-Ruiz, A.; Diéguez, E.; Sepúlveda-Valbuena, N.; Herrmann, F.; Cerdó, T.; López-Torrecillas, F.; De-Castellar, R.; Jiménez, J.; Pérez-García, M.; Miranda, M.T.; et al. The Effects of an Infant Formula Enriched with Milk Fat Globule Membrane, Long-Chain Polyunsaturated Fatty Acids and Synbiotics on Child Behavior up to 2.5 Years Old: The COGNIS Study. Nutrients 2020, 12, E3825. [Google Scholar] [CrossRef]
- Sepúlveda-Valbuena, N.; Nieto-Ruiz, A.; Diéguez, E.; Herrmann, F.; Escudero-Marín, M.; De-Castellar, R.; Rodríguez-Palmero, M.; Miranda, M.T.; García-Santos, J.A.; Bermúdez, M.G.; et al. Growth patterns and breast milk/infant formula energetic efficiency in healthy infants up to 18 months of life: The COGNIS study. Br. J. Nutr. 2021, 126, 1809–1822. [Google Scholar] [CrossRef]
- Diéguez, E.; Nieto-Ruiz, A.; Martín-Pérez, C.; Sepúlveda-Valbuena, N.; Herrmann, F.; Jiménez, J.; De-Castellar, R.; Catena, A.; García-Santos, J.A.; Bermúdez, M.G.; et al. Association study between hypothalamic functional connectivity, early nutrition, and glucose levels in healthy children aged 6 years: The COGNIS study follow-up. Front. Nutr. 2022, 9, 935740. [Google Scholar] [CrossRef]
- Growth Reference 5–19 Years—Application Tools. Available online: https://www.who.int/tools/growth-reference-data-for-5to19-years/application-tools (accessed on 19 October 2022).
- Norton, K. Standards for Anthropometry Assessment. In Kinanthropometry and Exercise Physiology; University of South Australia: Adelaide, Australia, 2018; pp. 68–137. [Google Scholar] [CrossRef]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; Loan, M.D.V.; Bemben, D.A. Skinfold Equations for Estimation of Body Fatness in Children and Youth. Hum. Biol. 1988, 60, 709–723. [Google Scholar]
- On Behalf of the IDEFICS Consortium; Nagy, P.; Kovacs, E.; Moreno, L.A.; Veidebaum, T.; Tornaritis, M.; Kourides, Y.; Siani, A.; Lauria, F.; Sioen, I.; et al. Percentile reference values for anthropometric body composition indices in European children from the IDEFICS study. Int. J. Obes. 2014, 38, S15–S25. [Google Scholar] [CrossRef] [PubMed]
- Moreira, O.C.; Alonso-Aubin, D.A.; de Oliveira, C.E.P.; Candia-Luján, R.; De Paz, J.A. Métodos de evaluación de la composición corporal: Una revisión actualizada de descripción, aplicación, ventajas y desventajas. Arch. De Med. Del Deporte 2015, 32, 387–394. [Google Scholar]
- Manual Tanita BC-418 (23 Páginas). Available online: https://www.manualpdf.es/tanita/bc-418/manual (accessed on 19 October 2022).
- McCarthy, H.D.; Cole, T.J.; Fry, T.; Jebb, S.A.; Prentice, A.M. Body fat reference curves for children. Int. J. Obes. 2006, 30, 598–602. [Google Scholar] [CrossRef] [PubMed]
- Sistema Flash de Monitorización de Glucosa|FreeStyle Libre. Available online: https://www.freestylelibre.es/libre/index.html (accessed on 24 February 2022).
- Villalobos, J.; Hernández-Sandoval, G.; Paz, J.J.; Finol, M.; Colina, J.L.; Villalobos, J.; Hernández-Sandoval, G.; Paz, J.J.; Finol, M.; Colina, J.L. Variabilidad glucémica como parámetro de control metabólico en pacientes con diabetes tipo 1. Rev. Venez. Endocrinol. Metab. 2020, 18, 107–120. [Google Scholar]
- Zhang, X.D.; Zhang, Z.; Wang, D. CGManalyzer: An R package for analyzing continuous glucose monitoring studies. Bioinformatics 2018, 34, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Sun, S.; Cao, Z.; Shi, Y.; Sun, B.; Zhang, X.D. A comprehensive comparison and overview of R packages for calculating sample entropy. Biol. Methods Protoc. 2019, 4, bpz016. [Google Scholar] [CrossRef]
- Fabris, C.; Sparacino, G.; Sejling, A.-S.; Goljahani, A.; Duun-Henriksen, J.; Remvig, L.S.; Juhl, C.B.; Cobelli, C. Hypoglycemia-related electroencephalogram changes assessed by multiscale entropy. Diabetes Technol. Ther. 2014, 16, 688–694. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Preparation and use of food-based dietary guidelines. Report of a joint FAO/WHO consultation. FAO/WHO. World Health Organ. Tech. Rep. Ser. 1998, 880, i–vi, 1–108. [Google Scholar]
- ALCE INGENIERIA. Available online: https://www.alceingenieria.net/nutricion.htm (accessed on 24 February 2022).
- Institute of Medicine (US) Subcommittee on Interpretation and Uses of Dietary Reference Intakes; Institute of Medicine (US) Standing Committee on the Scientific Evaluation of Dietary Reference Intakes. DRI Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press (US): Washington, DC, USA, 2000. Available online: http://www.ncbi.nlm.nih.gov/books/NBK222890/ (accessed on 24 February 2022).
- Cheshmeh, S.; Nachvak, S.M.; Hojati, N.; Elahi, N.; Heidarzadeh-Esfahani, N.; Saber, A. The effects of breastfeeding and formula feeding on the metabolic factors and the expression level of obesity and diabetes-predisposing genes in healthy infants. Physiol. Rep. 2022, 10, e15469. [Google Scholar] [CrossRef]
- Wong, W.W.; Hachey, D.L.; Insull, W.; Opekun, A.R.; Klein, P.D. Effect of dietary cholesterol on cholesterol synthesis in breast-fed and formula-fed infants. J. Lipid Res. 1993, 34, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Zubin Maslov, P.; Hill, J.A.; Lüscher, T.F.; Narula, J. High-sugar feeding and increasing cholesterol levels in infants. Eur. Heart J. 2021, 42, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Maggio, C.A.; Pi-Sunyer, F.X. Obesity and type 2 diabetes. Endocrinol. Metab. Clin. N. Am. 2003, 32, 805–822. [Google Scholar] [CrossRef] [PubMed]
- Ghane, N.; Broadney, M.M.; Davis, E.K.; Trenschel, R.W.; Collins, S.M.; Brady, S.M.; Yanovski, J.A. Estimating plasma glucose with the FreeStyle Libre Pro continuous glucose monitor during oral glucose tolerance tests in youth without diabetes. Pediatr. Diabetes 2019, 20, 1072–1079. [Google Scholar] [CrossRef]
- Flokas, M.E.; Zeymo, A.; Mete, M.; Anhalt, H.; Rother, K.I.; Gourgari, E. Overweight and obese children with optimal control in the T1D Exchange Registry: How are they different from lean children with optimal control? J. Diabetes Complicat. 2020, 34, 107513. [Google Scholar] [CrossRef]
- Lipsky, L.M.; Gee, B.; Liu, A.; Nansel, T.R. Glycemic control and variability in association with body mass index and body composition over 18months in youth with type 1 diabetes. Diabetes Res. Clin. Pract. 2016, 120, 97–103. [Google Scholar] [CrossRef]
- DuBose, S.N.; Li, Z.; Sherr, J.L.; Beck, R.W.; Tamborlane, W.V.; Shah, V.N. Effect of Exercise and Meals on Continuous Glucose Monitor Data in Healthy Individuals Without Diabetes. J. Diabetes Sci. Technol. 2021, 15, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar]
- Stephenson, E.J.; Smiles, W.; Hawley, J.A. The Relationship between Exercise, Nutrition and Type 2 Diabetes. In Medicine and Sport Science; Goedecke, J.H., Ojuka, E.O., Eds.; S. KARGER AG: Basel, Switzerland, 2014; pp. 1–10. Available online: https://www.karger.com/Article/FullText/357331 (accessed on 18 May 2022).
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Bröer, S. Amino acid transporters as modulators of glucose homeostasis. Trends Endocrinol. Metab. 2022, 33, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Harrison, S.; Couture, P.; Lamarche, B. Diet Quality, Saturated Fat and Metabolic Syndrome. Nutrients 2020, 12, E3232. [Google Scholar] [CrossRef]
- Edge, J.; Acerini, C.; Campbell, F.; Hamilton-Shield, J.; Moudiotis, C.; Rahman, S.; Randell, T.; Smith, A.; Trevelyan, N. An alternative sensor-based method for glucose monitoring in children and young people with diabetes. Arch. Dis. Child 2017, 102, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Sundberg, F.; Forsander, G. Continuous Glucose Monitoring in Healthy Children Aged 2–8 Years. Diabetes Technol. Ther. 2018, 20, 113–116. [Google Scholar] [CrossRef] [PubMed]
- DuBose, S.N.; Kanapka, L.G.; Bradfield, B.; Sooy, M.; Beck, R.W.; Steck, A.K. Continuous Glucose Monitoring Profiles in Healthy, Nondiabetic Young Children. J. Endocr. Soc. 2022, 6, bvac060. [Google Scholar] [CrossRef]


| SF (n = 32) | EF (n = 32) | BF (n = 28) | p2 | ||
|---|---|---|---|---|---|
| Mother | |||||
| Age (years) | 30.61 ± 6.60 a,b | 30.81 ± 4.64 a | 34.04 ± 4.54 b | 0.015 | |
| IQ (points) | 106.03 ± 12.29 | 102.94 ± 14.86 | 107.54 ± 14.63 | 0.43 | |
| pBMI (kg/m2) | 24.33 (4.79) | 25.28 (7.24) | 24.29 (3.76) | 0.74 | |
| GWG (kg) | 5.60 ± 5.43 | 6.36 ± 5.02 | 6.32 ± 3.28 | 0.79 | |
| GDM | No | 32 (100.00%) | 30 (93.80%) | 28 (100.00%) | 0.33 |
| Yes | 0 (0%) | 2 (6.30%) | 0 (0%) | ||
| Smoking during pregnancy | No | 25 (78.10%) | 29 (90.60%) | 26 (92.90%) | 0.23 |
| Yes | 7 (21.90%) | 3 (9.40%) | 2 (7.10%) | ||
| Type of delivery | Vaginal | 24 (75.00%) | 22 (68.80%) | 22 (78.60%) | 0.68 |
| Cesarean | 8 (25.00%) | 10 (31.30%) | 6 (21.40%) | ||
| Postpartum Depression | No | 24 (75.00%) | 27 (87.10%) | 24 (85.70%) | 0.39 |
| Yes | 8 (25.00%) | 4 (12.90%) | 4 (14.30%) | ||
| Educational level | NS/Primary | 2 (6.30%) | 9 (29.00%) | 2 (7.10%) | 0.002 |
| Secondary | 10 (31.30%) a | 7 (22.60%) a,b | 1 (3.60%) b | ||
| VT | 13 (40.60%) | 8 (25.80%) | 9 (32.10%) | ||
| University/ PhD | 7 (21.90%) a | 7 (22.60%) a | 16 (57.10%) b | ||
| Employment status | Unemployed | 6 (18.80%) | 3 (9.40%) | 4 (14.30%) | 0.32 |
| Domestic work | 1 (3.10%) | 3 (9.40%) | 0 (0%) | ||
| TC | 2 (6.30%) | 7 (21.90%) | 4 (14.30%) | ||
| SE | 23 (71.90%) | 19 (59.40%) | 20 (71.40%) | ||
| Father | |||||
| Age (years) | 32.30 ± 7.24 | 33.48 ± 6.43 | 36.36 ± 4.38 | 0.055 | |
| IQ (points) | 108.93 ± 11.39 | 103.00 ± 16.75 | 109.28 ± 10.73 | 0.16 | |
| Educational level | NS/Primary | 6 (18.80%) a,b | 13 (41.90%) b | 3 (10.70%) a | 0.005 |
| Secondary | 16 (50.00%) | 8 (25.80%) | 7 (25.00%) | ||
| VT | 4 (12.50%) | 7 (22.60%) | 6 (21.40%) | ||
| University/ PhD | 6 (18.80%) a,b | 3 (9.70%) b | 12 (42.90%) a | ||
| Employment status | Unemployed | 5 (15.60%) | 2 (6.90%) | 1 (3.60%) | 0.53 |
| Domestic work | 0 (0%) | 0 (0%) | 0 (0%) | ||
| TC | 4 (12.50%) | 2 (6.90%) | 3 (10.70%) | ||
| SE | 23 (71.90%) | 25 (86.20%) | 24 (85.70%) | ||
| Parents | |||||
| Socioeconomic status | Low | 5 (15.60%) a,b | 6 (18.80%) b | 0 (0%) a | 0.004 |
| Middle-Low | 16 (50.00%) | 14 (43.80%) | 7 (25.00%) | ||
| Middle-High | 9 (28.10%) | 10 (31.30%) | 11 (39.30%) | ||
| High | 2 (6.30%) a | 2 (6.30%) a | 10 (35.70%) b | ||
| Place of residence | Urban | 11 (34.40%) | 11 (34.40%) | 6 (21.40%) | 0.46 |
| Rural | 21 (65.60%) | 21 (65.60%) | 22 (78.60%) | ||
| Neonate | |||||
| GA at delivery (weeks) | 40.00 (2.00) | 40.00 (3.00) | 39.50 (3.00) | 0.71 | |
| Birth Weight (kg) | 3.34 ± 0.44 | 3.46 ± 0.53 | 3.39 ± 0.41 | 0.57 | |
| Birth Length (cm) | 51.00 (3.00) | 51.00 (3.60) | 51.00 (2.30) | 0.91 | |
| Birth HC (cm) | 35.00 (2.00) | 34.50 (1.10) | 35.00 (2.00) | 0.45 | |
| Breastfeeding (days) | 14.50 (21.50) a | 13.50 (33.00) a | 390.00 (270.00) b | <0.001 | |
| Sex | Boy | 21 (65.60%) | 19 (59.40%) | 11 (39.30%) | 0.11 |
| Girl | 11 (34.40%) | 13 (40.60%) | 17 (60.70%) | ||
| Siblings | 0 | 7 (21.90%) | 7 (22.60%) | 4 (14.30%) | 0.68 |
| ≥1 | 25 (78.10%) | 24 (77.40%) | 24 (85.70%) |
| Parameter | SF (n = 32) | EF (n = 32) | BF (n = 28) | p2 | padj3 | |
|---|---|---|---|---|---|---|
| Age (years) | 6.08 (0.11) | 6.08 (0.14) | 6.06 (0.11) | 0.76 | - | |
| BMI (kg/m2) | 16.19 (2.68) | 16.40 (2.42) | 16.02 (2.21) | 0.86 | 0.65 | |
| Weight (kg) | 22.74 ± 4.19 | 22.57 ± 3.59 | 22.95 ± 3.71 | 0.93 | 0.80 | |
| Height (cm) | 116.76 ± 4.43 | 115.86 ± 4.65 | 117.42 ± 4.84 | 0.43 | 0.80 | |
| BAZ | 0.61 ± 1.28 | 0.80 ± 1.14 | 0.66 ± 1.02 | 0.80 | 0.93 | |
| WAZ | 0.51 ± 1.22 | 0.55 ± 1.05 | 0.63 ± 1.08 | 0.91 | 0.90 | |
| HAZ | 0.09 ± 0.86 | −0.04 ± 0.84 | 0.28 ± 0.94 | 0.38 | 0.91 | |
| HC (cm) | 51.71 ± 1.75 | 51.63 ± 1.60 | 51.68 ± 1.15 | 0.98 | 0.26 | |
| TS (mm) | 9.80 (4.80) | 9.90 (4.70) | 9.84 (4.45) | 0.93 | 0.84 | |
| SS (mm) | 5.70 (2.68) | 6.05 (1.95) | 5.83 (2.10) | 0.94 | 0.85 | |
| Skinfolds’ BFM (%) | 16.18 (10.35) | 16.32 (7.84) | 20.36 (9.66) | 0.89 | 0.96 | |
| Skinfolds’ BFM classification | Thin | 1 (3.20%) | 0 (0%) | 0 (0%) | 0.87 | - |
| Normoweight | 22 (71.00%) | 25 (78.10%) | 21 (75.00%) | |||
| Overweight | 5 (16.10%) | 3 (9.40%) | 5 (17.90%) | |||
| Obesity | 3 (9.70%) | 4 (12.50%) | 2 (7.10%) | |||
| TANITA® BFM (%) | 19.41 ± 4.57 | 19.56 ± 4.59 | 21.04 ± 4.36 | 0.35 | 0.35 | |
| TANITA® BFM classification | Thin | 0 (0%) | 0 (0%) | 0 (0%) | 0.99 | - |
| Normoweight | 21 (65.60%) | 21 (67.70%) | 16 (64.00%) | |||
| Overweight | 6 (18.80%) | 5 (16.10%) | 5 (20.00%) | |||
| Obesity | 5 (15.60%) | 5 (16.10%) | 4 (16.00%) | |||
| Glucose Data | SF (n = 32) | EF (n = 32) | BF (n = 28) | p2 | padj3 |
|---|---|---|---|---|---|
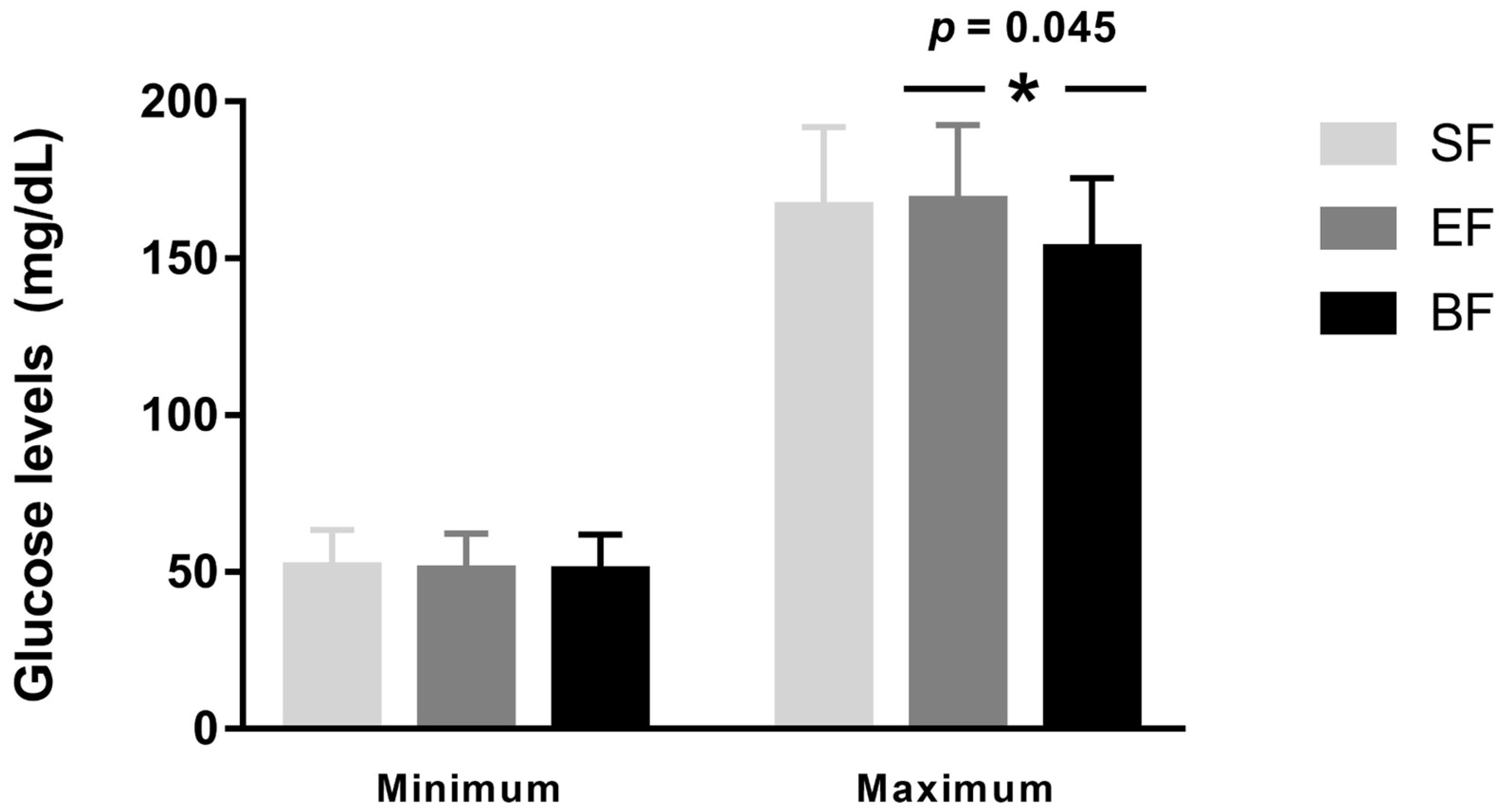
| Mean glucose levels (mg/dL) | 95.98 ± 9.38 a | 94.13 ± 9.08 a,b | 90.10 ± 8.26 b | 0.040 | 0.026 |
| Adjusted mean glucose levels (mg/dL) | 101.80 ± 9.50 a | 99.99 ± 9.05 a,b | 94.96 ± 8.78 b | 0.015 | 0.005 |
| 3′–30′ MSE increment | 0.194 ± 0.089 a | 0.236 ± 0.096 a | 0.254 ± 0.104 a | 0.048 | 0.081 |
| Glucose CV | 0.185 ± 0.037 a,b | 0.190 ± 0.035 a | 0.169 ± 0.025 b | 0.046 | 0.014 |
| Glucose CV (%) | 18.49 ± 3.69 a,b | 19.02 ± 3.55 a | 16.92 ± 2.46 b | 0.046 | 0.014 |
| Minutes | SF (n = 32) | EF (n = 32) | BF (n = 28) | p1 | p2 | p3 |
|---|---|---|---|---|---|---|
| MSE 3′ | 0.175 ± 0.057 | 0.186 ± 0.047 | 0.196 ± 0.043 | 0.40 | 0.11 | 0.40 |
| MSE 6′ | 0.217 ± 0.071 | 0.233 ± 0.059 | 0.249 ± 0.059 | 0.31 | 0.059 | 0.30 |
| MSE 9′ | 0.244 ± 0.084 | 0.267 ± 0.074 | 0.285 ± 0.072 | 0.25 | 0.045 | 0.33 |
| MSE 12′ | 0.270 ± 0.094 | 0.296 ± 0.086 | 0.321 ± 0.089 | 0.25 | 0.034 | 0.28 |
| MSE 15′ | 0.290 ± 0.106 | 0.319 ± 0.097 | 0.343 ± 0.100 | 0.24 | 0.048 | 0.35 |
| MSE 18′ | 0.304 ± 0.109 | 0.344 ± 0.105 | 0.364 ± 0.107 | 0.14 | 0.037 | 0.47 |
| MSE 21′ | 0.316 ± 0.109 | 0.363 ± 0.117 | 0.388 ± 0.113 | 0.10 | 0.016 | 0.41 |
| MSE 24′ | 0.342 ± 0.124 | 0.390 ± 0.126 | 0.407 ± 0.120 | 0.13 | 0.045 | 0.59 |
| MSE 27′ | 0.361 ± 0.128 | 0.407 ± 0.129 | 0.428 ± 0.134 | 0.15 | 0.054 | 0.56 |
| MSE 30′ | 0.369 ± 0.132 | 0.423 ± 0.135 | 0.450 ± 0.132 | 0.11 | 0.021 | 0.45 |
| Minutes | NWGV (n = 57) | RWGV (n = 12) | SWGV (n = 19) | p1 | p2 | p3 |
|---|---|---|---|---|---|---|
| MSE 3′ | 0.191 ± 0.050 | 0.159 ± 0.041 | 0.195 ± 0.040 | 0.026 | 0.75 | 0.023 |
| MSE 6′ | 0.238 ± 0.066 | 0.205 ± 0.047 | 0.243 ± 0.053 | 0.058 | 0.71 | 0.047 |
| MSE 9′ | 0.271 ± 0.082 | 0.232 ± 0.052 | 0.277 ± 0.066 | 0.045 | 0.75 | 0.045 |
| MSE 12′ | 0.301 ± 0.096 | 0.256 ± 0.056 | 0.312 ± 0.076 | 0.037 | 0.63 | 0.026 |
| MSE 15′ | 0.326 ± 0.108 | 0.270 ± 0.063 | 0.331 ± 0.083 | 0.025 | 0.81 | 0.029 |
| MSE 18′ | 0.345 ± 0.113 | 0.288 ± 0.069 | 0.357 ± 0.095 | 0.030 | 0.65 | 0.025 |
| MSE 21′ | 0.363 ± 0.119 | 0.298 ± 0.071 | 0.380 ± 0.109 | 0.019 | 0.58 | 0.017 |
| MSE 24′ | 0.388 ± 0.131 | 0.321± 0.074 | 0.405 ± 0.110 | 0.022 | 0.57 | 0.017 |
| MSE 27′ | 0.410 ± 0.139 | 0.347 ± 0.082 | 0.409 ± 0.112 | 0.045 | 0.98 | 0.085 |
| MSE 30′ | 0.424 ± 0.142 | 0.364 ± 0.094 | 0.432 ± 0.116 | 0.085 | 0.81 | 0.087 |
| Adj. glucose | 97.83 ± 9.51 | 102.13 ± 7.45 | 101.06 ± 10.33 | 0.10 | 0.24 | 0.74 |
| Minutes | SF (n = 22) | EF (n = 25) | BF (n = 21) | p1 |
|---|---|---|---|---|
| MSE 3′ | 0.167 ± 0.056 a | 0.176 ± 0.043 a | 0.197 ± 0.047 a | 0.045 |
| MSE 6′ | 0.206 ± 0.070 a | 0.221 ± 0.054 a | 0.251 ± 0.065 a | 0.019 |
| MSE 9′ | 0.233 ± 0.081 a | 0.253 ± 0.068 a | 0.288 ± 0.078 a | 0.018 |
| MSE 12′ | 0.257 ± 0.090 a | 0.281 ± 0.077 a,b | 0.325 ± 0.096 b | 0.012 |
| MSE 15′ | 0.274 ± 0.100 a | 0.302 ± 0.088 a,b | 0.352 ± 0.105 b | 0.011 |
| MSE 18′ | 0.287 ± 0.101 a | 0.325 ± 0.094 a,b | 0.373 ± 0.108 b | 0.006 |
| MSE 21′ | 0.299 ± 0.103 a | 0.340 ± 0.102 a,b | 0.398 ± 0.114 b | 0.003 |
| MSE 24′ | 0.323 ± 0.113 a | 0.364± 0.103 a,b | 0.419 ± 0.123 b | 0.007 |
| MSE 27′ | 0.337 ± 0.113 a | 0.388 ± 0.116 a,b | 0.441 ± 0.131 b | 0.006 |
| MSE 30′ | 0.346 ± 0.116 a | 0.403 ± 0.121 a,b | 0.464 ± 0.134 b | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diéguez, E.; Nieto-Ruiz, A.; Sepúlveda-Valbuena, N.; Herrmann, F.; Agil, A.; De-Castellar, R.; Jiménez, J.; Azaryah, H.; García-Santos, J.A.; García-Bermúdez, M.; et al. Long-Term Effects and Potential Impact of Early Nutrition with Breast Milk or Infant Formula on Glucose Homeostasis Control in Healthy Children at 6 Years Old: A Follow-Up from the COGNIS Study. Nutrients 2023, 15, 852. https://doi.org/10.3390/nu15040852
Diéguez E, Nieto-Ruiz A, Sepúlveda-Valbuena N, Herrmann F, Agil A, De-Castellar R, Jiménez J, Azaryah H, García-Santos JA, García-Bermúdez M, et al. Long-Term Effects and Potential Impact of Early Nutrition with Breast Milk or Infant Formula on Glucose Homeostasis Control in Healthy Children at 6 Years Old: A Follow-Up from the COGNIS Study. Nutrients. 2023; 15(4):852. https://doi.org/10.3390/nu15040852
Chicago/Turabian StyleDiéguez, Estefanía, Ana Nieto-Ruiz, Natalia Sepúlveda-Valbuena, Florian Herrmann, Ahmad Agil, Roser De-Castellar, Jesús Jiménez, Hatim Azaryah, José Antonio García-Santos, Mercedes García-Bermúdez, and et al. 2023. "Long-Term Effects and Potential Impact of Early Nutrition with Breast Milk or Infant Formula on Glucose Homeostasis Control in Healthy Children at 6 Years Old: A Follow-Up from the COGNIS Study" Nutrients 15, no. 4: 852. https://doi.org/10.3390/nu15040852
APA StyleDiéguez, E., Nieto-Ruiz, A., Sepúlveda-Valbuena, N., Herrmann, F., Agil, A., De-Castellar, R., Jiménez, J., Azaryah, H., García-Santos, J. A., García-Bermúdez, M., & Campoy, C. (2023). Long-Term Effects and Potential Impact of Early Nutrition with Breast Milk or Infant Formula on Glucose Homeostasis Control in Healthy Children at 6 Years Old: A Follow-Up from the COGNIS Study. Nutrients, 15(4), 852. https://doi.org/10.3390/nu15040852









