Prognostic Impact of Nutritional Status on Overall Survival and Health-Related Quality of Life in Men with Advanced Prostate Cancer
Abstract
1. Introduction
2. Patients and Methods
3. Study Design
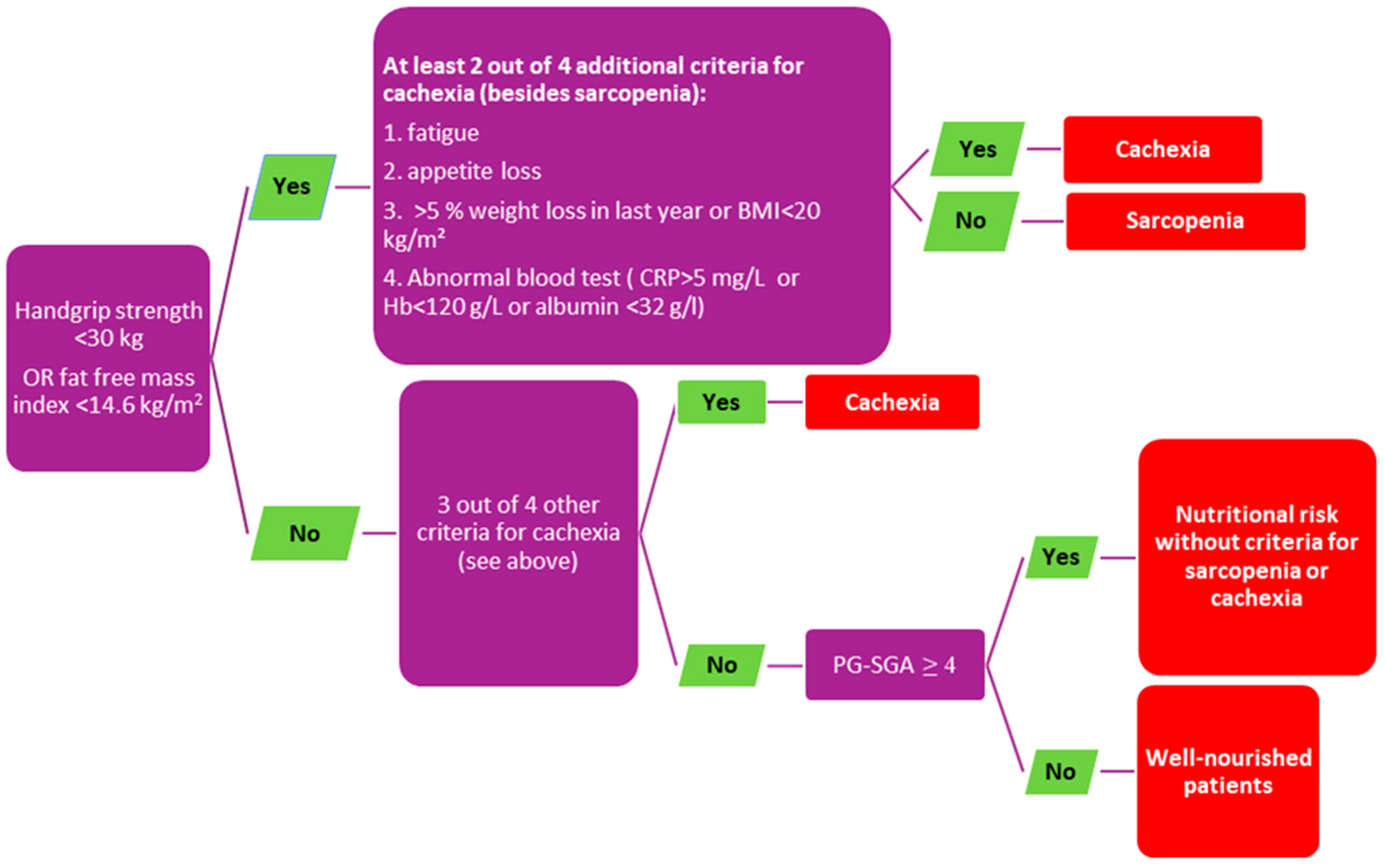
4. Assessment of the NS
5. HRQoL Assessment
6. Statistical Analysis
7. Results
Patients’ Characteristics
8. Prognostic Role of Baseline NS for Estimated HRQoL
9. Prognostic Role of Baseline NS for OS
10. Discussion
11. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bergius, S.; Torvinen, S.; Muhonen, T.; Roine, R.P.; Sintonen, H.; Taari, K. Health-related quality of life among prostate cancer patients: Real-life situation at the beginning of treatment. Scand. J. Urol. 2017, 51, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART I. J. Urol. 2021, 205, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Kretschmer, A.; Ploussard, G.; Heidegger, I.; Tsaur, I.; Borgmann, H.; Surcel, C.; Mathieu, R.; de Visschere, P.; Valerio, M.; van den Bergh, R.C.N.; et al. Health-related Quality of Life in Patients with Advanced Prostate Cancer: A Systematic Review. Eur. Urol. Focus 2021, 7, 742–751. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART II. J. Urol. 2021, 205, 22–29. [Google Scholar] [CrossRef]
- Cavka, L.; Pohar Perme, M.; Zakotnik, B.; Rotovnik Kozjek, N.; Seruga, B. Nutritional Status and Health-Related Quality of Life in Men with Advanced Castrate-Resistant Prostate Cancer. Nutr. Cancer 2021, 74, 472–481. [Google Scholar] [CrossRef] [PubMed]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S.; ESMO Guidelines Committee. Prostate cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Cushen, S.J.; Power, D.G.; Murphy, K.P.; McDermott, R.; Griffin, B.T.; Lim, M.; Daly, L.; MacEneaney, P.; O’ Sullivan, K.; Prado, C.M.; et al. Impact of body composition parameters on clinical outcomes in patients with metastatic castrate-resistant prostate cancer treated with docetaxel. Clin. Nutr. ESPEN 2016, 13, e39–e45. [Google Scholar] [CrossRef]
- Planas, M.; Álvarez-Hernández, J.; León-Sanz, M.; Celaya-Pérez, S.; Araujo, K.; Abelardo García de Lorenzo on behalf of the PREDyCES researchers. Prevalence of hospital malnutrition in cancer patients: A sub-analysis of the PREDyCES® study. Support Care Cancer 2016, 24, 429–435. [Google Scholar] [CrossRef]
- Gellrich, N.C.; Handschel, J.; Holtmann, H.; Kruskemper, G. Oral Cancer Malnutrition Impacts Weight and Quality of Life. Nutrients 2015, 7, 2145–2160. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; Initiative, S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Cavka, L.; Perme, M.P.; Zakotnik, B.; Kozjek, N.R.; Seruga, B. 676P Prognostic role of nutritional status (NS) for health-related quality of life (HRQoL) in men with advanced prostate cancer. Ann. Oncol. 2020, 31, S540. [Google Scholar] [CrossRef]
- Cella, D.; Petrylak, D.P.; Fishman, M.; Teigland, C.; Young, J.; Mulani, P. Role of quality of life in men with metastatic hormone-refractory prostate cancer: How does atrasentan influence quality of life? Eur. Urol. 2006, 49, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Roydhouse, J.K.; Gutman, R.; Keating, N.L.; Mor, V.; Wilson, I.B. Proxy and patient reports of health-related quality of life in a national cancer survey. Health Qual. Life Outcomes 2018, 16, 6. [Google Scholar] [CrossRef]
- Morgans, A.K.; van Bommel, A.C.; Stowell, C.; Abrahm, J.L.; Basch, E.; Bekelman, J.E.; Berry, D.L.; Bossi, A.; Davis, I.D.; de Reijke, T.M.; et al. Development of a Standardized Set of Patient-centered Outcomes for Advanced Prostate Cancer: An International Effort for a Unified Approach. Eur. Urol. 2015, 68, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Arndt, V.; Merx, H.; Stegmaier, C.; Ziegler, H.; Brenner, H. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: A population-based study. J. Clin. Oncol. 2004, 22, 4829–4836. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, M.; Basch, E.; Denis, F.; Fallowfield, L.J.; Ganz, P.A.; Howell, D.; Kowalski, C.; Perrone, F.; Stover, A.M.; Sundaresan, P.; et al. The role of patient-reported outcome measures in the continuum of cancer clinical care: ESMO Clinical Practice Guideline. Ann. Oncol. 2022, 33, 878–892. [Google Scholar] [CrossRef]
- Di Maio, M.; Basch, E.; Bryce, J.; Perrone, F. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat. Rev. Clin. Oncol. 2016, 13, 319–325. [Google Scholar] [CrossRef]
- Wheelwright, S.; Darlington, A.S.; Hopkinson, J.B.; Fitzsimmons, D.; White, A.; Johnson, C.D. A systematic review of health-related quality of life instruments in patients with cancer cachexia. Support Care Cancer 2013, 21, 2625–2636. [Google Scholar] [CrossRef]
- Lis, C.G.; Gupta, D.; Lammersfeld, C.A.; Markman, M.; Vashi, P.G. Role of nutritional status in predicting quality of life outcomes in cancer—A systematic review of the epidemiological literature. Nutr. J. 2012, 11, 27. [Google Scholar] [CrossRef]
- Ryan, A.M.; Power, D.G.; Daly, L.; Cushen, S.J.; Bhuachalla, E.N.; Prado, C.M. Cancer-associated malnutrition, cachexia and sarcopenia: The skeleton in the hospital closet 40 years later. Proc. Nutr. Soc. 2016, 75, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Clements, S.; McWilliam, A.; Green, A.; Descamps, T.; Oing, C.; Gillessen, S. Influence of abiraterone and enzalutamide on body composition in patients with metastatic castration resistant prostate cancer. Cancer Treat Res. Commun. 2020, 25, 100256. [Google Scholar] [CrossRef]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Senesse, P.; Gioulbasanis, I.; Antoun, S.; Bozzetti, F.; Deans, C.; Strasser, F.; Thoresen, L.; Jagoe, R.T.; Chasen, M.; et al. Diagnostic criteria for the classification of cancer-associated weight loss. J. Clin. Oncol. 2015, 33, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.; Birdsell, L.; Macdonald, N.; Reiman, T.; Clandinin, M.T.; McCargar, L.J.; Murphy, R.; Ghosh, S.; Sawyer, M.B.; Baracos, V.E. Cancer cachexia in the age of obesity: Skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J. Clin. Oncol. 2013, 31, 1539–1547. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, A.R.; Kamal, A.H.; LeBlanc, T.W.; Ma, J.D.; Baracos, V.E.; Roeland, E.J. Cancer Cachexia: Beyond Weight Loss. J. Oncol. Pract. 2016, 12, 1163–1171. [Google Scholar] [CrossRef]
- Ramos Chaves, M.; Boléo-Tomé, C.; Monteiro-Grillo, I.; Camilo, M.; Ravasco, P. The diversity of nutritional status in cancer: New insights. Oncologist 2010, 15, 523–530. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef]
- Cushen, S.; Power, D.; McEneaney, P.; McLaughlin, P.; Fitzpatrick, F.; Ryan, A. A prospective investigation of nutritional status of ambulatory Irish oncology patients undergoing chemotherapy: Prevalence of malnutrition, cachexia, sarcopenia and impact on quality of life. Eur. J. Cancer 2013, 49, S283–S284. [Google Scholar]
- Guo, Z.Q.; Yu, J.M.; Li, W.; Fu, Z.M.; Lin, Y.; Shi, Y.Y.; Hu, W.; Ba, Y.; Li, S.Y.; Li, Z.N.; et al. Survey and analysis of the nutritional status in hospitalized patients with malignant gastric tumors and its influence on the quality of life. Support Care Cancer 2020, 28, 373–380. [Google Scholar] [CrossRef]
- Baracos, V.E.; Arribas, L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann. Oncol. 2018, 29, ii1–ii9. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C. Cancer cachexia: Developing multimodal therapy for a multidimensional problem. Eur. J. Cancer 2008, 44, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Solheim, T.S.; Laird, B.J.A.; Balstad, T.R.; Bye, A.; Stene, G.; Baracos, V.; Strasser, F.; Griffiths, G.; Maddocks, M.; Fallon, M.; et al. Cancer cachexia: Rationale for the MENAC (Multimodal-Exercise, Nutrition and Anti-inflammatory medication for Cachexia) trial. BMJ Support. Palliat. Care 2018, 8, 258–265. [Google Scholar] [CrossRef]
- Campbell, K.L.; Cormie, P.; Weller, S.; Alibhai, S.M.H.; Bolam, K.A.; Campbell, A.; Cheville, A.L.; Dalzell, M.A.; Hart, N.H.; Higano, C.S.; et al. Exercise Recommendation for People With Bone Metastases: Expert Consensus for Health Care Providers and Exercise Professionals. JCO Oncol. Pract. 2022, 18, e697–e709. [Google Scholar] [CrossRef]
- Galvao, D.A.; Newton, R.U.; Gardiner, R.A.; Girgis, A.; Lepore, S.J.; Stiller, A.; Mihalopolous, C.; Occhipinti, S.; Chambers, S.K. Compliance to exercise-oncology guidelines in prostate cancer survivors and associations with psychological distress, unmet supportive care needs, and quality of life. Psycho-Oncol. 2015, 24, 1241–1249. [Google Scholar] [CrossRef]
- Vanhoutte, G.; van de Wiel, M.; Wouters, K.; Sels, M.; Bartolomeeussen, L.; De Keersmaecker, S.; Verschueren, C.; De Vroey, V.; De Wilde, A.; Smits, E.; et al. Cachexia in cancer: What is in the definition? BMJ Open Gastroenterol. 2016, 3, e000097. [Google Scholar] [CrossRef]
- Grašič Kuhar, C.; Gortnar Cepeda, T.; Kovač, T.; Kukar, M.; Ružić Gorenjec, N. Mobile App for Symptom Management and Associated Quality of Life During Systemic Treatment in Early Stage Breast Cancer: Nonrandomized Controlled Prospective Cohort Study. JMIR Mhealth Uhealth 2020, 8, e17408. [Google Scholar] [CrossRef] [PubMed]



| WN (N = 59) | NR (N = 24) | Sarcopenia (N = 42) | Cachexia (N = 16) | |
|---|---|---|---|---|
| 1st line | N = 59 (100%) | N = 24 (100%) | N = 40 (95.2%) | N = 14 (87.5%) |
| Docetaxel (11) ARSI (38) Cabazitaxel (0) Radium-223 (3) | Docetaxel (6) ARSI (16) Cabazitaxel (0) Radium-223 (2) | Docetaxel (5) ARSI (31) Cabazitaxel (0) Radium (4) | Docetaxel (1) ARSI (10) Cabazitaxel (0) Radium-223 (3) | |
| 2nd line | N = 42 (71.7%) | N = 15 (62.5%) | N = 22 (52.3%) | N = 7 (43.8%) |
| Docetaxel (16) ARSI (14) Cabazitaxel (3) Radium-223 (6) Other * (3) | Docetaxel (7) ARSI (4) Cabazitaxel (1) Radium-223 (3) Other * (0) | Docetaxel (7) ARSI (10) Cabazitaxel (2) Radium-223 (3) Other * (0) | Docetaxel (1) ARSI (4) Cabazitaxel (0) Radium-223(2) Other * (0) | |
| 3rd line | N = 27 (45.7%) | N = 11 (45.8%) | N = 16 (38.1%) | N = 4 (25%) |
| Docetaxel (5) ARSI (11) Cabazitaxel (9) Radium-223 (2) Other * (0) | Docetaxel (1) ARSI (3) Cabazitaxel (5) Radium-223 (2) Other * (0) | Docetaxel (2) ARSI (5) Cabazitaxel (6) Radium-223 (2) Other * (1) | Docetaxel (1) ARSI (0) Cabazitaxel (2) Radium-223 (1) Other * (0) | |
| ˃3 lines | N = 25 (42.4%) | N = 11 (45.8%) | N = 11 (26.2%) | N = 1 (6.2%) |
| Docetaxel (3) ARSI (11) Cabazitaxel (7) Radium-223 (0) Other * (4) | Docetaxel (2) ARSI (4) Cabazitaxel (1) Radium-223 (2) Other * (2) | Docetaxel (2) ARSI (6) Cabazitaxel (1) Radium-223 (2) Other * (0) | Docetaxel (0) ARSI (1) Cabazitaxel (0) Radium-223 (0) Other * (0) |
| Variable | OR [95% CI] | p-Value |
|---|---|---|
| NR vs. WN | 3.45 [1.28 to 9.09] | 0.01 |
| Sarcopenia vs. WN | 1.69 [0.73 to 3.84] | 0.22 |
| Cachexia vs. WN | 4.17 [1.28 to 12.5] | 0.02 |
| Variable | HR [95% CI] | p-Value |
|---|---|---|
| NR vs. WN | 2.04 [1.19–3.49] | ˂0.01 |
| Sarcopenia vs. WN | 2.21 [0.84–2.21] | 0.21 |
| Cachexia vs. WN | 5.54 [1.56–5.41] | ˂0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavka, L.; Pohar Perme, M.; Rotovnik Kozjek, N.; Seruga, B. Prognostic Impact of Nutritional Status on Overall Survival and Health-Related Quality of Life in Men with Advanced Prostate Cancer. Nutrients 2023, 15, 1044. https://doi.org/10.3390/nu15041044
Cavka L, Pohar Perme M, Rotovnik Kozjek N, Seruga B. Prognostic Impact of Nutritional Status on Overall Survival and Health-Related Quality of Life in Men with Advanced Prostate Cancer. Nutrients. 2023; 15(4):1044. https://doi.org/10.3390/nu15041044
Chicago/Turabian StyleCavka, Luka, Maja Pohar Perme, Nada Rotovnik Kozjek, and Bostjan Seruga. 2023. "Prognostic Impact of Nutritional Status on Overall Survival and Health-Related Quality of Life in Men with Advanced Prostate Cancer" Nutrients 15, no. 4: 1044. https://doi.org/10.3390/nu15041044
APA StyleCavka, L., Pohar Perme, M., Rotovnik Kozjek, N., & Seruga, B. (2023). Prognostic Impact of Nutritional Status on Overall Survival and Health-Related Quality of Life in Men with Advanced Prostate Cancer. Nutrients, 15(4), 1044. https://doi.org/10.3390/nu15041044






