Highlights
What are the main findings?
- Beer has a diverse composition, with many ingredients that may interact positively with the gut microbiota such as polyphenols, fibers, and melanoidins; they can stimulate gut microorganisms mainly through their prebiotic actions, leading to anti-inflammatory and antioxidant effects.
- While beer has potential health benefits, chronic alcohol consumption is harmful to the gut microbiota. Hence, alcohol-free or low-alcohol beers are suggested as healthier alternatives.
What is the implication of the main finding?
- There are strong arguments for developing functional beers, mainly through fortification with additional fibers, antioxidants, and probiotics to enhance the health benefits while minimizing alcohol content.
Abstract
Beer is one of the most consumed drinks worldwide. It contains numerous categories of antioxidants, phenolic products, traces of group B vitamins, minerals (selenium, silicon, potassium), soluble fibers and microorganisms. Low or moderate beer consumption, with or without alcohol, showed positive effects on health by stimulating the development of a healthy microbiota. In the present review we focused on four components responsible with interaction with gut microbiota: microorganisms, polyphenols, fiber and melanoidins, their presence in usual beers and on perspectives of development of fortified beers with enhanced effects on gut microbiota. Though microorganisms rarely escape pasteurization of beer, there are new unpasteurized types that might bring strains with probiotic effects. The polyphenols from beer are active on the gut microbiota stimulating its development, with consequent local anti-inflammatory and antioxidant effects. Their degradation products have prebiotic action and may combat intestinal dysbiosis. Beer contains dietary fiber such as non-starchy, non-digestible carbohydrates (β-glucans, arabinoxylans, mannose, fructose polymers, etc.) that relate with gut microbiota through fermentation, serving as a nutrient substrate. Another type of substances that are often considered close to fiber because they have an extremely low digestibility, melanoidins (melanosaccharides), give beer antioxidant and antibacterial properties. Though there are not many research studies in this area, the conclusion of this review is that beer seems a good candidate for a future functional food and that there are many pathways by which its ingredients can influence in a positive manner the human gut microbiota. Of course, there are many technological hinderances to overcome. However, designing functional beers fortified with fiber, antioxidants and probiotics, with a very low or no alcoholic content, will counteract the negative perception of beer consumption, will nullify the negative effects of alcohol, while simultaneously exerting a positive action on the gut microbiota.
1. Introduction
One of the most consumed drinks on the whole world is represented by beer. With a consumption history of several millennia, proven by very old archaeological discoveries, having a manufacturing technology that has evolved over time to the current form of the drink, it can have a significant impact on the health of people and the effects can be major, due to the enormous area where beer is drunk. In the past, beer also had medicinal values, as a stimulant or analgesic [1]. Beer was already widely consumed in ancient Egypt [2]. If until recently the history of beer went all the way to Babylonia, around 6000 BC, today there is evidence that a product similar to beer was consumed in China 9000 years ago, in a ritualic context [3]. Like other alcoholic beverages, beer is viewed with caution in the medical world. However, similar to other fermented products, it has health potential and beers without alcohol can be easily produced. It contains numerous categories of antioxidants, especially phenolic products from both hops and malt [4,5], traces of vitamins (especially from group B) [6] and minerals (selenium, silicon, potassium) [6] and soluble fibers [7]. Some bacteria and fungi have also been identified in the beer, the most encountered being lactic acid bacteria (Lactobacillales order, Bifidobacterium genus) and Saccharomyces spp. [8,9,10].
Alcohol itself can have dual effects, depending, of course, on the amount consumed. The composition, as well as the versatility of the manufacturing process that allows obtaining beer with minimal or no alcohol, identifies it as a potential functional food as such, but above all it can become functional through the addition of biologically active substances. We exclude excessive consumption of alcoholic beer from the start, due to harmful effects. Numerous studies have followed the action of low or moderate beer consumption, with and without alcohol, on health. The identified effects were positive in the following directions: cardio-protective effect [11], bone health [12], positive stimulation of microbiota [13], etc. In the entire evaluation of beer, the current consumption trends must be taken into account, in which the individual is interested in the health benefits of the products consumed, beyond the satisfaction and covering of basic survival needs. The highlighting of the natural presence of some sanogenic ingredients or of the enrichment of the product in a specific ingredient with positive action on health can be beneficial for both the producer and the consumer [14].
Microbiota, the living microorganisms from oral cavity, gut, skin and other sites such as vagina or lungs was studied by numerous researchers since it was discovered back in early 1900s [15]. It harbors a whole range of microorganisms, which from the most studied are bacteria and fungi that are symbiotic with the human body generating its optimal functioning [16,17,18,19], Table 1.

Table 1.
Types of microorganism from human gut microbiota.
In the human microbiota there are also Archaea and Viruses which roles are not yet completely understood [20]. The gut microbiota, composed of more than 100 trillion microorganisms [21], is the most studied, and several roles such as food fermentation, production of vitamins or even immune roles have been attributed to it. In humans, gut microbiota differs at the individual level, by localization into the gastro-intestinal tract and by age [22]. There are different conditions (pH and level of oxygen) that induce colonization with bacterial types: in the small intestine Proteobacteria (Enterobacteriaceae) are found and in the colon Bacteriodetes (Bacteroidaceae, Prevotellaceae and Rikenellaceae) were detected [23]. Gut microbiota becomes relatively stable from the age of three [24], but in people over seventy its diversity changes, with low levels of Bifidobacterium and high levels of Clostridium and Proteobacteria [25]. The alteration of healthy gut microbiota can determine an unbalanced composition of bacterial population, leading to various diseases such as cardiovascular diseases, cancer, diabetes mellitus, inflammatory bowel diseases, chronic liver diseases and chronic kidney diseases [26].
The relationship between beer or some of its ingredients and the intestinal microbiota is interesting and has been revealed in several studies to date, offering generous premises for future research. The influence of food ingredients on the composition, diversity and functionality of the intestinal microbiota is still incompletely deciphered, especially when it comes to lasting effects over time (Figure 1). It should be noted that chronic alcohol consumption has negative effects on the diversity of the microbiota, causing intestinal dysbiosis [13], among other ill effects on human metabolism. In the present narrative review, we targeted four components of beer that might interact with gut microbiota, according to research: microorganisms, antioxidants, fiber and melanoidins. By means of pre and probiotic mechanisms, they show potential to enhance the development of a healthy gut microbiota, with predominant saccharolytic, short chain fatty acids-producing bacteria.
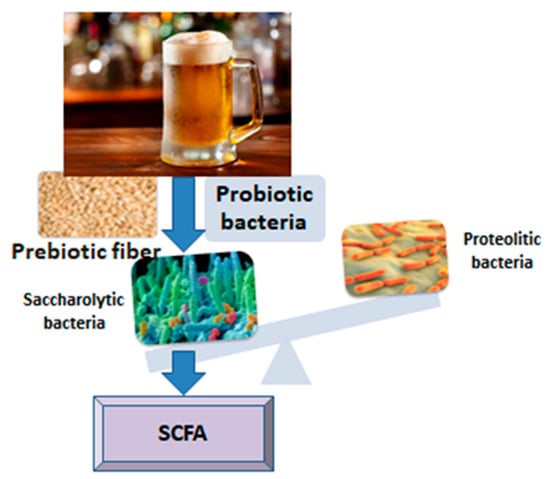
Figure 1.
From beer to short chain fatty acids (SCFA).
2. Methodology
Major databases (PubMed, Scopus, Web of Science, Google Scholar) were searched for items followed in this review and results are presented in Table 2.

Table 2.
Summary of the cited papers according to their reference number.
3. Beer and its Principal Interactions with the Microbiota
3.1. The Microorganisms from Beer and Their Probiotic Potential
Beer is usually a pasteurized product, but there are crafted beers that have a potential of influencing the gut microbiota because they contain bacteria. Studies of the effect of beer consumption on the gut microbiota are few. A study from 2019 detected by sequencing of 16S rDNA eighteen genera of bacteria present in the rice beer, Lactobacillus being the dominant group (90%). Other types were Acetobacter, Acinetobacter, Bacillus, Dickeya, Enterococcus, Enterobacter, Exiguobacterium, Gluconobacter, Janibacteria, Klebsiella, Lactococcus, Leuconostoc, Pseudomonas, Pediococcus, Rothia, Staphylococcus and Weissella. Based on the detected bacteria and their bacterial profiles metabolic pathways were revealed as being influenced by the consumption of the rice beer, such as metabolisms of carbohydrate, amino acid, vitamins and cofactors, as well as xenobiotic biodegradation [89].
The main effect of alcohol intake on gut microbiota is dysbiosis [13,31], by changing the balance of the dominant bacterial from Phyla Bacteroidetes, Firmicutes and Phylum proteobacteria. However, this is not the case with usual beer, when consumed with moderation, because it contains about 5% alcohol. Low-alcohol and alcohol-free beers are popular and widely consumed. So, when considering beer, it is important to choose a type of beer with low or without alcohol, which gives the benefits of fermented foods.
Beer enriched with Saccharomyces cerevisiae strain intake may modulate gut microbiota and have beneficial influence the symptoms in Alzheimer’s disease, generating a neuroprotective effect by ameliorating cognition and increasing the concentration of anti-inflammatory cytokines, as new data revealed in 2022 [9]. Another type of beer rich in bacterial composition is Belgian lambic beer. The different bacteria present throughout the production process result because of the spontaneous inoculation of microorganisms from the environmental air and the inner surfaces of the wooden barrels [86]. Several new bacterial species such as Acetobacter lambici and Gluconobacter cerevisiae have been described in lambic beer. In the process of production of this type of beer, the following bacteria are present: Enterobacteria (Enterobacter cloacae; Klebsiella oxitoca), acetic acid bacteria (Acetobacter spp.; Gluconobacter cerevisiae) and lactic acid bacteria (Pediacoccus spp.), along with different yeasts (Hanseniaspora uvarum; Saccharomyces spp.; Brettanomyces spp.), with possible, not-yet-studied influence on gut microbiota. Crafted beers with several enhanced tastes, such as fruits, herbs, honey, spices and vegetables, have become more popular lately, but because they are not always pasteurized or sterilized by filtration they are subject to spoilage, due to the microbiota associated with the organic raw ingredients added to obtain the special tastes. Even if there is not enough knowledge available about the microbiota diversity in craft breweries it is known that some lactic acid bacteria from beer can produce biogenic amines such as histamine, tyrosine, putrescine and cadaverine, which can alter the beer and have possible toxic effects. Biogenic amines can also be found in sausages, fermented vegetables, fishery products, cheese and wine [32]. Published studies of sixty monitored points inside the craft brewery revealed that Lactobacillus, Pediococcus and Leuconostoc genera, are responsible for biogenic amines production, especially two isolates of Lactobacillus brevis that are able to be cultured into acidic conditions, with more hop and alcohol, these isolates had presented horA, horC and hitA genes, and the highest production of biogenic amines [90]. Corn beer has potential sources of probiotic lactic acid bacteria with cholesterol lowering activity, the strains identified by sequencing the 16S rRNA gene were Levilactobacillus brevis and Enterococcus faeccium, NCBI genbank accension numbers ON454506 and ON908682; isolates that effectively lowered LDL-c and increased HDL-c in rat sera, which are the main risk factors for cardiovascular diseases [84]. Beer is a fermented beverage that has enhanced nutritional and functional properties due to transformation of substrates, formation of bioactive end-products and presence of living microorganisms, genetically similar to strains used as probiotics [27]. Some studies revealed that beer components may have antimicrobial properties, as well as microbiological spoilage risks [91].
The microbial community (bacteria and fungi) from beer differs in time because it is influenced by its initial composition, the quantity of alcohol and the type of barrel where it is kept. Studies that used amplicon sequencing of the V4 region of the bacterial 16S rRNA gene and the fungal ITS1 region have shown using PerMANOVA analysis that during the process of beer maturation significant higher levels in the bacterial and fungal population appeared. The lactic acid bacteria became dominant in the moderately hopped beers and remained fairly constant in high-bitterness beer; with similar composition of the traditional beers, Pediococcus damnosus, Lactobacillus brevis and Acetobacter spp., and the fungi were influenced by the presence of alcohol [85]. There are even studies that introduced the idea of non-Saccharomyces yeast beers, and these types of beers may enter on the market in the future, after investigations and guideline for the safety assessment of yeasts are carried out [92].
So, bacteria and fungi encountered in the beer fabrication process, enriched or crafted beer, produce an array of compounds such as vitamins, bacteriocins and organic acids which confer health benefits to the consumers and can modulate the indigenous intestinal flora of the host.
3.2. Polyphenols and Microbiota
Beer is an important vehicle for polyphenols, which, together with bitter acids, form beer’s antioxidants. Most of them come from malt and only about 20% from hops. There are in vitro studies that confirm the action of polyphenols on the microbiota [93] (Figure 2). Animal studies support this interaction. For example, a polyphenol well represented in beer, ferulic acid, amplifies the biodiversity of the microbiota and stimulates the multiplication of bacteria that produce propionate and butyrate in the colon of rats [36]. Although limited in number, studies on human subjects also confirm the interaction under discussion. Studies show that after the ingestion of polyphenols, the production of short chain fatty acids (SCFA) increases, with consequent local anti-inflammatory effects [37]. The increase in SCFA synthesis confirms the action on the microbiota, an effect already demonstrated in the case of red wine consumption, which is itself a source of polyphenols. Red wine increased the levels of Bifidobacterium in the microbiota, as well as Faecalibacterium prausnitzii and Roeburia. The development of Enterobacter or Escherichia coli strains was inhibited [38]. These positive effects were also observed for alcohol-free wine. So, although the absorption of polyphenols from alcohol-free products (beer, wine) decreases compared to the same products with alcohol [4,94], polyphenols not absorbed that reach the colon have important effects in situ with multiple repercussions through the action on the intestinal microbiota. It is about oligo and polymeric polyphenols that usually do not undergo transformations until the distal intestine [37]. Polyphenols are “activated” by certain populations of the microbiota, especially when it comes to phytoestrogens [39,40,95]. Polyphenols are transformed by bacteria into absorbable products that reach through the portal blood, to the liver or into prenylated products that have important sanogenic actions, such as the antiproliferative action on some cell lines, as prenyl naringenin and xanthohumol have [41]. The interrelation between polyphenols and microbiota is extremely complex. The microbiota increases the bioavailability of polyphenols which, in turn, modulate the composition of the populations in the colon, inhibiting pathogenic microorganisms and stimulating the development of healthy ones through a prebiotic action [42,43]. Quercitin, a flavonoid from beer, combated intestinal dysbiosis, improving the ratio between Firmicutes and Bacteroides populations and opposing the proliferation of microbiota species associated with excessive body weight [48,49]. It should be noted that by drinking non-alcoholic beer or wine, you not only avoid the negative effects determined by ethyl alcohol, including on the microbiota, but also increase the number of polyphenols that reach the intestine and which would have positive effects on the microbiota.
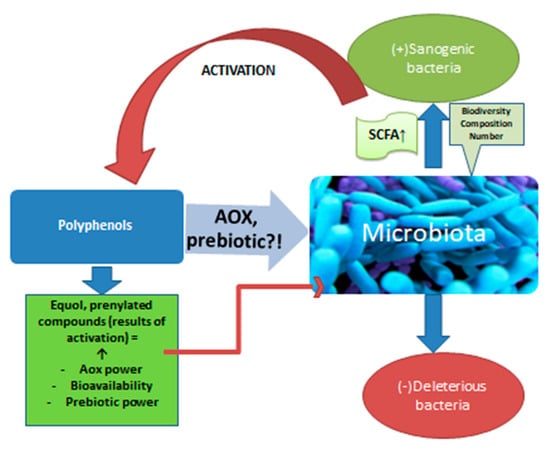
Figure 2.
The action of polyphenols on the microbiota.
In a study conducted by Hernández-Quiroz et al. [46], after the administration of a dose of 355 mL of beer per day for 30 days in healthy subjects, divided into a group that received beer without alcohol (n = 35) and one that received beer with alcohol (n = 33), a clear influence on the intestinal microbiota, as well as on the functionality of pancreatic β cells and fasting blood glucose, could be observed. The action on the microbiota consisted of increasing the diversity of the microbiota, by favoring species of the Bacteroides type, at the expense of Firmicutes. The authors attribute this effect to the polyphenols in beer, and it was found in beer without alcohol. Beer with alcohol did not have positive effects of the same scope and negatively impacted blood sugar and β cell functionality.
Another observational study [47] found an increase in butyric acid, a byproduct of the intestinal microbiota, in beer consumers, but without quantifying the intake of polyphenols. Positive effects were found only with wine in a large study on twins in Great Britain, in which non-alcoholic beer was not an element of investigation [96]. A recent clinical study by Martínez-Montoro et al. [97] worked on adults aged 30–60, divided into two groups (with or without metabolic syndrome) who were administered beer with different concentrations of polyphenols, consumed successively, after respective washout periods. In the beginning there were no radical differences in microbiota characteristics between the two groups. During the study, the authors report significant changes in the microbiota, with substantial changes in the entire profile, all the more important as the polyphenol content of the beer was higher. The changes were also influenced by the metabolic status of the individuals, being significant in the group of subjects with metabolic syndrome, where the abundance of streptococcus was highest after consumption of dark beer. A previous study showed that certain species of streptococci interact with gallic acid and catechins, amplifying their antioxidant effects [48]. Moreover, some streptococci can transform beer melanoidins into an isoflavone with estrogenic and antioxidant action called equol [49]. The authors attribute the effects found in the group with metabolic syndrome to the correction of intestinal dysbiosis, which is usually present in individuals with the syndrome in question. Since the most important changes were found after the consumption of dark beer, very rich in antioxidant polyphenols, the authors explain the influence also through the respective antioxidant action on the microbiota, excluding the possible interference of alcohol, which was found in equal quantities in lager beer (with fewer polyphenols) and in the black one (with maximum level of polyphenols).
There are still open study perspectives in which to possibly follow the impact of some types of beer enriched in polyphenols, with or without alcohol, on the intestinal microbiota and from here, on the entire metabolism [4]. An extensive review of the effects of beer polyphenols on the microbiota was carried out by Quesada-Molina et al. [50]. The authors analyze the existing studies, noting that in principle a detailed analysis of the microbiota-polyphenols interaction is needed and that the existing results so far are based on deduction rather than on concrete quantification of the effects.
3.3. Dietary Fibers in Beer and Gut Microbiota
In the tables of data on the composition of foods, beer is not mentioned as containing dietary fiber. In reality, beer contains a series of non-starchy, non-digestible carbohydrates, such as β-glucans (approximately 75%), arabinoxylans (approximately 20%), arabinogalactans and their fragments, mannose and fructose polymers as well as resistant starch [50,54,55]. Detailed tests performed on various types of beer showed the existence of arabinoxylan derivatives with a medium to high degree of polymerization and β-glucans with a significantly lower degree of polymerization. Numerous small oligosaccharides of β-glucosyl or pentosyl type (arabinoxylans with reduced polymerization) have also been identified as being present in a rather high quantity [56].
Scientists are working to develop analytical methods to quantify these compounds [57]. In this context, it is discussed to what extent the presence of soluble fiber in beer has or does not have an impact on the health of consumers through the intestinal microbiota [50]. Trials undertaken to date place the level of dietary fiber in beer in the range of 0.5–4 g/L [54], varying depending on the technological solutions applied and the assortment, more precisely, depending on the content in the wort extract [58]. The respective quantities are small compared to the quantity required to be able to use a nutritional claim on beer labels. Reid et al. [59], citing Li and Du [56], approximates the amounts of non-starchy carbohydrates as 0–1.5 g L−1 in blonde beers such as lager, 1.0–2.0 g L−1 in brown beers, the highest amount being in wheat beers (1.5–4.0 g L−1).
Recent studies argue that beer, including alcohol-free or low-alcohol beer, could contribute substantially to the intake of soluble fibers [56,57,59].
The connection of dietary fibers with the intestinal microbiota is very close and is related to the fermentability of the fibers. It is about their characteristic of serving as a nutrient substrate for the bacteria of the intestinal microbiota. Many of the dietary fibers have prebiotic action. Prebiotics are defined as “dietary ingredients selectively metabolized by certain phylogenetic groups of the intestinal microbiota, which cause specific changes—both composition and activity—of the human microbiome, conferring physiological and health benefits” [60]. The compounds considered to have prebiotic activity are of a carbohydrate nature, and, most often, they are from the category of inulins, fructo-oligosaccharides, galacto-oligosaccharides, soy-oligosaccharides, xylo-oligosaccharides, pyrodextrins, isomalto-oligosaccharides or lactulose [61]. Not every type of fiber is a prebiotic. In order to have an effect, prebiotics must keep their structure unmodified under the action of various factors that act along the digestive tract up to the level of the colon. It should be noted that because they are not altered by the enzymes of the human digestive system, prebiotics are considered soluble dietary fibers [62], but there are also dietary fibers without prebiotic action or prebiotic substances that are not fibers according to their classical definition. An indicator often used to monitor the effect on the microbiota is the production of SCFA through the fermentation of prebiotics, which are a source of energy for intestinal anaerobes and have various sanogenetic effects, from increasing the absorption of nutrients, to balancing glucose metabolism, stimulating immunity, influencing lipid metabolism, etc.
As stated about, beer contains some fiber; however, research has usually targeted effects of fiber found in beer, not beer by itself. For several years, an increased interest has been aroused by the prebiotic effect of the hydrolysis products of prebiotic fiber, i.e., arabinoxylo-oligosaccharides(AXOS), xylo-oligosaccharides (XOS) and β-glucano-oligosaccharides (βGOS). There are already available on the market ingredients with a high content of AXOS, XOS or βGOS, intended for the enrichment/fortification of bakery products or other cereal products, even beer, but only for the purpose of changing the palatability [33]. Generally, β-glucans from barley are already hydrolyzed during malting and mashing, in the process of beer fabrication, resulting in low molecular weight oligosaccharides (βGOS) that cannot be digested by S. cerevisiae. An in vitro study tested the ability of βGOS as a prebiotic [50] and showed that the substance can survive in conditions of pH and enzymatic stress similar with those in a human digestive tract. Different strains of Lactobacillus were able to survive by only using b GOS as source of carbon. Authors calculate that with a daily intake of 0.33 l of beer providing 4 mg/mL β-GOS [56], the quantity reaching the colon would be 1.34 g/L, which is far below the quantities used in the study. Other in vitro tests using colon models indicated a considerable prebiotic potential of oligosaccharides derived from β-glucans and pentosans from barley, wheat and oats. A study designed to compare the prebiotic activity of hydrolyzed β-glucan derivatives reveals a significant stimulating effect on the populations of the Lactobacillus-Enterococcus group for βGOS from barley with 3–4 monomer units, in contrast to non-hydrolyzed β-glucan fibers, which marginally favored the development these bacteria, but less compared to inulin [63]. XOS from barley stimulated the accumulation of short-chain fatty acids—especially butyrate—in the vessels of the colon model and demonstrated a greater ability to stimulate the growth of species of the Bifidobacterium lactis group compared to FOS and inulin, favoring B. longum [64]. Perhaps more than in the case of other fibers, the action of β-glucans on the microbiota is well described. Their origin in beer can be different, most of them coming from the cereal raw material and a small part from the walls of the yeast cells, the two origins having different structural aspects [65]. In a review of Jayachandran et al. [34], in vitro, in vivo and clinical studies confirming the prebiotic action of these fibers are presented. In particular, β-glucans from barley and oats have been proven to reduce the levels of LDL and total cholesterol by modulating the microbial populations in the microbiota. Gut microbiota undergoes a shift in the direction of saccharolytic metabolism, producing SCFA, with the consequent decrease in protein metabolism products, such as p-cresyl sulfate (pCS) and indoxyl sulfate [66]. Β-glucans from oats have proven a greater power in stimulating the development of Lactobacilli and Bifidobacterium populations, compared to those from barley [67]. In a clinical study, 52 healthy subjects taking low doses of β-glucans from barley led to a significant increase in the number of Bifidobacterium [73,74]. Of course, the amount of β-glucans in beer is not large, most of it being degraded to glucose during the technological production processes. Moreover, the presence of β-glucans in barley generates technological problems. However, the remaining small amounts have the potential, along with other prebiotic elements in beer, to contribute to the modulation of the microbiota in a sanogenetic sense.
Arabinoxylans are another major component of beer’s fiber. Lynch et al. [69] used an extract of arabinoxylans from brewer’s spent grain to see the possible effects on the microbiota. Probiotic effects were obtained, consisting in the multiplication of lactobacilli populations by 2 times and bifidobacterial populations by 3.5 times. The same raw material, rich in arabinoxylans and β-glucans, was administered as a feed supplement to ruminants, causing the growth the activity of Bifidobacterium, Enterococcus and Lactobacillus [29]. In a study on human subjects, the effect of consuming bread enriched with oligosaccharides of arabinoxylans was followed [29]. Butyrate-producing colon bacteria and Bifidobacterium faecalis levels increased. In an in vitro study, arabinoxylans from maize species with various genotypes increased SCFA production, but in a genotype-dependent manner [70]. After drinking beer, an increase in the number of bacteria from the Lachnospira genus was found, which are usually SCFA producers with consecutive sanogenetic effects [30]. Different researchers emphasize the fact that the action on the microbiota is closely related to the degree of aggregation and branching of the arabinoxylans [71], their structure being very different depending on the source, its state of germination and the extraction methods.
It is difficult to generalize the effects on the microbiota because in vitro studies are not always reproduced in vivo, and most in vivo studies are based on supplements or products “designed” in the laboratory. In the real world, the action of arabinoxylans can be modified by the food matrix that they themselves influence, as in the case of beer. In vivo studies are required, using food products, possibly fortified, as long as the fortification does not make the product unacceptable from an organoleptic point of view [72]. Another element of variability is the subjects’ own microbiota that may or may not ferment such complex polysaccharides, some researchers finding very different effects depending on the geographic area from which the subjects came [73]. There are species from the microbiota genetically adapted (Bacteroides) to the digestion of complex arabinoxylans [98]. Moreover, when the excretion of SCFA is taken into account as an indicator of the in vivo action on the microbiota, it can be neglected that part of the SCFA has already been absorbed by the intestinal epithelium and thus the action of arabinoxylans can appear as negligible [99,100]. The fiber–microbiota relationship is bidirectional and both actors must be able to interact, so that possible positive effects can be highlighted.
From the multitude of contradictory results, a clear conclusion is that arabinoxylans and other soluble fibers present in beer are likely to positively influence the intestinal microbiota of consumers. Since arabinoxylans can bring organoleptic benefits to beer (increasing viscosity, foam stability), especially important in alcohol-free beers, it is assumed that an addition of arabinoxylans could be used intentionally, with collateral sanogenetic benefits [51,55]. However, producers must take into consideration the fact that consumers have a particular reluctance regarding the addition of additives and technological adjuvants in food, and beer is generally considered to be a drink based strictly on natural raw materials [74,101].
3.4. Melanoidins in Beer and Gut Microbiota
A type of substances that are often considered close to fiber because they have an extremely low digestibility [52] are the melanoidins. Their presence in beer originates from malted barley, they are formed in Maillard-type reactions. The structure of melanoidins in malt is very complex and is influenced by both the raw material, and the environmental conditions during the forming reaction [75,76]. Beer is dominated by a type of melanoidins called melanosaccharides, which are water-soluble substances [53]. Ingestion of melanoidins is variable in different populations, and beer contributes with relatively low levels compared to other foods [53]. Melanoidins in beer are valued especially for their organoleptic role, being involved in the color, texture and aroma of beer [28]. The amount of melanoidins in beer varies between 0.06 and 10.3 g/100 mL [77]. Rivero et al. [78] found, obviously, the highest amounts of melanoidin in dark beer, followed by blonde beer and Zhao et al. [80] described very large differences depending on the raw material and manufacturing methods. Alcohol-free beer had the lowest amounts (0.58 g/L). In recent years, however, numerous studies have focused on highlighting the influence of melanoidins on health, especially on their antioxidant, reducing capacity. Melanoidins in beer too have antioxidant roles [78,102]. However, because melanoidins reach the intestine largely undigested, it is assumed that they can also have a prebiotic role [80], being fermented by the intestinal microbiota [81]. The studies carried out in vitro and in vivo showed that the products of the Maillard reactions are decomposed by the intestinal flora, but so far there are no clear data on melanoidins as such. However, some studies argue that they have been shown to influence the intestinal flora, stimulating the growth of some local populations, fact that prove that bacteria can use the fermentation of melanoidins as a source of energy [103]. Other experiments support the fiber-like effect of melanoidins, but only after a long period of their administration, probably because the intestinal flora needs time to adapt to the digestion of substances with such a complex structure [104,105]. Moreover, the antioxidant influence of melanoidins on the digestive tract, demonstrated in various studies, could also be achieved through mechanisms mediated by the intestinal microbiota [106]. Although the studies on melanoidins as prebiotic fibers are few, they are not missing, and support the interaction with the gut microbiota. Aljahdali et al. administered to rats either malt without melanoidins (control group), or malt with increasing concentrations of melanoidins, for 3 weeks. The effects on the microbiota were important and differentiated, depending on the microbial species. The influence on SCFA production was also measured [107]. The populations of Firmicutes (Dorea, Oscillibacter and Alisitpes) decreased, but the first to respond were the populations of Bacteroidetes, then those of Lactobacilli, Verrucomicrobia Acinobacteria and Proteobacteria (Parasutterella), which increased. Two beneficial species, Bifidobacterium and Akkermansia, responded significantly only towards the end of the 3-week experiment, which supports the hypothesis of the need to adapt to melanoidins as an energy source. Stimulation of Bifidobacterium has been demonstrated in other studies, in which melanoidins from other food sources were used [79].
It should be noted that melanoidins also have an antibacterial role [82], being able to influence the microbiota in this way as well. They inhibit the development of pathogenic species, such as Salmonella typhimurium, Staphylococcus aureus, Escherichia coli, Bacillus cereus and Pseudomonas aeruginosa [83,108,109,110]. Melanoidins inhibited the ability of Streptococcus mutans to form adherent biofilms [107]. Of course, the intake of melanoidins through beer is not as high as the intake from other foods. Beer contribution is extremely variable, depending on the differences in beer consumption from one country to another and depending on the type of beer mostly consumed. However, the potential for interaction with the microbiota should not be neglected, and this process is still waiting to be defined more precisely through future studies.
4. Alcohol in Beer and Gut Microbiota
The content of alcohol is variable, and beer has the potential to be produced with minimal or no alcohol at all. However, widely consumed types of beer have generally around 5% alcohol. There are studies that discuss the link between alcohol and the gut–brain axis and the associated disorders such as alcoholic hepatitis, liver cirrhosis, anxiety, depression and impaired cognition performance that are a major cause of personal death and disability worldwide [111]. It is proven that the higher the percentage of alcohol in beer, the bigger the harmful impact of beer on gut microbiota and overall health. Nowadays, no level of alcohol intake can be considered safe. Alcohol modifies the gut microbiota composition and contribute to alcohol-induced oxidative stress, intestinal hyperpermeability to luminal bacterial products, and the subsequent development of alcoholic liver disease [112]. Alcohol is a factor that alters the normal function of the gut, it destroys the permeability of the intestinal membrane and by this it allows bacteria to enter the blood stream. A high constant alcohol intake has harmful effects on gut microbiota and the immune system [87,88,113,114,115]. Bacterial products that get through the non-intact intestinal barrier cause central inflammation; modify gut microbiota and impair enterohepatic circulation of bile acids; alcohol abuse causes shortage of vital nutrients such as thiamine [111]. The increased gut inflammation and intestinal hyperpermeability also induces endotoxemia, systemic inflammation and tissue damage/organ pathologies [112]. These arguments lead to the conclusion that only a minimal level of alcohol can be tolerated in functional beers and that the best alternative will be to fortify an alcohol-free beer.
5. Conclusions
The conclusions of this review are that beers (especially low or alcohol-free types) seem good candidates for future functional products and that there are many paths in which beer’s ingredients can influence in a positive way the human microbiota.
Combined actions of pre and probiotic factors can stimulate the proliferation or shift of the bacteria population towards a glycolytic one, normalizing its profile. Adding fiber, antioxidants and/or probiotics to beer are active ways to boosts its sanogenic properties but technological effects have to be balanced in such a way that fortification does not lower the acceptability of the product by consumers. Moreover, designing functional beers with a low or no alcoholic content will counteract the negative perception of beer consumption and will nullify the negative effects of alcohol. Even beer as it is, without any fortification, contributes at the intake of microbiota stimulating ingredients, that add to the sum of factors ingested by humans form a wholesome diet. However, we should aways take into consideration that no amount of alcohol is considered safe for health, and research has to target the development of functional beers without alcohol, that can be consumed at all ages and at of all groups of peoples. There is much research to be carried out in future but what it is known in this area up to now is encouraging for further positive results.
Author Contributions
Conceptualization, C.-A.Z.; methodology, C.M.; investigation, L.S.C.M.; resources, C.-A.Z.; data curation, L.S.C.M.; writing—original draft preparation, C.-A.Z.; writing—review and editing, L.S.C.M. and C.C.; visualization, L.S.C.M. and C.C.; supervision, C.M. All authors have read and agreed to the published version of the manuscript.
Funding
Publication fee of the article was partially funded by the Center for Beer, Health and Nutrition from Romania.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosso, A.M. Beer and wine in antiquity: Beneficial remedy or punishment imposed by the Gods? Acta Med. Hist. Adriat. 2012, 10, 237–262. [Google Scholar] [PubMed]
- Bamforth, C.W. Nutritional aspects of beer—A review. Nutr. Res. 2022, 22, 227–237. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, L.; Sun, H. Early evidence for beer drinking in a 9000-year-old platform mound in southern China. PLoS ONE 2021, 16, e0255833. [Google Scholar] [CrossRef] [PubMed]
- Ambra, R.; Pastor, G.; Lucchetti, S. The Role of Bioactive Phenolic Compounds on the Impact of Beer on Health. Molecules 2021, 26, 486. [Google Scholar] [CrossRef] [PubMed]
- Zugravu, C.A.; Bohiltea, R.E.; Salmen, T.; Pogurschi, E.; Otelea, M.R. Antioxidants in Hops: Bioavailability, Health Effects and Perspectives for New Products. Antioxidants 2022, 11, 241. [Google Scholar] [CrossRef] [PubMed]
- Sohrabvandi, S.; Mortazavian, A.M.; Rezaei, K. Health-Related Aspects of Beer: A Review. Int. J. Food Prop. 2012, 15, 350–373. [Google Scholar] [CrossRef]
- Díaz-Rubio, E.; Saura-Calixto, F. Dietary Fiber Complex in Beer. J. Am. Soc. Brew. Chem. 2009, 67, 38–43. [Google Scholar] [CrossRef]
- Radu, M.C.; Boeru, C.; Marin, M.; Manolescu, L.S.C. SARS-CoV-2 Infection in Seven Childbearing Women at the Moment of Delivery, a Romanian Experience. Cureus 2021, 13, e12811. [Google Scholar] [CrossRef]
- Cecarini, V.; Gogoi, O.; Bonfili, L.; Veneruso, I.; Pacinelli, G.; De Carlo, S.; Benvenuti, F.; D’Argenio, V.; Angeletti, M.; Cannella, N.; et al. Modulation of Gut Microbiota and Neuroprotective Effect of a Yeast-Enriched Beer. Nutrients 2022, 14, 2380. [Google Scholar] [CrossRef]
- De Roos, J.; Van der Veken, D.; De Vuyst, L. The Interior Surfaces of Wooden Barrels Are an Additional Microbial Inoculation Source for Lambic Beer Production. Appl. Environ. Microbiol. 2018, 85, e02226-18. [Google Scholar] [CrossRef]
- Marcos, A.; Serra-Majem, L.; Pérez-Jiménez, F.; Pascual, V.; Tinahones, F.J.; Estruch, R. Moderate Consumption of Beer and Its Effects on Cardiovascular and Metabolic Health: An Updated Review of Recent Scientific Evidence. Nutrients 2021, 13, 879. [Google Scholar] [CrossRef]
- Tucker, K.L.; Jugdaohsing, R.; Powell, J.J.; Qiao, N.; Hannan, M.T.; Sripanyakorn, S.; Cupples, L.A.; Kie, D.P. Effects of beer, wine, and liquor intakes on bone mineral density in older men and women. Am. J. Clin. Nutr. 2009, 89, 1188–1196. [Google Scholar] [CrossRef]
- Redond, N.; Nova, E.; Díaz-Prieto, L.E.; Marco, A. Effects of moderate beer consumption on health. Nutr. Hosp. 2018, 35, 41–44. [Google Scholar] [CrossRef]
- Peng, M.Z.; Tabashsum, M.; Anderson, A.; Truong, A.K.; Houser, J.; Padilla, A.; Akmel, J.; Bhatti, S.O.; Rahaman, O.; Biswas, D. Effectiveness of probiotics, prebiotics, and prebiotic-like components in common functional foods. Compr. Rev. Food Sci. 2020, 19, 1908–1933. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Thomas, C.; Minty, M.; Vinel, A.; Canceill, T.; Loubières, P.; Burcelin, R.; Kaddech, M.; Blasco-Baque, V.; Laurencin-Dalicieux, S. Oral Microbiota: A Major Player in the Diagnosis of Systemic Diseases. Diagnostics 2021, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Costello, E.K.; Lauber, C.L.; Hamady, M.; Fierer, N.; Gordon, J.I.; Knight, R. Bacterial community variation in human body habitats across space and time. Science 2009, 326, 1694–1697. [Google Scholar] [CrossRef]
- Hillman, E.T.; Lu, H.; Yao, T.; Nakatsu, C.H. Microbial Ecology along the Gastrointestinal Tract. Microbes Environ. 2017, 32, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Auchtung, T.A.; Fofanova, T.Y.; Stewart, C.J.; Nash, A.K.; Wong, M.C.; Gesell, J.R.; Petrosino, J.F. Investigating colonization of the healthy adult gastrointestinal tract by fungi. mSphere 2018, 3, e00092-18. [Google Scholar] [CrossRef] [PubMed]
- Man, W.H.; de Steenhuijsen Piters, W.A.; Bogaert, D. The microbiota of the respiratory tract: Gatekeeper to respiratory health. Nat. Rev. Microbiol. 2017, 15, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Moissl-Eichinger, C.; Pausan, M.; Taffner, J.; Berg, G.; Bang, C.; Schmitz, R.A. Archaea Are Interactive Components of Complex Microbiomes. Trends Microbiol. 2018, 26, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms. 2019, 7, 14. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Louis, P.; Duncan, S.H. The role of the gut microbiota in nutrition and health. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 577–589. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Gordon, J.I. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y.; Doré, J.; Schiffrin, E.J. The inflammatory status of old age can be nurtured from the intestinal environment. Curr. Opin. Clin. Nutr. Metab. Care. 2008, 11, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Fossi, B.T.; Ekabe, D.E.; Toukam, L.L.; Tatsilong Pambou, H.O.; Gagneux-Brunon, A.; Nguefeu, C.N.; Bongue, B. Probiotic lactic acid bacteria isolated from traditional cameroonian palm wine and corn beer exhibiting cholesterol lowering activity. Heliyon 2022, 8, e11708. [Google Scholar] [CrossRef]
- Fay, L.B.; Brevard, H. Contribution of mass spectrometry to the study of the Maillard reaction in food. Mass Spectrom. Rev. 2005, 24, 487–507. [Google Scholar] [CrossRef]
- La, E.J.; Dimoso, N.; Raymond, J.; Mbega, E.R. The prebiotic potential of brewers’ spent grain on livestock’s health: A review. Trop. Anim. Health Prod. 2020, 52, 461–472. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Chen, T.; Ma, D.; Yao, T.; Gu, F.; Lim, J.; Tuinstra, M.R.; Hamaker, B.R. High arabinoxylan fine structurespecificity to gut bacteria driven by corn genotypes but not environment. Carbohydr. Polym. 2021, 257, 117667. [Google Scholar] [CrossRef]
- Das, S.; Deb, D.; Adak, A.; Khan, M.R. Exploring the microbiota and metabolites of traditional rice beer varieties of Assam and their functionalities. 3 Biotech 2019, 9, 174. [Google Scholar] [CrossRef] [PubMed]
- Bongaerts, D.; De Roos, J.; De Vuyst, L. Technological and environmental features determine the uniqueness of the lambic beer microbiota and production process. Appl. Environ. Microbiol. 2021, 87, e00612-21. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Courtin, C.M.; Verbeke, K.; Van de Wiele, T.; Verstraete, W.; Delcour, J.A. Prebiotic and other health-related effects of cereal-derived arabinoxylans, arabinoxylanoligosaccharides, and xylooligosaccharides. Crit. Rev. Food Sci. Nutr. 2011, 51, 178–194. [Google Scholar] [CrossRef] [PubMed]
- Speers, Y.-L.; Paulson, R.A.; Stewart, R.J. Barley beta-glucans and their degradation during malting and brewing. Tech. Q.-Master Brew. Assoc. Am. 2004, 41, 231–240. [Google Scholar]
- De Angelis, M.; Montemurno, E.; Vannini, L.; Cosola, C.; Cavallo, N.; Gozzi, G.; Gesualdo, L. Effect of whole-grain barley on the human fecal microbiota and metabolome. Appl. Environ. Microbiol. 2015, 81, 7945–7956. [Google Scholar] [CrossRef] [PubMed]
- Takagak, A.; Nanjo, F. Bioconversion of (−)-Epicatechin, (+)-Epicatechin, (−)-Catechin, and (+)-Catechin by (−)-Epigallocatechin-Metabolizing Bacteria. Biol. Pharm. Bull. 2015, 38, 789–794. [Google Scholar] [CrossRef]
- Ou, J.; Huang, J.; Song, Y.; Yao, S.; Pen, X.; Wang, M.; Ou, S. Feruloylated Oligosaccharides from Maize Bran Modulated the Gut Microbiota in Rats. Plant Foods Hum. Nutr. 2016, 71, 123–128. [Google Scholar] [CrossRef]
- Cardona, F.; Andrés-Lacueva, C.; Tulipani, S.; Tinahones, F.J.; Queipo-Ortuño, M.I. Benefits of polyphenols on gut microbiota and implications in human health. J. Nutr. Biochem. 2013, 24, 1415–1422. [Google Scholar] [CrossRef]
- Ghiselli, A.; Natella, F.; Guidi, A.; Montanar, L.; Fantozzi, P.; Scaccini, C. Beer increases plasma antioxidant capacity in humans. J. Nutr. Biochem. 2000, 11, 76–80. [Google Scholar] [CrossRef]
- Possemiers, S.; Heyerick, A.; Robbens, V.; De Keukeleire, D.; Verstraete, W. Activation of Proestrogens from Hops (Humulus lupulus L.) by Intestinal Microbiota; Conversion of Isoxanthohumol into 8-Prenylnaringenin. J. Agric. Food Chem. 2005, 53, 6281–6628. [Google Scholar] [CrossRef]
- Marín, L.; Miguélez, E.M.; Villar, C.J.; Lombó, F. Bioavailability of Dietary Polyphenols and Gut Microbiota Metabolism: Antimicrobial Properties. BioMed Res. Int. 2015, 15, 905215. [Google Scholar] [CrossRef] [PubMed]
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly Cancer Selective Antiproliferative Activity of Natural Prenylated Flavonoids. Molecules 2018, 23, 2922. [Google Scholar] [CrossRef] [PubMed]
- Ozdal, T.; Sela, D.A.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 2, 78. [Google Scholar] [CrossRef]
- Hui, C.L.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar]
- Tomás-Barberán, F.A.; Selma, M.V.; Espín, J.C. Interactions of gut microbiota with dietary polyphenols and consequences to human health. Curr. Opin. Clin. Nutr. Metab. Care. 2016, 19, 471–476. [Google Scholar] [CrossRef]
- Hernández-Quiroz, F.; Nirmalkar, K.; Villalobos-Flores, L.E.; Murugesan, S.; Cruz-Narváez, Y.; Rico-Arzate, E.; Hoyo-Vadillo, C.; Chavez-Carbajal, A.; Pizano-Zárate, M.L.; García-Mena, J. Influence of moderate beer consumption on human gut microbiota and its impact on fasting glucose and β-cell function. Alcohol 2020, 85, 77–94. [Google Scholar] [CrossRef]
- González-Zancada, N.; Redondo-Useros, N.; Díaz, L.E.; Gómez-Martínez, S.; Marcos, A.; Nova, E. Association of Moderate Beer Consumption with the Gut Microbiota and SCFA of Healthy Adults. Molecules 2020, 25, 4772. [Google Scholar] [CrossRef]
- Khalil, R.K.S. Influence of gallic acid and catechin polyphenols on probiotic properties of Streptococcus thermophilus CHCC 3534 strain. World J. Microbiol. Biotechnol. 2010, 26, 2069–2079. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Rajakaruna, S.; Pastoriza, S.; Paliy, O.; Ángel Rufián-Henares, J. Bioactivity of food melanoidins is mediated by gut microbiota. Food Chem. 2020, 316, 126309. [Google Scholar] [CrossRef]
- Quesada-Molina, M.; Muñoz-Garach, A.; Tinahones, F.J.; Moreno-Indias, I. A New Perspective on the Health Benefits of Moderate Beer Consumption: Involvement of the Gut Microbiota. Metabolites 2019, 9, 272. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Deehan, E.C.; Zhang, Z.; Jin, M.; Baskota, N.; Perez-Muñoz, M.E.; Cole, J.; Tuncil, Y.E.; Seethaler, B.; Wang, T.; et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 2020, 8, 118. [Google Scholar] [CrossRef]
- Zugravu, C.A.; Pogurschi, E.N.; Pătrașcu, D.; Iacob, P.D.; Nicolae, C. Attitudes towards Food Additives: A Pilot Study. Ann. Univ. Dunarea Jos Galati Fascicle VI—Food Technol. 2017, 41, 50–61. [Google Scholar]
- Ekielski, A.; Mishra, P.K.; Zelazinski, T. Assessing the influence of roasting process parameters on mepiquat and chlormequat formation in dark barley malts. Food Bioprocess Technol. 2018, 11, 1177–1187. [Google Scholar] [CrossRef]
- Kanyer, A.J.; Bornhors, G.M.; Marco, M.L.; Bamfort, C.W. Is beer a source of prebiotics? J. Inst. Brew. 2017, 3, 361–365. [Google Scholar] [CrossRef]
- Li, M.; Du, M.J.; Zhang, K. Profiling of carbohydrates in commercial beers and their influence on beer quality. J. Sci. Food Agric. 2020, 100, 3062–3070. [Google Scholar] [CrossRef] [PubMed]
- Li, M.J.; Du, M.J.; Zheng, Y. Non-starch polysaccharides in wheat beers and barley malt beers: A comparative study. Foods 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Kanauchi, M.; Ishikura, W.; Bamforth, C.W. Beta-glucans and pentosans and their degradation products in commercial beers. J. Inst. Brew. 2011, 117, 120–124. [Google Scholar] [CrossRef]
- Goni, I.; Diaz-Rubio, M.E.; Saura-Calixt, F. Dietary Fiber in Beer: Content, Composition, Colonic Fermentability, and Contribution to the Diet, in Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier: London, UK, 2009. [Google Scholar]
- Reid, J.; Yakubov Gleb, E.; Lawrence, S.J. Non-starch polysaccharides in beer and brewing: A review of their occurrence and significance. Crit. Rev. Food Sci. Nutr. 2022; ahead of print. [Google Scholar] [CrossRef]
- Diaz-Rubio, M.E.; Saura-Calixto, F. Beverages have an appreciable contribution to the intake of soluble dietary fibre: A study in the Spanish diet. Int. J. Food Sci. Nutr. 2011, 62, 715–718. [Google Scholar] [CrossRef]
- Gibson, G.R.; Probert, H.M.; Van Lo, J.; Rastall, R.A.; Roberfroid, M. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef]
- Delcour, J.A.; Roua, X.; Courti, C.M.; Poutanen, K.; Ranieri, R. Technologies for enhanced exploitation of the health-promoting potential of cereals. Trends Food Sci. Technol. 2012, 14, 78–86. [Google Scholar] [CrossRef]
- Hughes, S.A.; Shewry, P.R.; Gibson, G.R.; McCleary, B.V.; Rastall, R.A. In vitro fermentation of oat and barley derived beta-glucans by human faecal microbiota. FEMS Microbiol. Ecol. 2008, 64, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Makelainen, H.; Forssten, S.; Saarinen, M.; Stowell, J.; Rautonen, N.; Ouwehand, A.C. Xylooligosaccharides enhance the growth of bifidobacteria and Bifidobacterium lactis in asimulated colon model. Benef. Microbes. 2010, 1, 81–91. [Google Scholar] [CrossRef]
- Jayachandran, M.; Chen, J.; Sum, S.; Chung, M.; Xu, B. A critical review on the impacts of β-glucans on gut microbiota and human health. J. Nutr. Biochem. 2018, 61, 101–110. [Google Scholar] [CrossRef]
- Cosola, C.; De Angelis, M.; Rocchetti, M.T.; Montemurno, E.; Maranzano, V.; Dalfin, G.; Gesualdo, L. beta-Glucans supplementation associates with reduction in P-cresyl sulfate levels and improved endothelial vascular reactivity in healthy individuals. PLoS ONE 2017, 12, e0169635. [Google Scholar] [CrossRef]
- Shen, X.J.; Rawls, J.F.; Randall, T.; Burcal, L.; Mpande, C.N.; Jenkins, N.; Keku, T.O. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes 2010, 1, 138–147. [Google Scholar] [CrossRef]
- Lynch, K.M.; Strain, C.R.; Johnson, C.; Patangia, D.; Stanton, C.; Koc, F.; Gil-Martinez, J.; O’riordan, P.; Sahin, A.W.; Ross, R.P.; et al. Extraction and characterisation of arabinoxylan from brewers spent grain and investigation of microbiome modulation potential. Eur. J. Nutr. 2021, 60, 4393–4411. [Google Scholar] [CrossRef] [PubMed]
- Walton, G.E.; Lu, C.; Trogh, I.; Arnaut, F.; Gibson, G.R. A randomised, double-blind, placebo controlled cross-over study to determine the gastrointestinal effects of consumption of arabinoxylan-oligosaccharides enriched bread in healthy volunteers. Nutr. J. 2012, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Fu, Y.; Liu, J.; Lin, S.; Zhang, Q.; Liu, Y.; Wu, D.; Lin, D.; Han, G.; et al. Structure, Antioxidant, and Hypoglycemic Activities of Arabinoxylans Extracted by Multiple Methods from Triticale. Antioxidants 2019, 8, 584. [Google Scholar] [CrossRef] [PubMed]
- Zannini, E.; Bravo Núñez, Á.; Sahin, A.W.; Arendt, E.K. Arabinoxylans as Functional Food Ingredients: A Review. Foods 2022, 11, 1026. [Google Scholar] [CrossRef]
- Li, J.; Du, J. Molecular Characterization of Arabinoxylan from Wheat Beer, Beer Foam and Defoamed Beer. Molecules 2019, 24, 1230. [Google Scholar] [CrossRef] [PubMed]
- Salmen, T.; Bohiltea, R.; Mihai, B.M.; Nedelescu, M.; Zugravu, C.A. Changes in Attitudes towards Food Environment in Romania. Ann. Univ. Dunarea Jos Galati Fascicle VI—Food Technol. 2021, 45, 168–179. [Google Scholar] [CrossRef]
- Pérez-Jiménez, J.; Díaz-Rubio, M.E.; Mesías, M.; Morales, F.J.; Saura-Calixto, F. Evidence for the formation of maillardized insoluble dietary fiber in bread: A specific kind of dietary fiber in thermally processed food. Food Res. Int. 2014, 55, 391–396. [Google Scholar] [CrossRef]
- Pastoriza, S.; Rufián-Henares, J.A. Contribution of melanoidins to the antioxidant capacity of the Spanish diet. Food Chem. 2014, 164, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Rivero, D.; Pérez-Magariño, S.; González-Sanjosé, M.L.; Valls-Belles, V.; Codoñer, P.; Muñiz, P. Inhibition of induced DNA oxidative damage by beers: Correlation with the content of polyphenols and melanoidins. J. Agric. Food Chem. 2005, 53, 3637–3642. [Google Scholar] [CrossRef] [PubMed]
- Tagliazucchi, D.; Bellesia, A. The gastro-intestinal tract as the major site of biological action of dietary melanoidins. Amino Acids 2015, 47, 1077–1089. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, H.; Sun, G.; Yang, B.; Zhao, M. Assessment of endogenous antioxidative compounds and antioxidant activities of lager beers. J. Sci. Food Agric. 2013, 93, 910–917. [Google Scholar] [CrossRef]
- Alves, G.; Xavier, P.; Limoeiro, R.; Perrone, D. Contribution of melanoidins from heat-processed foods to the phenolic compound intake and antioxidant capacity of the Brazilian diet. J. Food Sci. Technol. 2020, 57, 3119–3131. [Google Scholar] [CrossRef]
- Aljahdali, N.; Gadonna-Widehem, P.; Anton, P.M.; Carbonero, F. Gut microbiota modulation by dietary barley malt melanoidins. Nutrients 2020, 12, 241. [Google Scholar] [CrossRef]
- Rufián-Henares, J.A.; de la Cueva, S.P. Antimicrobial activity of coffee melanoidins. A study of their metal-chelating properties. J. Agric. Food Chem. 2009, 57, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Saavedra, M.; de Llano, D.G.; Moreno-Arribas, M.V. Beer spoilage lactic acid bacteria from craft brewery microbiota: Microbiological quality and food safety. Food Res. Int. 2020, 138 Pt A, 109762. [Google Scholar] [CrossRef]
- Kordialik-Bogacka, E. Biopreservation of beer: Potential and constraints. Biotechnol. Adv. 2022, 58, 107910. [Google Scholar] [CrossRef] [PubMed]
- Engen, P.A.; Green, S.J.; Voigt, R.M.; Forsyth, C.B.; Keshavarzian, A. The Gastrointestinal Microbiome: Alcohol Effects on the Composition of Intestinal Microbiota. Alcohol. Res. 2015, 37, 223–236. [Google Scholar] [PubMed]
- Wang, S.-C.; Chen, Y.-C.; Chen, S.-J.; Lee, C.-H.; Cheng, C.-M. Alcohol Addiction, Gut Microbiota, and Alcoholism Treatment: A Review. Int. J. Mol. Sci. 2020, 21, 6413. [Google Scholar] [CrossRef]
- Calleja-Conde, J.; Echeverry-Alzate, V.; Bühler, K.-M.; Durán-González, P.; Morales-García, J.Á.; Segovia-Rodríguez, L.; Rodríguez de Fonseca, F.; Giné, E.; López-Moreno, J.A. The Immune System through the Lens of Alcohol Intake and Gut Microbiota. Int. J. Mol. Sci. 2021, 22, 7485. [Google Scholar] [CrossRef]
- Dragomirescu, C.C.; Lixandru, B.E.; Coldea, I.L.; Palade, A.M.; Baltoiu, M.; Dinu, S.; Cristea, V.C.; Manolescu, L.; Popa, M.I. Comparative analysis of different phenotypic and molecular methods used for the taxonomic identification of Corynebacterium spp. isolated from clinical samples in Romania. Rom. Biotechnol. Lett. 2017, 22, 12926–12933. [Google Scholar]
- Ovalle-Marmolejo, X.Y.; Redondo-Solano, M.; Granados-Chinchilla, F.; Miranda-Castilleja, D.E.; Arvizu-Medrano, S.M. Effect of stress factors on the production of biogenic amines by lactic acid bacteria isolated from fermented Mexican foods (cheese and beer). Food Control 2023, 146, 109553. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.I.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Bossaert, S.; Winne, V.; Van Opstaele, F.; Buyse, J.; Verreth, C.; Herrera-Malaver, B.; Van Geel, M.; Verstrepen, K.J.; Crauwels, S.; De Rouck, G.; et al. Description of the temporal dynamics in microbial community composition and beer chemistry in sour beer production via barrel ageing of finished beers. Int. J. Food Microbiol. 2021, 339, 109030. [Google Scholar] [CrossRef]
- Miguel, G.A.; Carlsen, S.; Arneborg, N.; Saerens, S.M.C.; Laulund, S.; Knudsen, G.M. Non-Saccharomyces yeasts for beer production: Insights into safety aspects and considerations. Int. J. Food Microbiol. 2022, 383, 109951. [Google Scholar] [CrossRef]
- Queipo-Ortuñ, M.I.; Boto-Ordóñez, M.; Murri, M.; Gómez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef]
- Moreno Indias, I. Beneficios de los polifenoles contenidos en la cerveza sobre la microbiota intestinal [Benefits of the beer polyphenols on the gut microbiota]. Nutr. Hosp. 2017, 34, 41–44. [Google Scholar] [CrossRef]
- Le Roy, C.I.; Wells, P.M.; Si, J.; Raes, J.; Bell, J.T.; Spector, T.D. Red Wine Consumption Associated with Increased Gut Microbiota α-Diversity in 3 Independent Cohorts. Gastroenterology 2020, 158, 270–272. [Google Scholar] [CrossRef]
- Martínez-Montoro, J.I.; Quesada-Molina, M.; Gutiérrez-Repiso, C.; Ruiz-Limón, P.; Subiri-Verdugo, A.; Tinahones, F.J.; Moreno-Indias, I. Effect of Moderate Consumption of Different Phenolic-Content Beers on the Human Gut Microbiota Composition: A Randomized Crossover Trial. Antioxidants 2022, 11, 696. [Google Scholar] [CrossRef] [PubMed]
- Flint, H.J.; Duncan, S.; Louis, P. The impact of nutrition on intestinal bacterial communities. Curr. Opin. Microbiol. 2017, 38, 59–65. [Google Scholar] [CrossRef]
- Wu, G.D.; Compher, C.; Chen, E.Z.; Smith, S.A.; Shah, R.D.; Bittinger, K.; Chehoud, C.; Albenberg, L.G.; Nessel, L.; Gilroy, E.; et al. Comparative metabolomics in vegans and omnivores reveal constraints on diet-dependent gut microbiota metabolite production. Gut 2016, 65, 63–72. [Google Scholar] [CrossRef]
- Pereira, G.V.; Abdel-Hamid, A.M.; Dutta, S.; D’alessandro-Gabazza, C.N.; Wefers, D.; Farris, J.A.; Bajaj, S.; Wawrzak, Z.; Atomi, H.; Mackie, R.I.; et al. Degradation of complex arabinoxylans by human colonic Bacteroidetes. Nat. Commun. 2021, 12, 459. [Google Scholar] [CrossRef]
- Millet, S.; Van Oeckel, M.J.; Aluwé, M.; Delezie, E.; De Brabander, D.L. Prediction of In Vivo Short-Chain Fatty Acid Production in Hindgut Fermenting Mammals: Problems and Pitfalls. Crit. Rev. Food Sci. Nutr. 2010, 50, 605–619. [Google Scholar] [CrossRef]
- Martinez-Gomez, A.; Caballero, I.; Blanco, C.A. Phenols and melanoidins as natural antioxidants in beer. Structure, reactivity and antioxidant activity. Biomolecules 2020, 10, 400. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Visconti, A.; Mennella, C.; Anese, M.; Fogliano, V. Chemical characterization and antioxidant properties of coffee melanoidins. J. Agric. Food Chem. 2002, 50, 6527–6533. [Google Scholar] [CrossRef]
- Borrelli, R.C.; Fogliano, V. Bread crust melanoidins as potential prebiotic ingredients. Mol. Nutr. Food Res. 2005, 49, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Ames, J.M.; Wynne, A.; Hofmann, A.; Plos, S.; Gibson, G.R. The effect of a model melanoidin mixture on faecal bacterial populations in vitro. Br. J. Nutr. 1999, 82, 489–495. [Google Scholar] [CrossRef]
- Cann, I.; Bernardi, R.C.; Mackie, R.I. Cellulose degradation in the human gut: Ruminococcus champanellensis expands the cellulosome paradigm. Environ. Microbiol. 2016, 18, 307–310. [Google Scholar] [CrossRef]
- Moraïs, S.; David, Y.B.; Bensoussan, L.; Duncan, S.H.; Koropatkin, N.M.; Martens, E.C.; Bayer, E.A. Enzymatic profiling of cellulosomal enzymes from the human gut bacterium, Ruminococcus champanellensis, reveals a fine-tuned system for cohesin-dockerin recognition. Environ. Microbiol. 2016, 18, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.J.; Somoza, V.; Fogliano, V. Physiological relevance of dietary melanoidins. Amino Acids 2012, 42, 1097–1109. [Google Scholar] [CrossRef]
- Langner, E.; Rzeski, W. Biological Properties of Melanoidins: A. Review. Int. J. Food Prop. 2014, 17, 344–353. [Google Scholar] [CrossRef]
- Malita, M.A.; Manolescu, L.S.C.; Pîrvu, C.F.; Costea, R.C.; Marcov, E.C.; Burlibasa, M.; Pîrvu, D.A.; Burliba, L.; Radu, M.C.; Prasacu, I.; et al. Cumulative Antibiogram—A Rapid Method to Hinder Transmission of Resistant Bacteria to Oral Cavity of Newborn Babies. Antibiotics 2023, 12, 80. [Google Scholar] [CrossRef] [PubMed]
- Muntean, M.M.; Muntean, A.-A.; Preda, M.; Manolescu, L.S.C.; Dragomirescu, C.; Popa, M.I.; Popa, G.L. Phenotypic and genotypic detection methods for antimicrobial resistance in ESKAPE pathogens. Exp. Ther. Med. 2022, 24, 508. [Google Scholar] [CrossRef]
- Preda, M.; Mihai, M.M.; Popa, L.I.; Diţu, L.M.; Holban, A.M.; Manolescu, L.S.C.; Popa, G.L.; Muntean, A.-A.; Gheorghe, I.; Chifiriuc, C.M.; et al. Phenotypic and genotypic virulence features of staphylococcal strains isolated from difficult-to-treat skin and soft tissue infections. PLoS ONE 2021, 16, e0246478. [Google Scholar] [CrossRef] [PubMed]
- Mutlu, E.; Keshavarzian, A.; Engen, P.; Forsyth, C.B.; Sikaroodi, M.; Gillevet, P. Intestinal dysbiosis: A possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin. Exp. Res. 2009, 33, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Niță, I.; Nițipir, C.; Toma, S.A.; Limbău, A.M.; Pîrvu, E.; Bădărău, A.I.; Suciu, I.; Suciu, G.; Manolescu, L.S.C. Correlation between Androgen Receptor Expression and Immunohistochemistry Type as Prognostic Factors in a Cohort of Breast Cancer Patients: Result from a Single-Center, Cross Sectional Study. Healthcare 2021, 9, 277. [Google Scholar] [CrossRef]
- Medar, C.; Cristache, C.M.; Mihut, T.; Marcov, E.C.; Furtunescu, F.L.; Burlibasa, M.; Burlibasa, L. Defensive dentistry from normal medical practice to safeguard from malpractice litigations. New rules in COVID-19 pandemic. Rom. J. Leg. Med. 2020, 28, 465–469. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).