Abstract
A long-term complication of obesity is the development of type 2 diabetes (T2D). Patients with T2D have been described as having epigenetic modifications. Epigenetics is the post-transcriptional modification of DNA or associated factors containing genetic information. These environmentally-influenced modifications, maintained during cell division, cause stable changes in gene expression. Epigenetic modifications of T2D are DNA methylation, acetylation, ubiquitylation, SUMOylation, and phosphorylation at the lysine residue at the amino terminus of histones, affecting DNA, histones, and non-coding RNA. DNA methylation has been shown in pancreatic islets, adipose tissue, skeletal muscle, and the liver. Furthermore, epigenetic changes have been observed in chronic complications of T2D, such as diabetic nephropathy, diabetic retinopathy, and diabetic neuropathy. Recently, a new drug has been developed which acts on bromodomains and extraterminal (BET) domain proteins, which operate like epigenetic readers and communicate with chromatin to make DNA accessible for transcription by inhibiting them. This drug (apabetalone) is being studied to prevent major adverse cardiovascular events in people with T2D, low HDL cholesterol, chronic kidney failure, and recent coronary events. This review aims to describe the relationship between obesity, long-term complications such as T2D, and epigenetic modifications and their possible treatments.
1. Introduction
The current view of epigenetics considers DNA modifications or associated factors with information content other than the DNA sequence itself. These modifications are maintained during cell division, influenced by the environment, and cause stable changes in gene expression. Hence, the epigenetic definition is now contemplated more dynamically than when Conrad Waddington first described it as the science that analyzes the link between genes and the events that give birth to the phenotype [1]. Acknowledgment of the profound effect of the environment on developmental plasticity, particularly with aging and susceptibility to common diseases, has brought major changes in epigenetics [2]. Some epigenetic DNA modifications that affect gene transcription are at least partially reversible, which may be enzymatically reversed after cessation of exposure to environmental factors, but some epigenetic changes persist over time [3].
The first hypothesis suggesting the role of epigenetics in the development of metabolic diseases described how early life events affect health outcomes in adulthood. However, this field of knowledge has reached enormous relevance with new laboratory technologies, especially with regard to epigenetic marks [4]. The significant epigenetic changes are constituted by DNA methylation, histone modifications (acetylation is the most known, but there are other mechanisms such as phosphorylation, ribosylation, ubiquitylation, SUMOylation, and citrullination) [5], and non-coding RNAs. In contrast, DNA and histone modifications close or open the chromatin structure regulating access to the transcription factors, and non-coding RNAs control gene expression at the RNA level [6]. DNA methylation, known as CpG (cytosine and guanine separated by only one phosphate group) methylation, silences gene expression, condensing chromatin [7]. Conversely, depending on the site of methylation and the number of methyl groups, histone methylation can either increase or decrease gene transcription, and acetylation relaxes the chromatin structure, allowing access to transcription factors for gene transcription [8,9]. Thus, histone acetylation is generally associated with active gene expression [8,9]. Non-coding RNAs are not considered epigenetic components; however, they are involved in epigenetic modifications [4,10,11]. MicroRNAs (miRNAs), for example, are one of the more abundant classes of gene regulatory molecules, and their dysregulation is associated with several diseases, including cancer and T2D [12]. Long non-coding RNAs (LncRNAs) regulate metabolic processes, and recent research has found that they play a determinant role in controlling pathophysiological processes in many chronic diseases, including cancer and T2D, and their complications [6].
Genetic and external factors are then involved in many diseases, and, in some cases, environmental changes result in adaptive epigenetic changes. These changes are heritable and can be altered in response to developmental signals or environmental exposures associated with adverse health conditions, although it is challenging to establish a causal role [3,13]. Some of these exposures have been studied under different conditions, such as maternal caloric restriction (starvation), caloric excess (diabetes) during gestation, and their association with obesity in offspring. Other conditions include ingesting endocrine-disrupting agents such as pesticides or plastics related to reproductive health and certain hormone-dependent cancers, inhalation of smoke and air pollutants with respiratory illnesses, and possible effects of stressful experiences [3,14,15]. Tumorigenesis is also partially regulated by epigenetic phenomena such as nucleosome remodeling by histone modifications, DNA methylation, and miRNA-mediated targeting of various genes [16].
In the same way, there is evidence that alterations in DNA methylation can contribute to the increased prevalence of obesity and both type 1 (T1D) and T2D [16]. Visceral fat (VF) is known to be associated with prediabetes and insulin resistance [17], and lipids secreted from obese adipose tissue accumulate in peripheral tissues such as the liver, pancreas, and muscle and alter insulin sensitivity causing T2D [18]. Studies included in a 2019 review have reported an epigenetic association with obesity, particularly with altered BMI and waist circumference, reporting differential DNA methylation and increased epigenetic variability in obese people [19]. In T2D, the development of micro- and macrovascular complications is correlated with DNA methylation, histone modifications, and several non-coding RNAs, such as miRNAs and LncRNAs, that affect many biochemical pathways. As these phenomena are being explored as therapeutic targets in the cancer field, they have also opened up the possibility of using them to treat T2D and prevent or slow down its complications [8,20,21,22,23]. This review is focused on various epigenetic factors, their involvement in the development of obesity and T2D, and the prescribed drugs in altering those mechanisms.
2. Obesity, Inflammation, and Epigenetics
Patients with obesity have been shown to have epigenetic alterations associated with CpG methylation and the expression pattern of miRNAs at all stages of adipocyte differentiation, affecting their lipid metabolism and insulin sensitivity in adipose tissue and causing β-cell dysfunction [23,24]. These epigenetic alterations persist even without causal stimuli (high-fat diet and sedentary lifestyle), leading to persistent inflammation, and are called metabolic memory. In turn, this causes vascular damage and the development of cardiovascular disease (CVD), affecting the immune system of patients with obesity and favoring infections, morbidity, and mortality [25].
Variabilities in DNA methylation at the HIF3A locus have been reported in the blood cells and adipose tissue of people with obesity [26]. Reprogramming of DNA methylation in PPARGC1A (the gene encoding PGC1, a master regulator of biogenesis and mitochondrial function) has also been shown in subjects with obesity of all ages, as well as hypermethylation of the proopiomelanocortin (POMC) promoter of intron 2/exon 3 in the melanocortin system. These results imply that the development of obesity depends critically on mitochondrial function [27,28].
As described above, histone modifications are another epigenetic process linked to obesity. Among the histones related to TNF-α and IL6 is H3K4, and these proinflammatory cytokines are involved in insulin signaling [29]. miRNAs are regulators of adipogenesis, which involves processes such as hyperglycemia or insulin regulation. The secretion of CCL2 (a gene linked to proinflammatory cytokines such as TNF-α and IL-1 β) is influenced by approximately 20 miRNAs. The low regulation of this gene produces adipose inflammation that increases the secretion of proinflammatory cytokines such as TNF-α and IL1β present in the pathophysiology of T2D [29] (Figure 1).
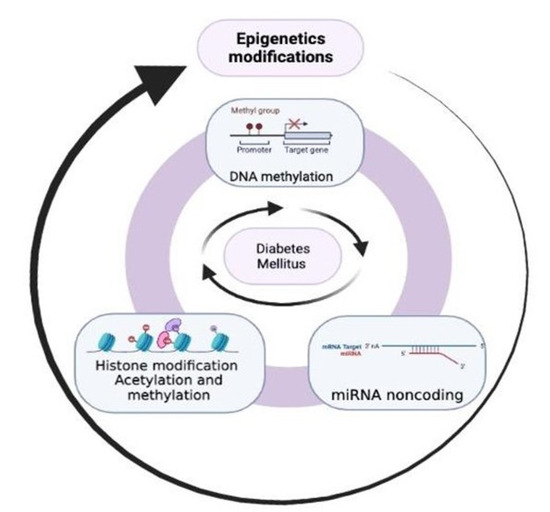
Figure 1.
The role of epigenetics in the pathogenesis of type 2 diabetes. DNA methylation, histone modifications (acetylation/methylation), and noncoding RNA are present through different pathways of the pathophysiology of the disease.
The permanence of genetic and environmental factors, such as a high-fat diet, a sedentary lifestyle, or toxic agent exposure, leads to chronic metabolic inflammation, which can cause hyperglycemia, IR, β-cell dysfunction, and as a consequence, T2D [30,31]. Obesity is known as a promoter of T2D. One of the best-proven hypotheses is the chronic inflammatory mechanism in people with obesity that interferes with insulin signaling, or IR processes. Epigenetic mechanisms control the expression of proinflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukins such as IL6, or cells such as monocytes and granulocytes [31,32].
Several genes are involved in the presence of T2D and are associated with obesity, as shown by the GWAS studies (ADCY5, FTO, HHEX, IRS1, KCNQ1, PPARG, and TCF7L2) [33]. Among the epigenetic processes involved in DNA methylation in patients with obesity, they were seen to be mainly mediated by DNA methyltransferase DNMT3A. Together with the Fabp4 promoter, it increased the expression of proinflammatory cytokines linked to M1 macrophages (IL-6 and TNF-α). Moreover, the methylation of SCD1 and SLCA1 is also related to inflammatory processes with increased cytokine production. Moreover, the inhibition of anti-inflammatory processes and increased secretion of proinflammatory cytokines are linked to DNMT1 methylation [34,35]. In addition, hypoxia or periconceptional malnutrition modulate epigenetic processes such as DNA methylation in genes such as HIF in hypoxia and LEP, INSIGF, and GNASAs in periconceptional malnutrition, promoting metabolic or inflammatory processes that lead to obesity or diseases such as T2D [36].
3. Epigenetics in Diabetes Mellitus
The incidence of T2D in the world is proliferating, with it becoming a public health problem that represents enormous costs for individuals who suffer from it and society [37,38]. The need to extensively know the pathophysiology and, therefore, find preventive and treatment measures has led to the search for different processes that allow us to understand the disease better, one of them being epigenetics. The clinical manifestations of T2D induced by alterations in gene expression that are not associated with specific changes in the DNA sequence [10] are produced by the interaction between genetic and environmental factors. Among the latter are intrauterine environments, stress, inadequate nutrient intake, low birth weight, older age [10,39], physical inactivity, diet, and obesity [4]. The last one is the most critical factor for T2D. As a result of these alterations, changes in glucose–insulin metabolism may occur, including impaired pancreatic β-cell function and liver glucose metabolism [40]. These disturbances lead to subsequent insulin insufficiency and resistance in the target organs (the liver, muscle, and adipose tissue) [41], which exhibit inappropriate expression of profibrotic and proapoptotic genes [10,40]. It has been suggested that DNA methylation may be a reversible and dynamic process that requires continuous regulation, and modifications have been described in the methylation of some genes of the insulin pathway, which has provided information on the possible relevant role of epigenetics as a key factor in positive changes in metabolic control parameters in T2DM patients [42,43].
Previously it was mentioned that the main mechanisms involved in the epigenetic modification of T2D are histone modifications, DNA methylation/demethylation within CpG islands, and non-coding RNA [10]. A vital point is the interrelationship between DNA methylation and histone modification, which influence gene transcription factors [40]. DNA methylation can alter the expression of specific genes. CpG islands play an essential role in this process. DNA methylation occurs primarily at CpG islands that impact gene expression (promoting gene silencing), cell differentiation, and molecular response. In a case–control study of Asian Indian and European T2D patients to determine whether DNA methylation is associated with the incidence of T2D, it was shown that methylation markers in seven genetic regions (TXNIP, PROC, C7orF29, SREBF1, PHOSPHO1, COCS3, and ABCG1) were associated with a higher incidence of T2D and four times higher risk of presenting the pathology in cases versus controls [24].
DNA methylation also causes alterations in pancreatic islets, adipose tissue, skeletal muscle, and the liver [40]. DNA methylation was identified at the TCF7L2, KCNQ1, THADA, FTO, IRS1, and PPARG loci in islets from T2D donors [33], and some of them were associated with altered expressions of mRNA, implying that illness development is linked to a change in transcriptional activity [33]. Other DNA-methylation-associated alterations include the pancreatic and duodenal homeobox 1 (PDX1), TCF7L2 genes, the peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A), PAX4 genes, and the glucagon-like peptide-1 receptor (GLP1R) gene that deregulate insulin secretion in β cells and therefore reduce expression in pancreatic islets; in addition to nuclear factor-kappa β (NF-kβ), osteopontin and Toll-like receptors that generate proinflammatory signals [33].
In 2013, a complete genome sequencing identified 25,820 differentially methylated regions (DMRs) covering the promoters of PDX1, TCF7L2, and adenylate cyclase 5 (ADCY5) [44]. In 2014, genome-wide methylation quantitative trait locus (metQTL) analysis included genotype data for 574,553 single nucleotide polymorphisms (SNPs) with DNA methylation. Data were extracted for 468,787 genes, and pancreatic islet CpG sites from 89 donors showed that SNPs influence gene methylation (adenylate cyclase 2 (ADCY2), potassium inwardly rectifying channel subfamily J member 11 (KCNJ11), traits of glucose-like growth factor receptor bound protein 10 (GRB10), and PDX1), all of which are responsible for epigenetic deregulation in pancreatic islets of T2D patients [45]. Recently, the analysis of 27,578 CpG sites from 14,495 genes from obese people with T2D detected a more significant amount of DMRs in visceral adipose tissues (340 DMRs), and the liver (185 DMRs), which favors insulin resistance (IR), adipogenesis, fat storage, and pro-inflammation in these obese patients [46]. Later, hypermethylation of nuclear receptor subfamily 4 group A member 1 (NR4A1), a transcriptional regulator of glucose metabolism in the skeletal muscle and liver, was observed in blood samples from diabetic patients [47].
Acetylation and methylation at the amino-terminal lysine residue is the primary mechanism of epigenetic modification of histones in muscle, the clinical result of which leads to IR being expressed in two ways: in peripheral use (GLUT 4 alteration) and hepatic noninhibition of gluconeogenesis; there is hepatic resistance to inhibit this last process [10]. The consequence is an increase in fasting blood glucose that causes greater stimulation of the islet [48]. GLUT-4 is an insulin-regulated glucose transporter found in adipocytes, skeletal muscle, the liver, and myocardium, which favors glucose’s movement into the cell. Epigenetic modifications can decrease the expression of this transporter and, as a consequence, glucose transport [10,48].
The stability of gene expression occurs due to the balance between the histone acetylation and deacetylation processes without affecting DNA sequencing [10,49]. Histone modification is associated with pancreatic β-cell death due to H3K9 deacetylation that inactivates chromatin, leading to the silencing of the PDX1 gene [10,49]. At the muscle level, H3K9 deacetylation produces peripheral inactivation of the GLUT 4 transporter, leading to hyperinsulinemia and hyperglycemia [10,49].
Another mechanism that regulates gene expression at both the transcriptional and post-transcriptional levels is ncRNA [50]. miRNAs are ncRNAs that bind with messenger RNA to regulate physiological and pathological events [15,50,51]. Currently, miRNAs act on target cells responsible for hepatic glycogen metabolism and insulin secretion, causing destruction and apoptosis of β cells and IR in peripheral organs [50]. The following miRNAs give these metabolic disorders: let-7b, miR-103, miR-142, miR-144, miR-223, miR-29, and the miR124a and miR-375 genes that have been considered to have a higher risk of causing T2D, altered fasting glucose, and IR [40,51,52]. Furthermore, exosomes that carry miRNAs contribute to the progression and complication of T2D. These epigenomic processes are also regulated by DNA methylation, histone modification, and vice versa [50,53,54].
4. From the Bench to the Bedside
The importance of intensive glucose control in diabetic patients is challenging. Complications in this pathology involve microangiopathic, macroangiopathic, musculoskeletal, neurological, and other events [55]. In this chapter, the epigenetic changes of chronic complications of diabetes mellitus (DM) focusing on T2D will be reviewed (Table 1). In addition, epidemiologic data on epigenetic changes will be reviewed.

Table 1.
Specific epigenetic modifications in T2D complications.
A multicenter clinical trial enrolled 500 participants with a mean age of 26.4 +/− 2.8 years and a diagnosis of youth-onset T2D 13.3 +/− 1.8 years ago. This observational follow-up study (performed from 2011 to 2020) was conducted in two phases; at the end of the second phase of follow-up, the trial pointed out that the risk of micro- and macrovascular complications increased over time and that the risk factors for the development of such complications were hyperglycemia, minority race, hypertension, and dyslipidemia [56].
In 499 participants in the Diabetes Control and Complications Trial (DCCT) and the Epidemiology of Diabetes Interventions and Complications (EDIC) follow-up study using blood DNA, the association between methylation of DNA in CpG and HbAb1 was demonstrated. These results suggested that DNA methylation in CpG may play an essential role in hyperglycemia and complications caused by metabolic memory (it negatively increases long-term complications if there was a previous period of hyperglycemia) [57]. In another cohort study in a rural Chinese population with 286 cases of T2D and matched controls, they found an increase of 16% (OR = 1.16, 95% CI = 1.02–1.31) in the incidence of T2D. These findings were identified when detecting an increase in DNA methylation levels at the ABCG1, CpG13, and CpG14 loci. Similarly, this risk increased to 78% when there was a gain in DNA methylation at the CpG15 locus, suggesting an etiological model for T2D [58].
4.1. Diabetic Nephropathy and Epigenetics
Microangiopathies result from the thickening of the basement membrane and glycosylation of structures resulting from T2D. Diabetic nephropathies (DNe) are associated with glomerulosclerosis, caused by an inflammatory process secondary to hyperglycemia. In addition, it is characterized by the presence of albuminuria and a decrease in the glomerular filtration rate generated by apoptosis and functional loss or alteration of podocytes, endothelial cells, and mesangial cells [59,60].
DNA methylation in cytosine in the CpG island is present in renal damage in diabetic patients. This methylation process is mediated by DNMT1 and DNMT3a/3b [61]. In an exploratory study of 107 cases, 253 controls, and 14 experimental controls, several genes were associated with end-stage kidney disease in T1D patients (AFF3, ARID5B, CUX1, ELMO1, FKBP5, HDAC4, ITGAL, LY9, PIM1, RUNX3, SEPTING9, and UTF3A) [62].
Histone modification is another process that plays an essential role in the epigenetics of DNe and metabolic memory. These processes are mediated by hyperglycemia and in response to the presence of transforming growth factor-beta (TGF-β), triggering active methylation of histones such as H3K4, H3K27, and H3K9 [63,64].
Multiple miRNAs regulate the characteristics of DNe. In a study carried out on 68 patients with T2D and 11 healthy subjects, whose objective was to establish a relationship between podocyte damage and proximal tubule dysfunction, it was shown that there is an association between podocyte damage and impaired proximal tubule function and excreted miRNA. Among the multiple regression models that showed correlation were miRNA192 with synaptopodin, uNAG, and eGFR (R2 = 0.902; p < 0.0001), miRNA124 with synaptopodin, uNAG, UACR, and eGFR (R2 = 0.881; p < 0.0001), and miRNA21 with podocalyxin, uNAG, UACR, and eGFR (R2 = 0.882; p < 0.0001) [65]. Together with the action of miRNAs, LncRNA is a group of transcription materials that lack a complete open reading frame and have no protein-coding functions [66]. A cross-sectional study of 136 participants with T2D and 25 healthy subjects demonstrated that LncRNAs contribute to the pathogenesis of DNe, with LncRNA MALAT1 and LncRNA NEAT1 through miRNA methylation associated with biomarkers of proximal tubule and podocyte injury [66].
Another factor related to the severity and presence of DNe is S-adenosylhomocysteine (SAH), a product of DNA methylation and histone modification [67]. Although the pathophysiological process of nephron damage is unknown, a study carried out in C57BL/6 mice indicated that inhibiting S-adenosylhomocysteine hydrolase (SAHH) induces an increase in SAH, allowing for the accumulation of SAH involved in damage at the podocyte level (renal function). In addition, the rise in SAH deters H3N27 me3 and increases the early growth response promoter (EGR1) [67]. These changes lead to oxidative stress due to the activation of the protein that interacts with thioredoxin (TXNIP) and NLPR3 (a mediator of the innate immune system), thus aggravating DNe [67].
4.2. Diabetic Retinopathy and Epigenetics
Diabetic retinopathy (DR) is another microvascular alteration in patients with T2D, whose hyperglycemic state causes progressive and irreversible blindness, even in normoglycemic diabetic states [68]. In addition, their hyperglycemic state causes modifications in DNA methylation in the early stages of T2D due to the metabolic memory present in this condition [69]. Genome-wide studies identified that the vascular changes of DR are regulated by LncRNAs characterized by pathological proliferation of retinal vessels, thickening of the capillary basement membrane associated with increased vascular permeability, tissue ischemia, and release of vasoactive substances [70].
Three situations may occur: (a) retinal neovascularization with vitreous hemorrhage and retinal detachment, called proliferative DR; (b) a process without neovascularization called non-proliferative DR with the formation of microaneurysms and subtle dilation of retinal vessels, generally in the early stages; and (c) neurodegeneration that causes neuroapoptosis, resulting in the death of retinal ganglion cells, glial alteration, and abnormalities in the retinal pigment layer [71].
Recent research on the molecular mechanisms of epigenetics has revealed that oxidative stress is the promoter of these alterations in photoreceptor cells since they are rich in easily oxidized polyunsaturated fatty acids. Therefore, the epigenetic changes caused by hyperglycemia lead to an excessive accumulation of cytosolic reactive oxygen species (ROS) [72] due to the activation of Nox2 (NADPH oxidase 2 nicotinamide adenine dinucleotide phosphate) [73].
Five established pathways produce metabolic abnormalities due to oxidative damage: the increased flow of the polyol pathway (sorbitol–aldose reductase) and the hexosamine pathway, hyperactivation of protein kinase C isoforms and angiotensin II, and the concentration of advanced glycation end products (AGEs) [71,74]. In addition, the polyol pathway has been investigated as a pathogenic role of DR. This pathway is activated in endothelial cells, pericytes, and Müller cells to produce macular edema and subsequent retinal ischemia [75,76]. Wenliang Li et al. investigated 3000 subjects, 1500 patients without DR and 1500 with it, of which 750 had non-proliferative DR and 750 had proliferative DR. They found that ALR2 rs759853 polymorphism variants were significantly associated with an increased risk of DR, p < 0.01 [77].
DR patients may have hyperlipidemia associated with hyperglycemia that further increases Rac1–Nox2–ROS activation, causing progressive mitochondrial damage with the loss of capillary cells [73], cell apoptosis, inflammation, lipid peroxidation, and structural and functional alterations in the retina [71]. A recent study compared retinal epigenetic modifications at 2, 4, and 6 months in non-obese T1D-induced rats versus obese T2D rats. It concluded that obesity accelerates mitochondrial damage by causing a more significant accumulation of cytosolic ROS by Rac1–Nox2 signaling, p = <0.05, establishing DR in the short term [73].
4.3. Diabetic Neuropathy and Epigenetics
Diabetic neuropathy is a very complex complication of T2D that can appear as diabetic peripheral neuropathy (DPN), diabetic autonomic neuropathy, or uremic neuropathy [78,79,80]. DPN is caused by chronic hyperglycemia, leading to peripheral nerve damage and subsequent foot ulceration, Charcot neuropathy, possible amputation, and death [79].
miRNAs also play a role in the pathogenesis of diabetic neuropathy as there are genes that are expressed in the brain and peripheral neuronal tissue [81]. In 2017, Ying-Bo Li et al. published a study that included 60 patients, of whom 75% had an increase in the expression of the mir-199a-3p gene with a significant association with a longer duration of the disease (p = 0.041) [82]. Subsequently, the study carried out by Cinzia et al. describes the genetic variation of mir-499a as a probable association with diabetic neuropathy [83]. On the contrary, Yanzhuo et al. identified the mir-25 gene as a protective factor for diabetic neuropathy [84]. Another work with diabetic rodents reports the association of miRNA-29c and protein kinase C gene expression with distal neural damage [85].
LncRNA has been considered the epigenetic mechanism that mediates this damage [79]. In 2018, Muhamad et al. evaluated the epigenetic mechanisms of the expression of LncRNA present in DPN in samples from 18 different participants, evenly distributed between DPN and healthy groups. There, 17 genes were found to be differentially expressed between both types of patients (p = <0.0005), two of which (MTHFSD and LMAN2L) were partially non-coding. In addition, the TUBA4B gene was overexpressed in the DPN samples; however, this study has inconsistent results [78], and, like other publications, the studies are limited due to the variability of epigenetic mechanisms in these patients [79].
5. Is There a Possible Therapeutic Related to Epigenetics?
Epigenetic factors and signal transduction pathways contribute significantly to DM; they affect gene expression and phenotypes associated with DNe. Life-threatening complications, including DNe and significant cardiovascular problems, denote the need for a more molecular mechanism to clarify better therapies for DM [86]. Furthermore, epigenetic modifications can lead to severe phenotype changes, and these events can be reversible [87]. Likewise, with environmental and genetic stress, epigenetic changes push ahead or backward, and stress removal can revert to the original state. Ongoing work is building to develop novel drug candidates to treat DM; they can reverse the phenotype changes, primarily when therapy has been administered early in the disorder’s progression [10]. New and novel T1D and T2D management focuses on epigenetic modifications for glucose metabolism, and some new small molecules demonstrate epigenetic activity [88]. According to their epigenetic effects, they are called “epidrugs” and are still under clinical trials. In the event of chromatin modification, they are referred to as histone acetyltransferase inhibitors (HDAC inhibitors) and RNA interference molecules [89].
Recently, data proposed a detailed mechanism related to modifying gene expression to manipulate the etiology of T2D and its complications. One of the essential issues of diabetic stimuli triggers some epigenetic modifications to be the basis of metabolic memory [90,91,92]. Nevertheless, there remains a lack of information regarding epigenetic factors and the mechanism related to gene modification; this is probably the rationality behind the rapid growth and energetic field for new mechanisms and therapeutic targets [93].
Hyperglycemia and its related complications are the main objectives for conventional management, including diet, insulin, and/or anti-diabetic drugs. Their correct management favors patients; however, these traditional medications have substantial side effects, such as lactic acidosis, severe hypoglycemia, cardiovascular problems, and urinary tract infections [94]. Although hypoglycemia is a fear side effect, its relationship with patient mortality is currently under discussion [94]. Epigenetic drugs might improve T2D complications; the critical issue is epigenetic modification [19].
Epigenetic modulators are a brand-new drug therapy that modifies gene transcription [89]; BET domain proteins operate like epigenetic readers and communicate with chromatin to access DNA for transcription. BET proteins attach molecular scaffolds using chromatin and transcription machinery to promote transcription and mRNA production and provide maladaptive gene expression in CVD models [95]. BET protein inhibitors can modify the disease-driven cellular response in people with a probability of CVD, together with CKD [96,97]. Apabetalone, an oral BET inhibitor, has anti-inflammatory and alkaline-phosphatase-lowering attributes [92,97,98].
The leading causes of CKD are T2D and hypertension, accounting for more than 50% of the cases [99]. DM and CKD are related to coronary and cerebrovascular disease, heart failure, and death [100]. Inflammation is extremely crucial in controlling the cardiovascular risk of CKD, regardless of T2D and its etiology [101]. Conventional treatment has reduced cardiovascular risk in patients with moderate CKD, but the residual risk remains considerable [102]. Apabetalone has been studied for preventing major adverse cardiovascular events (MACE) compared with novel treatment vs. a placebo in patients with T2D, low HDL cholesterol, and recent coronary events. Apabetalone performs well in lowering MACE, but the effect did not reach statistical significance [91,92]. However, in a phase 3 controlled post-acute coronary syndrome trial in patients with T2D, the participants with CKD had a better response, with an almost 50% reduction in MACE up to 27 months of inhibition. This randomized, double-blind, placebo-controlled, multicenter trial was conducted in 13 countries with 2425 participants. In the apabetalone group, more individuals abandoned the study than the placebo group (9% vs. 6%) for medical reasons [91].
The placebo’s main results summarized that patients with CKD presented with acute coronary syndrome and myocardial infarction; the proportion without ST elevation was more significant in the placebo group. The main laboratory findings for apabetalone treatment were a decrease in serum alkaline phosphatase compared to the placebo group, especially in participants with CKD, and a modest increase in HDL cholesterol [103]. Participants who completed the follow-up period of the placebo group with CKD underwent a higher incidence of MACE than those without CKD. Table 2 summarizes the trial results showing a significant reduction in primary HRs and Cis outcomes for indicating composite and component endpoints [103]. Although, in a post-hoc analysis of the BETonMACE trial, including patients with T2D, recent acute coronary disease, and a moderate likelihood of NAFLD, apabetalone reduced the risk of MACE [104].

Table 2.
Specific epigenetic modifications in T2D complications.
Apabetalone was related to a declining hazard for MACE and fewer hospitalizations for heart failure [90]. Apabetalone trials in patients with T2D and CKD had a better response to the treatment, fewer hospitalizations, and a significant reduction in MACE [90]. This novel treatment is a promising trial of epigenetic modification with secure and efficient oral medication with inhibition of BET protein to reduce cardiovascular risk in individuals with CKD, T2D, and recent acute coronary syndrome. Apabetalone is considered a selective BET protein inhibitor with possibly healthy properties on pathways related to inflammation, thrombosis, and vascular calcification. Nevertheless, the mechanism underlying these benefits is still unclear [90,91].
6. Conclusions
Various epigenetic factors are involved in developing T2D, such as DNA methylation, which causes alterations in pancreatic islets, adipose tissue, skeletal muscle, and the liver. Many genes are implicated in deregulating insulin secretion in pancreatic β cells and inducing proinflammatory signals. Other epigenetic alterations in visceral adipose tissues and the liver favor IR. At the same time, acetylation and methylation of histones in muscle are expressed in two ways: in the peripheral insulin use and the hepatic noninhibition of gluconeogenesis, and, consequently, there is an increase in fasting blood glucose that causes greater stimulation of the islet.
Epigenetic modifications can also decrease the glucose transporter’s expression and alter glucose’s movement into the cell. Histone acetylation and deacetylation processes can also induce pancreatic β cell death or inactivation of transporters at the muscle level, causing hyperinsulinemia and hyperglycemia.
Currently, miRNAs act on the target cells responsible for hepatic glycogen metabolism and insulin secretion, causing destruction and apoptosis of β cells and IR in the peripheral organs. Therefore, the two general epigenetic modifications and ncRNA stated in this review have been described at different levels of glucose metabolism and IR, and so in T2D.
Some studies have found that epigenetic modifications in DM and microvascular complications that affect small blood vessels are also related. DNA methylation and histone modifications are processes of enormous significance in the epigenetics of DNe and metabolic memory. These processes are mediated by hyperglycemia. Multiple miRNAs and LncRNA regulate the characteristics of DNe, especially related to podocyte and proximal tubule injury. Research in animals has shown that SAH, a product of DNA methylation and histone modification, is related to the presence and severity of DNe. LncRNAs regulate vascular changes in DNE. On the other hand, DPN has been linked to miRNAs and LncRNA. These two epigenetic factors have been considered the mediating epigenetic mechanisms responsible for this complication of T2D.
Research in therapeutic advances in this area has been published with drugs that alter those mechanisms. In particular, in CVD and T2D, BET protein inhibitors are being studied in clinical trials due to their property to modify the disease-driven cellular response in people with the probability of CVD and CKD. The BD2-selective BET inhibitor apabetalone has been used in trials as a therapy for epigenetic changes, and it has shown that it might decrease cardiovascular risk in individuals with CKD, T2D, and recent acute coronary syndrome and also might modulate the pathways associated with inflammation, vascular calcification, and thrombosis, but the mechanism underlying this benefit is still unclear. Although the reported evidence between the association of complications related to obesity and diabetes and epigenetic modifications is unclear as to whether they are a cause or a consequence, it would seem that treatment with drugs that modulate said modifications could be a promising therapy. The results are inconsistent with subgroups of patients and post-hoc results, but much still needs to be explored in the future.
Author Contributions
Conceptualization, R.S. and S.P.C.; writing—original draft preparation, R.S., S.P.C. and E.F.-T.; writing—review and editing, R.S., S.P.C., L.Á.-C., L.V., E.B.-V., Y.S.-A. and E.F.-T.; supervision, G.S. and E.F.-T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The assistance of the staff is gratefully appreciated.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Noble, D. Conrad Waddington and the Origin of Epigenetics. J. Exp. Biol. 2015, 218, 816–818. [Google Scholar] [CrossRef]
- Teschendorff, A.E.; West, J.; Beck, S. Age-Associated Epigenetic Drift: Implications, and a Case of Epigenetic Thrift? Hum. Mol. Genet. 2013, 22, R7–R15. [Google Scholar] [CrossRef]
- Vineis, P.; Chatziioannou, A.; Cunliffe, V.T.; Flanagan, J.M.; Hanson, M.; Kirsch-Volders, M.; Kyrtopoulos, S. Epigenetic Memory in Response to Environmental Stressors. FASEB J. 2017, 31, 2241–2251. [Google Scholar] [CrossRef]
- Rönn, T.; Ling, C. DNA Methylation as a Diagnostic and Therapeutic Target in the Battle against Type 2 Diabetes. Epigenomics 2015, 7, 451–460. [Google Scholar] [CrossRef]
- Sadakierska-Chudy, A.; Filip, M. A Comprehensive View of the Epigenetic Landscape. Part II: Histone Post-Translational Modification, Nucleosome Level, and Chromatin Regulation by NcRNAs. Neurotox. Res. 2015, 27, 172–197. [Google Scholar] [CrossRef]
- Chen, Z.; Miao, F.; Braffett, B.H.; Lachin, J.M.; Zhang, L.; Wu, X.; Roshandel, D.; Carless, M.; Li, X.A.; Tompkins, J.D.; et al. DNA Methylation Mediates Development of HbA1c-Associated Complications in Type 1 Diabetes. Nat. Metab. 2020, 2, 744–762. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Z.; Chen, J.; Liu, W.; Lai, W.; Liu, B.; Li, X.; Liu, L.; Xu, S.; Dong, Q.; et al. Stella Safeguards the Oocyte Methylome by Preventing de Novo Methylation Mediated by DNMT1. Nature 2018, 564, 136–140. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Mohammad, G. Epigenetic Modifications in Diabetes. Metabolism 2022, 126, 154920. [Google Scholar] [CrossRef]
- Sarg, B.; Lopez, R.; Lindner, H.; Ponte, I.; Suau, P.; Roque, A. Identification of Novel Post-Translational Modifications in Linker Histones from Chicken Erythrocytes. J. Proteom. 2015, 113, 162–177. [Google Scholar] [CrossRef]
- Singh, R.; Chandel, S.; Dey, D.; Ghosh, A.; Roy, S.; Ravichandiran, V.; Ghosh, D. Epigenetic Modification and Therapeutic Targets of Diabetes Mellitus. Biosci. Rep. 2020, 40, BSR20202160. [Google Scholar] [CrossRef]
- Tanwar, V.S.; Reddy, M.A.; Natarajan, R. Emerging Role of Long Non-Coding RNAs in Diabetic Vascular Complications. Front. Endocrinol. 2021, 12, 665811. [Google Scholar] [CrossRef]
- Eliasson, L.; Esguerra, J.L.S. MicroRNA Networks in Pancreatic Islet Cells: Normal Function and Type 2 Diabetes. Diabetes 2020, 69, 804–812. [Google Scholar] [CrossRef]
- Ho, S.-M.; Cheong, A.; Adgent, M.A.; Veevers, J.; Suen, A.A.; Tam, N.N.C.; Leung, Y.-K.; Jefferson, W.N.; Williams, C.J. Environmental Factors, Epigenetics, and Developmental Origin of Reproductive Disorders. Reprod. Toxicol. 2017, 68, 85–104. [Google Scholar] [CrossRef]
- Sundar, I.K.; Rahman, I. Gene Expression Profiling of Epigenetic Chromatin Modification Enzymes and Histone Marks by Cigarette Smoke: Implications for COPD and Lung Cancer. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2016, 311, L1245–L1258. [Google Scholar] [CrossRef]
- Sundar, I.K.; Yin, Q.; Baier, B.S.; Yan, L.; Mazur, W.; Li, D.; Susiarjo, M.; Rahman, I. DNA Methylation Profiling in Peripheral Lung Tissues of Smokers and Patients with COPD. Clin. Epigenet. 2017, 9, 38. [Google Scholar] [CrossRef]
- Biswas, S.; Rao, C.M. Epigenetics in Cancer: Fundamentals and Beyond. Pharmacol. Ther. 2017, 173, 118–134. [Google Scholar] [CrossRef]
- Jadhav, R.A.; Maiya, G.A.; Shivashankara, K.N.; Umakanth, S. Measurement of Visceral Fat for Early Prediction of Prediabetes-Cross-Sectional Study from Southern India. J. Taibah Univ. Med. Sci. 2022, 17, 983–990. [Google Scholar] [CrossRef]
- Hong, J.; Kim, Y.-H. Fatty Liver/Adipose Tissue Dual-Targeting Nanoparticles with Heme Oxygenase-1 Inducer for Amelioration of Obesity, Obesity-Induced Type 2 Diabetes, and Steatohepatitis. Adv. Sci. 2022, 9, e2203286. [Google Scholar] [CrossRef]
- Ling, C.; Rönn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef]
- Parveen, N.; Dhawan, S. DNA Methylation Patterning and the Regulation of Beta Cell Homeostasis. Front. Endocrinol. 2021, 12, 651258. [Google Scholar] [CrossRef]
- Maude, H.; Sanchez-Cabanillas, C.; Cebola, I. Epigenetics of Hepatic Insulin Resistance. Front. Endocrinol. 2021, 12, 681356. [Google Scholar] [CrossRef]
- Edillor, C.R.; Parks, B.W.; Mehrabian, M.; Lusis, A.J.; Pellegrini, M. DNA Methylation Changes More Slowly Than Physiological States in Response to Weight Loss in Genetically Diverse Mouse Strains. Front. Endocrinol. 2019, 10, 882. [Google Scholar] [CrossRef]
- Arpón, A.; Milagro, F.I.; Santos, J.L.; García-Granero, M.; Riezu-Boj, J.I.; Martínez, J.A. Interaction among Sex, Aging, and Epigenetic Processes Concerning Visceral Fat, Insulin Resistance, and Dyslipidaemia. Front. Endocrinol. 2019, 10, 496. [Google Scholar] [CrossRef]
- Chambers, J.C.; Loh, M.; Lehne, B.; Drong, A.; Kriebel, J.; Motta, V.; Wahl, S.; Elliott, H.R.; Rota, F.; Scott, W.R.; et al. Epigenome-Wide Association of DNA Methylation Markers in Peripheral Blood from Indian Asians and Europeans with Incident Type 2 Diabetes: A Nested Case-Control Study. Lancet Diabetes Endocrinol. 2015, 3, 526–534. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Linares-Pineda, T.M.; Gonzalez-Jimenez, A.; Aguilar-Lineros, F.; Valdés, S.; Soriguer, F.; Rojo-Martínez, G.; Tinahones, F.J.; Morcillo, S. Epigenetic Biomarkers of Transition from Metabolically Healthy Obesity to Metabolically Unhealthy Obesity Phenotype: A Prospective Study. Int. J. Mol. Sci. 2021, 22, 10417. [Google Scholar] [CrossRef]
- Aslibekyan, S.; Demerath, E.W.; Mendelson, M.; Zhi, D.; Guan, W.; Liang, L.; Sha, J.; Pankow, J.S.; Liu, C.; Irvin, M.R.; et al. Epigenome-Wide Study Identifies Novel Methylation Loci Associated with Body Mass Index and Waist Circumference. Obesity 2015, 23, 1493–1501. [Google Scholar] [CrossRef]
- Kwon, E.J.; You, Y.-A.; Park, B.; Ha, E.H.; Kim, H.S.; Park, H.; Kim, Y.J. Association between the DNA Methylations of POMC, MC4R, and HNF4A and Metabolic Profiles in the Blood of Children Aged 7-9 Years. BMC Pediatr. 2018, 18, 121. [Google Scholar] [CrossRef]
- Obri, A.; Serra, D.; Herrero, L.; Mera, P. The Role of Epigenetics in the Development of Obesity. Biochem. Pharmacol. 2020, 177, 113973. [Google Scholar] [CrossRef]
- Jung, B.C.; Kang, S. Epigenetic Regulation of Inflammatory Factors in Adipose Tissue. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 159019. [Google Scholar] [CrossRef]
- Naidoo, V.; Naidoo, M.; Ghai, M. Cell and Tissue-Specific Epigenetic Changes Associated with Chronic Inflammation in Insulin Resistance and Type 2 Diabetes Mellitus. Scand. J. Immunol. 2018, 88, e12723. [Google Scholar] [CrossRef]
- Ramos-Lopez, O.; Milagro, F.I.; Riezu-Boj, J.I.; Martinez, J.A. Epigenetic Signatures Underlying Inflammation: An Interplay of Nutrition, Physical Activity, Metabolic Diseases, and Environmental Factors for Personalized Nutrition. Inflamm. Res. 2021, 70, 29–49. [Google Scholar] [CrossRef]
- Ndisang, J.F.; Rastogi, S.; Vannacci, A. Immune and Inflammatory Processes in Obesity, Insulin Resistance, Diabetes, and Related Cardiometabolic Complications. J. Immunol. Res. 2014, 2014, 579560. [Google Scholar] [CrossRef]
- Dayeh, T.; Tuomi, T.; Almgren, P.; Perfilyev, A.; Jansson, P.A.; de Mello, V.D.; Pihlajamäki, J.; Vaag, A.; Groop, L.; Nilsson, E.; et al. DNA Methylation of Loci within ABCG1 and PHOSPHO1 in Blood DNA Is Associated with Future Type 2 Diabetes Risk. Epigenetics 2016, 11, 482–488. [Google Scholar] [CrossRef]
- Malodobra-Mazur, M.; Dziewulska, A.; Kozinski, K.; Dobrzyn, P.; Kolczynska, K.; Janikiewicz, J.; Dobrzyn, A. Stearoyl-CoA Desaturase Regulates Inflammatory Gene Expression by Changing DNA Methylation Level in 3T3 Adipocytes. Int. J. Biochem. Cell Biol. 2014, 55, 40–50. [Google Scholar] [CrossRef]
- You, D.; Nilsson, E.; Tenen, D.E.; Lyubetskaya, A.; Lo, J.C.; Jiang, R.; Deng, J.; Dawes, B.A.; Vaag, A.; Ling, C.; et al. Dnmt3a Is an Epigenetic Mediator of Adipose Insulin Resistance. eLife 2017, 6, e30766. [Google Scholar] [CrossRef]
- Castellano-Castillo, D.; Denechaud, P.-D.; Fajas, L.; Moreno-Indias, I.; Oliva-Olivera, W.; Tinahones, F.; Queipo-Ortuño, M.I.; Cardona, F. Human Adipose Tissue H3K4me3 Histone Mark in Adipogenic, Lipid Metabolism and Inflammatory Genes Is Positively Associated with BMI and HOMA-IR. PLoS ONE 2019, 14, e0215083. [Google Scholar] [CrossRef]
- Hex, N.; Bartlett, C.; Wright, D.; Taylor, M.; Varley, D. Estimating the Current and Future Costs of Type 1 and Type 2 Diabetes in the UK, Including Direct Health Costs and Indirect Societal and Productivity Costs. Diabet. Med. 2012, 29, 855–862. [Google Scholar] [CrossRef]
- Bommer, C.; Sagalova, V.; Heesemann, E.; Manne-Goehler, J.; Atun, R.; Bärnighausen, T.; Davies, J.; Vollmer, S. Global Economic Burden of Diabetes in Adults: Projections From 2015 to 2030. Diabetes Care 2018, 41, 963–970. [Google Scholar] [CrossRef]
- Meng, R.; Lv, J.; Yu, C.; Guo, Y.; Bian, Z.; Yang, L.; Chen, Y.; Zhang, H.; Chen, X.; Chen, J.; et al. Prenatal Famine Exposure, Adulthood Obesity Patterns and Risk of Type 2 Diabetes. Int. J. Epidemiol. 2018, 47, 399–408. [Google Scholar] [CrossRef]
- Vaiserman, A.; Lushchak, O. Developmental Origins of Type 2 Diabetes: Focus on Epigenetics. Ageing Res. Rev. 2019, 55, 100957. [Google Scholar] [CrossRef]
- Sommese, L.; Benincasa, G.; Lanza, M.; Sorriento, A.; Schiano, C.; Lucchese, R.; Alfano, R.; Nicoletti, G.F.; Napoli, C. Novel Epigenetic-Sensitive Clinical Challenges Both in Type 1 and Type 2 Diabetes. J. Diabetes Complicat. 2018, 32, 1076–1084. [Google Scholar] [CrossRef]
- Liang, Y.-Z.; Dong, J.; Zhang, J.; Wang, S.; He, Y.; Yan, Y.-X. Identification of Neuroendocrine Stress Response-Related Circulating MicroRNAs as Biomarkers for Type 2 Diabetes Mellitus and Insulin Resistance. Front. Endocrinol. 2018, 9, 132. [Google Scholar] [CrossRef]
- Martínez-Ramírez, O.C.; Salazar-Piña, A.; Cerón-Ramírez, X.; Rubio-Lightbourn, J.; Torres-Romero, F.; Casas-Avila, L.; Castro-Hernández, C. Effect of Inulin Intervention on Metabolic Control and Methylation of INS and IRS1 Genes in Patients with Type 2 Diabetes Mellitus. Nutrients 2022, 14, 5195. [Google Scholar] [CrossRef]
- Volkov, P.; Bacos, K.; Ofori, J.K.; Esguerra, J.L.S.; Eliasson, L.; Rönn, T.; Ling, C. Whole-Genome Bisulfite Sequencing of Human Pancreatic Islets Reveals Novel Differentially Methylated Regions in Type 2 Diabetes Pathogenesis. Diabetes 2017, 66, 1074–1085. [Google Scholar] [CrossRef]
- Olsson, A.H.; Volkov, P.; Bacos, K.; Dayeh, T.; Hall, E.; Nilsson, E.A.; Ladenvall, C.; Rönn, T.; Ling, C. Genome-Wide Associations between Genetic and Epigenetic Variation Influence MRNA Expression and Insulin Secretion in Human Pancreatic Islets. PLoS Genet. 2014, 10, e1004735. [Google Scholar] [CrossRef]
- Barajas-Olmos, F.; Centeno-Cruz, F.; Zerrweck, C.; Imaz-Rosshandler, I.; Martínez-Hernández, A.; Cordova, E.J.; Rangel-Escareño, C.; Gálvez, F.; Castillo, A.; Maydón, H.; et al. Altered DNA Methylation in Liver and Adipose Tissues Derived from Individuals with Obesity and Type 2 Diabetes. BMC Med. Genet. 2018, 19, 28. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Liao, J.-W.; Tsai, Y.-C.; Tsai, F.-J. Inhibition of DNA Methyltransferase 1 Increases Nuclear Receptor Subfamily 4 Group A Member 1 Expression and Decreases Blood Glucose in Type 2 Diabetes. Oncotarget 2016, 7, 39162–39170. [Google Scholar] [CrossRef]
- Esau, C.; Kang, X.; Peralta, E.; Hanson, E.; Marcusson, E.G.; Ravichandran, L.V.; Sun, Y.; Koo, S.; Perera, R.J.; Jain, R.; et al. MicroRNA-143 Regulates Adipocyte Differentiation. J. Biol. Chem. 2004, 279, 52361–52365. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Sun, G. Histone Acetylation and Its Modifiers in the Pathogenesis of Diabetic Nephropathy. J. Diabetes Res. 2016, 2016, 4065382. [Google Scholar] [CrossRef]
- Morán, I.; Akerman, I.; van de Bunt, M.; Xie, R.; Benazra, M.; Nammo, T.; Arnes, L.; Nakić, N.; García-Hurtado, J.; Rodríguez-Seguí, S.; et al. Human β Cell Transcriptome Analysis Uncovers LncRNAs That Are Tissue-Specific, Dynamically Regulated, and Abnormally Expressed in Type 2 Diabetes. Cell Metab. 2012, 16, 435–448. [Google Scholar] [CrossRef]
- Akerman, I.; Tu, Z.; Beucher, A.; Rolando, D.M.Y.; Sauty-Colace, C.; Benazra, M.; Nakic, N.; Yang, J.; Wang, H.; Pasquali, L.; et al. Human Pancreatic β Cell LncRNAs Control Cell-Specific Regulatory Networks. Cell Metab. 2017, 25, 400–411. [Google Scholar] [CrossRef] [PubMed]
- Brennan, E.; Wang, B.; McClelland, A.; Mohan, M.; Marai, M.; Beuscart, O.; Derouiche, S.; Gray, S.; Pickering, R.; Tikellis, C.; et al. Protective Effect of Let-7 MiRNA Family in Regulating Inflammation in Diabetes-Associated Atherosclerosis. Diabetes 2017, 66, 2266–2277. [Google Scholar] [CrossRef]
- Voight, B.F.; Scott, L.J.; Steinthorsdottir, V.; Morris, A.P.; Dina, C.; Welch, R.P.; Zeggini, E.; Huth, C.; Aulchenko, Y.S.; Thorleifsson, G.; et al. Twelve Type 2 Diabetes Susceptibility Loci Identified through Large-Scale Association Analysis. Nat. Genet. 2010, 42, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Kooner, J.S.; Saleheen, D.; Sim, X.; Sehmi, J.; Zhang, W.; Frossard, P.; Been, L.F.; Chia, K.-S.; Dimas, A.S.; Hassanali, N.; et al. Genome-Wide Association Study in Individuals of South Asian Ancestry Identifies Six New Type 2 Diabetes Susceptibility Loci. Nat. Genet. 2011, 43, 984–989. [Google Scholar] [CrossRef] [PubMed]
- Nathan, D.M.; Cleary, P.A.; Backlund, J.-Y.C.; Genuth, S.M.; Lachin, J.M.; Orchard, T.J.; Raskin, P.; Zinman, B. Intensive Diabetes Treatment and Cardiovascular Disease in Patients with Type 1 Diabetes. N. Engl. J. Med. 2005, 353, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Drews, K.L.; Caprio, S.; Gubitosi-Klug, R.; Nathan, D.M.; Tesfaldet, B.; Tryggestad, J.; White, N.H.; Zeitler, P. Long-Term Complications in Youth-Onset Type 2 Diabetes. N. Engl. J. Med. 2021, 385, 416–426. [Google Scholar] [CrossRef]
- Lachin, J.M.; Nathan, D.M. Understanding Metabolic Memory: The Prolonged Influence of Glycemia During the Diabetes Control and Complications Trial (DCCT) on Future Risks of Complications During the Study of the Epidemiology of Diabetes Interventions and Complications (EDIC). Diabetes Care 2021, 44, 2216–2224. [Google Scholar] [CrossRef]
- Qie, R.; Chen, Q.; Wang, T.; Chen, X.; Wang, J.; Cheng, R.; Lin, J.; Zhao, Y.; Liu, D.; Qin, P.; et al. Association of ABCG1 Gene Methylation and Its Dynamic Change Status with Incident Type 2 Diabetes Mellitus: The Rural Chinese Cohort Study. J. Hum. Genet. 2021, 66, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.-W.; Hsu, Y.-C.; Shih, Y.-H.; Chang, P.-J.; Lin, C.-L. Glomerular Mesangial Cell and Podocyte Injuries in Diabetic Nephropathy. Nephrology 2018, 23 (Suppl. 4), 32–37. [Google Scholar] [CrossRef] [PubMed]
- Sugita, E.; Hayashi, K.; Hishikawa, A.; Itoh, H. Epigenetic Alterations in Podocytes in Diabetic Nephropathy. Front. Pharmacol. 2021, 12, 759299. [Google Scholar] [CrossRef]
- Hishikawa, A.; Hayashi, K.; Abe, T.; Kaneko, M.; Yokoi, H.; Azegami, T.; Nakamura, M.; Yoshimoto, N.; Kanda, T.; Sakamaki, Y.; et al. Decreased KAT5 Expression Impairs DNA Repair and Induces Altered DNA Methylation in Kidney Podocytes. Cell Rep. 2019, 26, 1318–1332.e4. [Google Scholar] [CrossRef] [PubMed]
- Smyth, L.J.; Kilner, J.; Nair, V.; Liu, H.; Brennan, E.; Kerr, K.; Sandholm, N.; Cole, J.; Dahlström, E.; Syreeni, A.; et al. Assessment of Differentially Methylated Loci in Individuals with End-Stage Kidney Disease Attributed to Diabetic Kidney Disease: An Exploratory Study. Clin. Epigenet. 2021, 13, 99. [Google Scholar] [CrossRef] [PubMed]
- Brasacchio, D.; Okabe, J.; Tikellis, C.; Balcerczyk, A.; George, P.; Baker, E.K.; Calkin, A.C.; Brownlee, M.; Cooper, M.E.; El-Osta, A. Hyperglycemia Induces a Dynamic Cooperativity of Histone Methylase and Demethylase Enzymes Associated with Gene-Activating Epigenetic Marks That Coexist on the Lysine Tail. Diabetes 2009, 58, 1229–1236. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Reddy, M.A.; Yuan, H.; Lanting, L.; Kato, M.; Natarajan, R. Epigenetic Histone Methylation Modulates Fibrotic Gene Expression. J. Am. Soc. Nephrol. 2010, 21, 2069–2080. [Google Scholar] [CrossRef] [PubMed]
- Milas, O.; Gadalean, F.; Vlad, A.; Dumitrascu, V.; Gluhovschi, C.; Gluhovschi, G.; Velciov, S.; Popescu, R.; Bob, F.; Matusz, P.; et al. Deregulated Profiles of Urinary MicroRNAs May Explain Podocyte Injury and Proximal Tubule Dysfunction in Normoalbuminuric Patients with Type 2 Diabetes Mellitus. J. Investig. Med. 2018, 66, 747–754. [Google Scholar] [CrossRef]
- Petrica, L.; Hogea, E.; Gadalean, F.; Vlad, A.; Vlad, M.; Dumitrascu, V.; Velciov, S.; Gluhovschi, C.; Bob, F.; Ursoniu, S.; et al. Long Noncoding RNAs May Impact Podocytes and Proximal Tubule Function through Modulating MiRNAs Expression in Early Diabetic Kidney Disease of Type 2 Diabetes Mellitus Patients. Int. J. Med. Sci. 2021, 18, 2093–2101. [Google Scholar] [CrossRef]
- Dai, X.; Liao, R.; Liu, C.; Liu, S.; Huang, H.; Liu, J.; Jin, T.; Guo, H.; Zheng, Z.; Xia, M.; et al. Epigenetic Regulation of TXNIP-Mediated Oxidative Stress and NLRP3 Inflammasome Activation Contributes to SAHH Inhibition-Aggravated Diabetic Nephropathy. Redox Biol. 2021, 45, 102033. [Google Scholar] [CrossRef]
- Andersen, N.; Hjortdal, J.Ø.; Schielke, K.C.; Bek, T.; Grauslund, J.; Laugesen, C.S.; Lund-Andersen, H.; Cerqueira, C.; Andresen, J. The Danish Registry of Diabetic Retinopathy. Clin. Epidemiol. 2016, 8, 613–619. [Google Scholar] [CrossRef]
- Zhong, Q.; Kowluru, R.A. Role of Histone Acetylation in the Development of Diabetic Retinopathy and the Metabolic Memory Phenomenon. J. Cell. Biochem. 2010, 110, 1306–1313. [Google Scholar] [CrossRef]
- Liu, J.-Y.; Yao, J.; Li, X.-M.; Song, Y.-C.; Wang, X.-Q.; Li, Y.-J.; Yan, B.; Jiang, Q. Pathogenic Role of LncRNA-MALAT1 in Endothelial Cell Dysfunction in Diabetes Mellitus. Cell Death Dis. 2014, 5, e1506. [Google Scholar] [CrossRef]
- Kang, Q.; Yang, C. Oxidative Stress and Diabetic Retinopathy: Molecular Mechanisms, Pathogenetic Role and Therapeutic Implications. Redox Biol. 2020, 37, 101799. [Google Scholar] [CrossRef] [PubMed]
- Lund, J.; Ouwens, D.M.; Wettergreen, M.; Bakke, S.S.; Thoresen, G.H.; Aas, V. Increased Glycolysis and Higher Lactate Production in Hyperglycemic Myotubes. Cells 2019, 8, 1101. [Google Scholar] [CrossRef] [PubMed]
- Kowluru, R.A. Retinopathy in a Diet-Induced Type 2 Diabetic Rat Model and Role of Epigenetic Modifications. Diabetes 2020, 69, 689–698. [Google Scholar] [CrossRef]
- Katagiri, M.; Shoji, J.; Inada, N.; Kato, S.; Kitano, S.; Uchigata, Y. Evaluation of Vitreous Levels of Advanced Glycation End Products and Angiogenic Factors as Biomarkers for Severity of Diabetic Retinopathy. Int. Ophthalmol. 2018, 38, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, M. The Polyol Pathway as a Mechanism for Diabetic Retinopathy: Attractive, Elusive, and Resilient. Exp. Diabetes Res. 2007, 2007, 61038. [Google Scholar] [CrossRef]
- Asnaghi, V.; Gerhardinger, C.; Hoehn, T.; Adeboje, A.; Lorenzi, M. A Role for the Polyol Pathway in the Early Neuroretinal Apoptosis and Glial Changes Induced by Diabetes in the Rat. Diabetes 2003, 52, 506–511. [Google Scholar] [CrossRef]
- Li, W.; Chen, S.; Mei, Z.; Zhao, F.; Xiang, Y. Polymorphisms in Sorbitol-Aldose Reductase (Polyol) Pathway Genes and Their Influence on Risk of Diabetic Retinopathy Among Han Chinese. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2019, 25, 7073–7078. [Google Scholar] [CrossRef]
- Jankovic, M.; Novakovic, I.; Nikolic, D.; Mitrovic Maksic, J.; Brankovic, S.; Petronic, I.; Cirovic, D.; Ducic, S.; Grajic, M.; Bogicevic, D. Genetic and Epigenomic Modifiers of Diabetic Neuropathy. Int. J. Mol. Sci. 2021, 22, 4887. [Google Scholar] [CrossRef]
- Fachrul, M.; Utomo, D.H.; Parikesit, A.A. LncRNA-Based Study of Epigenetic Regulations in Diabetic Peripheral Neuropathy. Silico Pharmacol. 2018, 6, 7. [Google Scholar] [CrossRef]
- Karki, D.B.; Yadava, S.K.; Pant, S.; Thusa, N.; Dangol, E.; Ghimire, S. Prevalence of Sensory Neuropathy in Type 2 Diabetes Mellitus and Its Correlation with Duration of Disease. Kathmandu Univ. Med. J. 2016, 14, 120–124. [Google Scholar]
- Huang, Y.; Liu, Y.; Li, L.; Su, B.; Yang, L.; Fan, W.; Yin, Q.; Chen, L.; Cui, T.; Zhang, J.; et al. Involvement of Inflammation-Related MiR-155 and MiR-146a in Diabetic Nephropathy: Implications for Glomerular Endothelial Injury. BMC Nephrol. 2014, 15, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-B.; Wu, Q.; Liu, J.; Fan, Y.-Z.; Yu, K.-F.; Cai, Y. MiR-199a-3p Is Involved in the Pathogenesis and Progression of Diabetic Neuropathy through Downregulation of SerpinE2. Mol. Med. Rep. 2017, 16, 2417–2424. [Google Scholar] [CrossRef] [PubMed]
- Ciccacci, C.; Latini, A.; Greco, C.; Politi, C.; D’Amato, C.; Lauro, D.; Novelli, G.; Borgiani, P.; Spallone, V. Association between a MIR499A Polymorphism and Diabetic Neuropathy in Type 2 Diabetes. J. Diabetes Complicat. 2018, 32, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Song, C.; Liu, J.; Bi, Y.; Li, H. Inhibition of MiR-25 Aggravates Diabetic Peripheral Neuropathy. Neuroreport 2018, 29, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Xourgia, E.; Papazafiropoulou, A.; Melidonis, A. Circulating MicroRNAs as Biomarkers for Diabetic Neuropathy: A Novel Approach. World J. Exp. Med. 2018, 8, 18–23. [Google Scholar] [CrossRef]
- Kato, M.; Natarajan, R. Epigenetics and Epigenomics in Diabetic Kidney Disease and Metabolic Memory. Nat. Rev. Nephrol. 2019, 15, 327–345. [Google Scholar] [CrossRef]
- Bansal, A.; Pinney, S.E. DNA Methylation and Its Role in the Pathogenesis of Diabetes. Pediatr. Diabetes 2017, 18, 167–177. [Google Scholar] [CrossRef]
- Arguelles, A.O.; Meruvu, S.; Bowman, J.D.; Choudhury, M. Are Epigenetic Drugs for Diabetes and Obesity at Our Door Step? Drug Discov. Today 2016, 21, 499–509. [Google Scholar] [CrossRef]
- Devaiah, B.N.; Case-Borden, C.; Gegonne, A.; Hsu, C.H.; Chen, Q.; Meerzaman, D.; Dey, A.; Ozato, K.; Singer, D.S. BRD4 Is a Histone Acetyltransferase That Evicts Nucleosomes from Chromatin. Nat. Struct. Mol. Biol. 2016, 23, 540–548. [Google Scholar] [CrossRef]
- Nicholls, S.J.; Schwartz, G.G.; Buhr, K.A.; Ginsberg, H.N.; Johansson, J.O.; Kalantar-Zadeh, K.; Kulikowski, E.; Toth, P.P.; Wong, N.; Sweeney, M.; et al. Apabetalone and Hospitalization for Heart Failure in Patients Following an Acute Coronary Syndrome: A Prespecified Analysis of the BETonMACE Study. Cardiovasc. Diabetol. 2021, 20, 13. [Google Scholar] [CrossRef]
- Ray, K.K.; Nicholls, S.J.; Buhr, K.A.; Ginsberg, H.N.; Johansson, J.O.; Kalantar-Zadeh, K.; Kulikowski, E.; Toth, P.P.; Wong, N.; Sweeney, M.; et al. Effect of Apabetalone Added to Standard Therapy on Major Adverse Cardiovascular Events in Patients With Recent Acute Coronary Syndrome and Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2020, 323, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Nicholls, S.J.; Ginsberg, H.D.; Johansson, J.O.; Kalantar-Zadeh, K.; Kulikowski, E.; Toth, P.P.; Wong, N.; Cummings, J.L.; Sweeney, M.; et al. Effect of Selective BET Protein Inhibitor Apabetalone on Cardiovascular Outcomes in Patients with Acute Coronary Syndrome and Diabetes: Rationale, Design, and Baseline Characteristics of the BETonMACE Trial. Am. Heart J. 2019, 217, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Kaimala, S.; Kumar, C.A.; Allouh, M.Z.; Ansari, S.A.; Emerald, B.S. Epigenetic Modifications in Pancreas Development, Diabetes, and Therapeutics. Med. Res. Rev. 2022, 42, 1343–1371. [Google Scholar] [CrossRef] [PubMed]
- The International Hypoglycaemia Study Group. Hypoglycaemia, Cardiovascular Disease, and Mortality in Diabetes: Epidemiology, Pathogenesis, and Management. Lancet Diabetes Endocrinol. 2019, 7, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Hajmirza, A.; Emadali, A.; Gauthier, A.; Casasnovas, O.; Gressin, R.; Callanan, M.B. BET Family Protein BRD4: An Emerging Actor in NFκB Signaling in Inflammation and Cancer. Biomedicines 2018, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Gilham, D.; Tsujikawa, L.M.; Sarsons, C.D.; Halliday, C.; Wasiak, S.; Stotz, S.C.; Jahagirdar, R.; Sweeney, M.; Johansson, J.O.; Wong, N.C.W.; et al. Apabetalone Downregulates Factors and Pathways Associated with Vascular Calcification. Atherosclerosis 2019, 280, 75–84. [Google Scholar] [CrossRef]
- Wasiak, S.; Dzobo, K.E.; Rakai, B.D.; Kaiser, Y.; Versloot, M.; Bahjat, M.; Stotz, S.C.; Fu, L.; Sweeney, M.; Johansson, J.O.; et al. BET Protein Inhibitor Apabetalone (RVX-208) Suppresses pro-Inflammatory Hyper-Activation of Monocytes from Patients with Cardiovascular Disease and Type 2 Diabetes. Clin. Epigenet. 2020, 12, 166. [Google Scholar] [CrossRef]
- Haarhaus, M.; Ray, K.K.; Nicholls, S.J.; Schwartz, G.G.; Kulikowski, E.; Johansson, J.O.; Sweeney, M.; Halliday, C.; Lebioda, K.; Wong, N.; et al. Apabetalone Lowers Serum Alkaline Phosphatase and Improves Cardiovascular Risk in Patients with Cardiovascular Disease. Atherosclerosis 2019, 290, 59–65. [Google Scholar] [CrossRef]
- Charles, C.; Ferris, A.H. Chronic Kidney Disease. Prim. Care 2020, 47, 585–595. [Google Scholar] [CrossRef]
- Huang, D.; Refaat, M.; Mohammedi, K.; Jayyousi, A.; Al Suwaidi, J.; Abi Khalil, C. Macrovascular Complications in Patients with Diabetes and Prediabetes. BioMed Res. Int. 2017, 2017, 7839101. [Google Scholar] [CrossRef]
- Stratton, I.M.; Adler, A.I.; Neil, H.A.; Matthews, D.R.; Manley, S.E.; Cull, C.A.; Hadden, D.; Turner, R.C.; Holman, R.R. Association of Glycaemia with Macrovascular and Microvascular Complications of Type 2 Diabetes (UKPDS 35): Prospective Observational Study. BMJ 2000, 321, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Gæde, P.; Oellgaard, J.; Carstensen, B.; Rossing, P.; Lund-Andersen, H.; Parving, H.-H.; Pedersen, O. Years of Life Gained by Multifactorial Intervention in Patients with Type 2 Diabetes Mellitus and Microalbuminuria: 21 Years Follow-up on the Steno-2 Randomised Trial. Diabetologia 2016, 59, 2298–2307. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Schwartz, G.G.; Nicholls, S.J.; Buhr, K.A.; Ginsberg, H.N.; Johansson, J.O.; Kulikowski, E.; Lebioda, K.; Toth, P.P.; Wong, N.; et al. Effect of Apabetalone on Cardiovascular Events in Diabetes, CKD, and Recent Acute Coronary Syndrome: Results from the BETonMACE Randomized Controlled Trial. Clin. J. Am. Soc. Nephrol. 2021, 16, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.P.; Schwartz, G.G.; Nicholls, S.J.; Khan, A.; Szarek, M.; Ginsberg, H.N.; Johansson, J.O.; Kalantar-Zadeh, K.; Kulikowski, E.; Lebioda, K.; et al. Reduction in the Risk of Major Adverse Cardiovascular Events with the BET Protein Inhibitor Apabetalone in Patients with Recent Acute Coronary Syndrome, Type 2 Diabetes, and Moderate to High Likelihood of Non-Alcoholic Fatty Liver Disease. Am. J. Prev. Cardiol. 2022, 11, 100372. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).