Impact of Chemotherapy Regimens on Body Composition of Breast Cancer Women: A Multicenter Study across Four Brazilian Regions
Abstract
1. Introduction
2. Materials and Methods
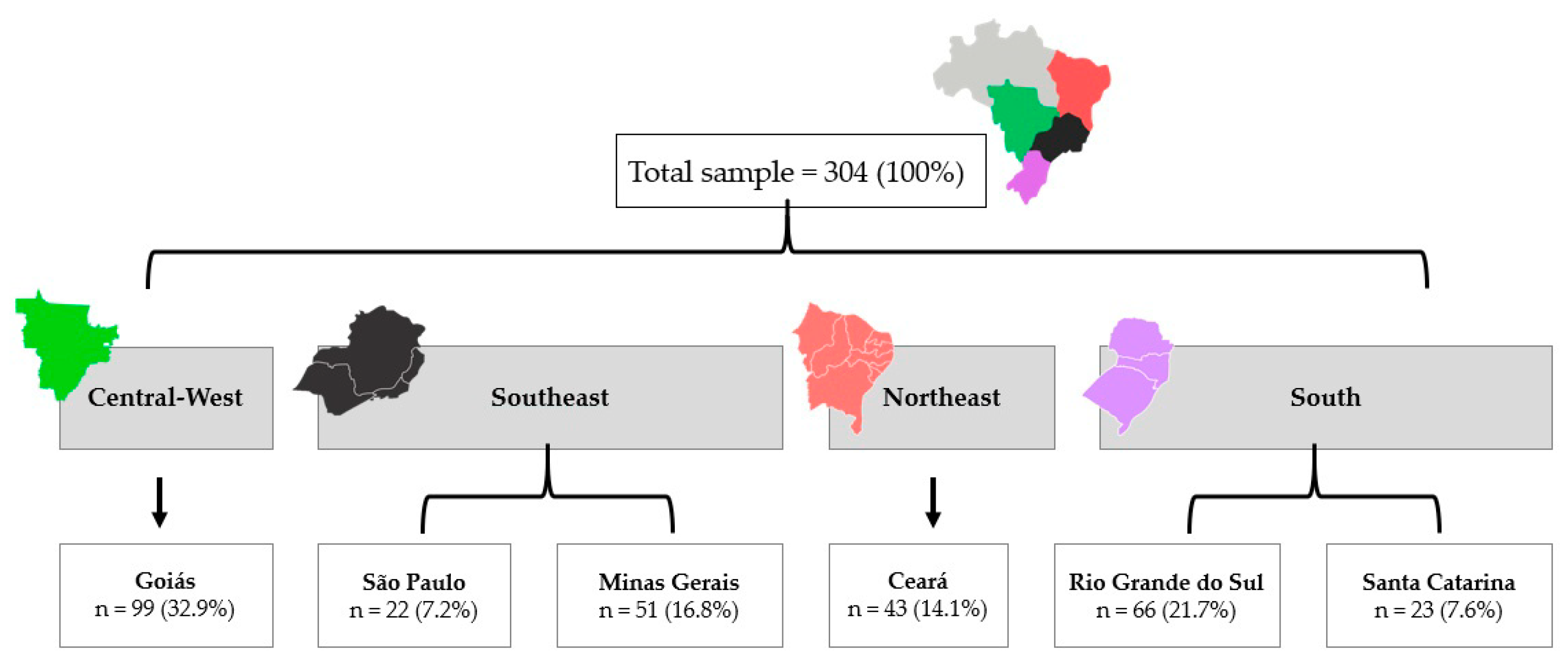
2.1. Study Design and Participants
2.2. Ethics Statement
2.3. Data Collection
2.4. Socioeconomic Status and Tumor-Related Characteristics
2.5. Behavioral Variables
2.6. Anthropometry and Body Composition
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrozo, L.V.; Fornaciali, M.; de André, C.D.S.; Morais, G.A.Z.; Mansur, G.; Cabral-Miranda, W. GeoSES: A socioeconomic index for health and social research in Brazil. PLoS ONE 2020, 15, e0232074. [Google Scholar] [CrossRef] [PubMed]
- Brasil, Ministério da Saúde, Instituto Nacional do Câncer. Estimativa 2023. Incidência de Câncer no Brasil; Instituto Nacional do Câncer: Rio de Janeiro, Brazil, 2022; p. 162.
- Guerra, M.R.; Nogueira, M.C.; Malta, D.C.; Côrrea, C.S.L.; de Souza, M.F.M.; Curado, M.P.; Felisbino-Mendes, M.S.; Mooney, M.; Naghavi, M.; Bustamante-Teixeira, M.T. Inequalities in the burden of female breast cancer in Brazil, 1990–2017. Popul. Health Metr. 2020, 18, 8. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; on behalf of the ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Fell, G.; ree, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.; Alexander, B.; Moy, B.; Isakoff, S.; et al. Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef]
- Fujii, T.; Le Du, F.; Xiao, L.; Kogawa, T.; Barcenas, C.H.; Alvarez, R.H.; Valero, V.; Shen, Y.; Ueno, N. Effectiveness of an Adjuvant Chemotherapy Regimen for Early-Stage Breast Cancer: A Systematic Review and Network Meta-analysis. JAMA Oncol. 2015, 1, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, N.B.; Salek, R.; Shandiz, F.H.; Shahidsales, S. Adjuvant Chemotherapy of Early Stage Breast Cancer in Community-based Cancer Treatment Fields: CMF Compared with Anthracycline/Taxane-based Regimens. Middle East J. Cancer 2017, 8, 83–91. [Google Scholar]
- Godinho-Mota, J.C.M.; Mota, J.F.; Gonçalves, L.V.; Soares, L.R.; Schincaglia, R.M.; Prado, C.M.; Martins, K.A.; Freitas-Junior, R. Chemotherapy negatively impacts body composition, physical function and metabolic profile in patients with breast cancer. Clin. Nutr. 2021, 40, 3421–3428. [Google Scholar] [CrossRef]
- Pedersen, B.; Delmar, C.; Bendtsen, M.D.; Bosaeus, I.; Carus, A.; Falkmer, U.; Groenkjaer, M. Changes in Weight and Body Composition among Women with Breast Cancer During and after Adjuvant Treatment: A Prospective Follow-up Study. Cancer Nurs. 2017, 40, 369–376. [Google Scholar] [CrossRef]
- Van den Berg, M.M.; Winkels, R.M.; de Kruif, J.T.; Van Laarhoven, H.W.; Visser, M.; de Vries, J.H.; de Vries, Y.C.; Kampman, E. Weight change during chemotherapy in breast cancer patients: A meta-analysis. BMC Cancer 2017, 17, 259. [Google Scholar] [CrossRef]
- Fang, Q.; Gan, L.; Chen, Y.Y.; Shen, K.W.; Wu, B.W. Percent Body Fat Change in Chinese Women After Adjuvant Chemotherapy for Breast Cancer. Med. Sci. Monit. 2018, 24, 5988–5995. [Google Scholar] [CrossRef]
- Freedman, R.J.; Aziz, N.; Albanes, D.; Hartman, T.; Danforth, D.; Hill, S.; Sebring, N.; Reynolds, J.C.; Yanovski, J.A. Weight and body composition changes during and after adjuvant chemotherapy in women with breast cancer. J. Clin. Endocrinol. Metab. 2004, 89, 2248–2253. [Google Scholar] [CrossRef]
- Makari-Judson, G.; Braun, B.; Jerry, D.J.; Mertens, W.C. Weight gain following breast cancer diagnosis: Implication and proposed mechanisms. World J. Clin. Oncol. 2014, 5, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Prado, C.M.; Baracos, V.E.; McCargar, L.J.; Reiman, T.; Mourtzakis, M.; Tonkin, K.; Mackey, J.R.; Koski, S.; Pituskin, E.; Sawyer, M.B. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin. Cancer Res. 2009, 15, 2920–2926. [Google Scholar] [CrossRef] [PubMed]
- de Kruif, J.; Visser, M.; Van den Berg, M.; Derks, M.J.M.; de Boer, M.R.; Van Laarhoven, H.W.M.; de Vries, J.H.M.; de Vries, Y.C.; Kampman, E.; Winkels, R.W.; et al. A longitudinal mixed methods study on changes in body weight, body composition, and lifestyle in breast cancer patients during chemotherapy and in a comparison group of women without cancer: Study protocol. BMC Cancer 2019, 19, 7. [Google Scholar] [CrossRef]
- Pedersen, B.; Delmar, C.; Lörincz, T.; Falkmer, U.; Grønkjær, M. Investigating Changes in Weight and Body Composition Among Women in Adjuvant Treatment for Breast Cancer: A Scoping Review. Cancer Nurs. 2019, 42, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Lipscombe, L.L.; Chan, W.W.; Yun, L.; Austin, P.C.; Anderson, G.M.; Rochon, P.A. Incidence of diabetes among postmenopausal breast cancer survivors. Diabetologia 2013, 56, 476–483. [Google Scholar] [CrossRef]
- Willett, W.C.; Manson, J.E.; Stampfer, M.J.; Colditz, G.A.; Rosner, B.; Speizer, F.E.; Hennekens, C.H. Weight, weight change, and coronary heart disease in women. Risk within the ‘normal’ weight range. JAMA 1995, 273, 461–465. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Rimer, B.K.; Winer, E.P. Weight gain in women diagnosed with breast cancer. J. Am. Diet. Assoc. 1997, 97, 519–529. [Google Scholar] [CrossRef]
- Vance, V.; Mourtzakis, M.; McCargar, L.; Hanning, R. Weight gain in breast cancer survivors: Prevalence, pattern and health consequences. Obes. Rev. 2011, 12, 282–294. [Google Scholar] [CrossRef]
- Kirch, W. Encyclopedia of Public Health; Springer: Dordrecht, Germany, 2008. [Google Scholar]
- Godinho-Mota, J.C.M.; Gonçalves, L.V.; Mota, J.F.; Soares, L.R.; Schincaglia, R.M.; Martins, K.A.; Freitas-Junio, R. Sedentary Behavior and Alcohol Consumption Increase Breast Cancer Risk Regardless of Menopausal Status: A Case-Control Study. Nutrients 2019, 11, 1871. [Google Scholar] [CrossRef]
- Wang, J.; Heng, Y.J.; Eliassen, A.H.; Tamimi, R.M.; Hazra, A.; Carey, V.J.; Ambrosone, C.B.; de Andrade, V.P.; Brufsky, A.; Couch, F.J.; et al. Alcohol consumption and breast tumor gene expression. Breast Cancer Res. 2017, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Sjostrom, M.A.B.; Bauman, A.; Bull, F.; Hamilton-Craig, C.; Sallis, J. Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ)–Short and Long Forms. 2005. pp. 1–15. Available online: https://www.researchgate.net/file.PostFileLoader.html?id=5641f4c36143250eac8b45b7&assetKey=AS%3A294237418606593%401447163075131 (accessed on 8 November 2022).
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International physical activity questionnaire: 12-country reliability and validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Habicht, J.-P. Estandarizacion de Metodos Epidemiologicos Cuantitativos Sobre el Terreno. Bol. Oficina Sanit. Panam. 1974, 76, 275–384. [Google Scholar]
- World Health Organization. Obesity: Preventing and Managing the Global Epidemic: Report of a WHO Consulation on Obesity; WHO Technical Report Series 894; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- Lipschitz, D.A. Screening for nutritional status in the elderly. Prim. Care: Clin. Off. Pract. 1994, 21, 55–67. [Google Scholar] [CrossRef]
- Ashwell, M.H.S.D. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int. J. Food Sci. Nutr. 2005, 56, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Valdez, R. A simple model-based index of abdominal adiposity. J. Clin. Epidemiol. 1991, 44, 955–956. [Google Scholar] [CrossRef]
- Vanitallie, T.B.; Yang, M.U.; Heymsfield, S.B.; Funk, R.C.; Boileu, R.A. Height-normalized indices of the body’s fat-free mass and fat mass: Potentially useful indicators of nutritional status. Am. J. Clin. Nutr. 1990, 56, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saúde. Vigitel Brasil 2020–Vigilância De Fatores De Risco E Proteção Para Doenças Crônicas Por Inquérito Telefônico; Ministério da Saúde: Brasília, Brazil, 2021.
- World Cancer Research Fund International. Diet, Nutrition, PHYSICAL activity and Breast Cancer; American Institute for Cancer Research: Arlington, VA, USA, 2018; p. 124. [Google Scholar]
- Godinho-Mota, J.C.M.; Gonçalves, L.V.; Soares, L.R.; Mota, J.F.; Martins, K.A.; Freitas-Junior, I.; Freitas-Junior, R. Abdominal Adiposity and Physical Inactivity Are Positively Associated with Breast Cancer: A Case-Control Study. BioMed Res. Int. 2018, 2018, 4783710. [Google Scholar] [CrossRef]
- Buch-Larsen, K.; Lund-Jacobsen, T.; Andersson, M.; Schwarz, P. Weight Change in Post-Menopausal Women with Breast Cancer during Chemotherapy-Perspectives on Nutrition, Activity and Bone Metabolism: An Interim Analysis of a 5-Year Prospective Cohort. Nutrients 2021, 13, 2902. [Google Scholar] [CrossRef]
- Dorling, J.; Broom, D.R.; Burns, S.F.; Clayton, D.J.; Deighton, K.; James, L.J.; King, J.A.; Miyashita, M.; Thackray, A.E.; Batterham, R.L.; et al. Acute and Chronic Effects of Exercise on Appetite, Energy Intake, and Appetite-Related Hormones: The Modulating Effect of Adiposity, Sex, and Habitual Physical Activity. Nutrients 2018, 10, 1140. [Google Scholar] [CrossRef]
- Makari-Judson, G.; Katz, D.; Barham, R.; Mertens, W. Deleterious Effect of Chemotherapy on Measures of Insulin Resistance in Patients with Newly-Diagnosed Invasive Breast Cancer. Cancer Res. 2009, 69, 24–33. [Google Scholar] [CrossRef]
- Melby, C.L.; Paris, H.L.; Sayer, R.D.; Bell, C.; Hill, J.O. Increasing Energy Flux to Maintain Diet-Induced Weight Loss. Nutrients 2019, 11, 2533. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.R.; Lovejoy, J.C.; Greenway, F.; Ryan, D.; DeJonge, L.; de la Bretonne, J.; Volafova, J.; Bray, G.A. Contributions of total body fat, abdominal subcutaneous adipose tissue compartments, and visceral adipose tissue to the metabolic complications of obesity. Metabolism 2001, 50, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Mourtzakis, M.; Prado, C.M.; Lieffers, J.R.; Reiman, T.; McCargar, L.J.; Baracos, V.E. A practical and precise approach to quantification of body composition in cancer patients using computed tomography images acquired during routine care. Appl. Physiol. Nutr. Metab. 2008, 33, 997–1006. [Google Scholar] [CrossRef]
- Sheean, P.M.; Hoskins, K.; Stolley, M. Body composition changes in females treated for breast cancer: A review of the evidence. Breast Cancer Res. Treat. 2012, 135, 663–680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.M.; Dou, Q.L.; Zeng, Y.; Yang, Y.; Cheng, A.S.K.; Zhang, W.W. Sarcopenia as a predictor of mortality in women with breast cancer: A meta-analysis and systematic review. BMC Cancer 2020, 20, 172. [Google Scholar] [CrossRef]
- Del Rio, G.; Zironi, S.; Valeriani, L.; Menozzi, R.; Bondi, M.; Bertolini, M.; Piccinini, L.; Banzi, M.C.; Federico, M. Weight gain in women with breast cancer treated with adjuvant cyclophosphomide, methotrexate and 5-fluorouracil. Analysis of resting energy expenditure and body composition. Breast Cancer Res. Treat. 2002, 73, 267–273. [Google Scholar] [PubMed]
- Van den Berg, M.; Kok, D.E.; Visser, M.; de Vries, J.H.M.; de Kruif, J.; de Vries, Y.; Posthuma, L.; Sommeijer, D.W.; Timmer-Bonte, A.; Los, M.; et al. Changes in body composition during and after adjuvant or neo-adjuvant chemotherapy in women with breast cancer stage I-IIIB compared with changes over a similar timeframe in women without cancer. Support. Care Cancer 2020, 28, 1685–1693. [Google Scholar] [CrossRef]
| Variables | n (%) |
|---|---|
| Socioeconomic status | |
| Age (Mean ± SD) | 51.30 ± 10.77 |
| Skin Color | |
| White | 162 (53.3) |
| Nonwhite | 142 (46.7) |
| Marital status | |
| With partner | 187 (61.5) |
| Without partner | 117 (38.5) |
| Level of education attained | |
| Grade school incomplete | 65 (21.4) |
| Grade school complete | 16 (5.3) |
| High school incomplete | 18 (5.9) |
| High school complete | 62 (20.4) |
| Undergraduate degree incomplete | 17 (5.6) |
| Undergraduate degree complete | 30 (9.9) |
| Unlettered | 10 (3.3) |
| NR | 86 (28.3) |
| Family income/month by minimum wage | |
| <1 | 54 (17.8) |
| >1 to ≤6 | 130 (42.8) |
| >6 to <10 | 16 (5.3) |
| ≥10 | 8 (2.6) |
| 0 | 3 (1.0) |
| NR | 93 (30.6) |
| Menopausal status | |
| Pre-menopausal | 89 (29.3) |
| Postmenopausal | 122 (40.1) |
| NR | 93 (30.6) |
| Variables | n (%) |
|---|---|
| Morphological types | |
| Ductal | 217 (71.4) |
| Lobular | 11 (3.6) |
| Special type (Mucinous) | 3 (1.0) |
| NR | 73 (24.0) |
| TNM Clinical stage | |
| I | 42 (13.8) |
| II | 176 (57.9) |
| III | 86 (28.3) |
| Grade | |
| G1 | 39 (12.8) |
| G2 | 97 (31.9) |
| G3 | 62 (20.4) |
| Gx | 4 (1.3) |
| NR | 102 (33.6) |
| Estrogen receptor | |
| Positive | 139 (45.7) |
| Negative | 55 (18.1) |
| NR | 110 (36.2) |
| Progesterone receptor | |
| Positive | 123 (40.5) |
| Negative | 71 (23.4) |
| NR | 110 (36.2) |
| HER2 | |
| Positive | 38 (12.5) |
| Negative | 147 (48.4) |
| NR | 119 (39.1) |
| Molecular subtypes | |
| Luminal | 115 (37.8) |
| HER2 | 29 (9.5) |
| Triple Negative | 38 (12.5) |
| NR | 122 (40.1) |
| CT regimen | |
| AC | 91 (29.9) |
| ACT | 148 (48.7) |
| CMF | 35 (11.5) |
| PD | 13 (4.3) |
| NR | 17 (5.6) |
| Surgery type | |
| No surgery | 22 (7.2) |
| Mastectomy | 90 (29.6) |
| Breast-conserving surgery | 102 (33.6) |
| NR | 90 (29.6) |
| Variables | n (%) |
|---|---|
| Alcohol consumption (grams of ethanol/week) | |
| ≥10 | 49 (16.1) |
| <10 | 142 (46.7) |
| NR | 113 (37.2) |
| Smoking status | |
| Current smokers | 25 (8.2) |
| Non-smokers | 167 (54.9) |
| Ex-smokers | 65 (21.4) |
| NR | 47 (15.5) |
| Physical activity (minutes/week) | |
| Active (≥150) | 67 (22.0) |
| Inactive (<150) | 106 (34.9) |
| NR | 131 (43.1) |
| CT Regimen | Interaction * | ||||||
|---|---|---|---|---|---|---|---|
| Dependent Variables | Overall Effect | AC | ACT | CMF | PD | Effects | p |
| BMI (n = 287) | 28.65 ± 0.56 | 28.36 ± 0.44 | 27.85 ± 0.94 | 27.93 ± 1.48 | Time | <0.001 | |
| Pre-CT | 27.92 ± 0.48 a | 28.34 ± 0.56 | 28.14 ± 0.45 | 27.63 ± 0.95 | 27.56 ± 1.49 | Regimen | 0.885 |
| Post-CT | 28.47 ± 0.48 b | 28.96 ± 0.57 | 28.58 ± 0.45 | 28.06 ± 0.95 | 28.29 ± 1.49 | Time * Regimen | 0.802 |
| ∆ | 0.50 ± 0.09 | 0.62 ± 0.13 | 0.45 ± 0.15 | 0.43 ± 0.18 | 0.71 ± 0.50 | ||
| WC * (n = 173) | 89.48 ± 2.54 | 91.61 ± 1.11 | 84.31 ± 5.17 | 92.16 ± 3.82 | Time | 0.248 | |
| Pre-CT | 88.92 ± 1.78 | 89.24 ± 2.57 | 91.05 ± 1.13 | 83.55 ± 5.25 | 91.84 ± 3.87 | Regimen | 0.495 |
| Post-CT | 89.86 ± 1.82 | 89.72 ± 2.64 | 92.16 ± 1.16 | 85.08 ± 5.37 | 92.49 ± 3.97 | Time * Regimen | 0.956 |
| ∆ | 1.02 ± 0.46 | 0.48 ± 0.91 | 1.14 ± 0.58 | 1.53 ± 1.70 | 0.65 ± 1.60 | ||
| WHtR * (n = 173) | 0.56 ± 0.02 | 0.58 ± 0.01 | 0.55 ± 0.04 | 0.59 ± 0.03 | Time | 0.950 | |
| Pre-CT | 0.57 ± 0.01 | 0.56 ± 0.02 | 0.58 ± 0.01 | 0.55 ± 0.04 | 0.59 ± 0.03 | Regimen | 0.554 |
| Post-CT | 0.57 ± 0.01 | 0.57 ± 0.02 | 0.59 ± 0.01 | 0.55 ± 0.04 | 0.58 ± 0.03 | Time * Regimen | 0.869 |
| ∆ | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.01 ± 0.01 | ||
| C-index * (n = 173) | 1.25 ± 0.02 | 1.26 ± 0.01 | 1.24 ± 0.04 | 1.25 ± 0.03 | Time | 0.730 | |
| Pre-CT | 1.25 ± 0.13 | 1.25 ± 0.02 | 1.26 ± 0.01 | 1.22 ± 0.04 | 1.26 ± 0.03 | Regimen | 0.807 |
| Post-CT | 1.25 ± 0.13 | 1.24 ± 0.02 | 1.26 ± 0.01 | 1.25 ± 0.04 | 1.25 ± 0.03 | Time * Regimen | 0.671 |
| ∆ | 0.00 ± 0.00 | −0.01 ± 0.01 | 0.00 ± 0.01 | 0.03 ± 0.02 | −0.01 ± 0.02 | ||
| FMI † (n = 213) | 10.54 ± 0.44 | 11.76 ± 0.35 | 9.97 ± 0.71 | 8.98 ± 2.12 | Time | 0.007 | |
| Pre-CT | 10.02 ± 0.59 a | 10.44 ± 0.45 | 11.48 ± 0.36 a | 9.90 ± 0.72 | 8.27 ± 2.16 | Regimen | 0.040 |
| Post-CT | 10.60 ± 0.59 b | 10.63 ± 0.45 | 12.04 ± 0.36 b | 10.05 ± 0.72 | 9.70 ± 2.16 | Time * Regimen | 0.121 |
| ∆ | 0.36 ± 0.10 | 0.17 ± 0.14 | 0.56 ± 0.15 | 0.15 ± 0.13 | 1.44 ± 2.24 | ||
| FFMI † (n = 213) | 18.04 ± 0.28 a | 14.87 ± 0.22 b | 18.21 ± 0.45 a | 16.40 ± 1.34 a,b | Time | 0.405 | |
| Pre-CT | 16.82 ± 0.37 | 17.87 ± 0.28 a | 14.89 ± 0.23 | 18.05 ± 0.46 | 16.46 ± 1.36 | Regimen | <0.001 |
| Post-CT | 16.95 ± 0.37 | 18.21 ± 0.29 b | 14.86 ± 0.23 | 18.38 ± 0.46 | 16.34 ± 1.36 | Time * Regimen | 0.06 |
| ∆ | 0.15 ± 0.07 | 0.34 ± 0.12 | −0.04 ± 0.10 | 0.34 ± 0.16 | −0.12 ± 0.12 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Godinho-Mota, J.C.M.; Vaz-Gonçalves, L.; Dias Custódio, I.D.; Schroeder de Souza, J.; Mota, J.F.; Gonzalez, M.C.; Rodrigues Vilella, P.; Anusca Martins, K.; Paiva Maia, Y.C.d.; Verde, S.M.M.L.; et al. Impact of Chemotherapy Regimens on Body Composition of Breast Cancer Women: A Multicenter Study across Four Brazilian Regions. Nutrients 2023, 15, 1689. https://doi.org/10.3390/nu15071689
Godinho-Mota JCM, Vaz-Gonçalves L, Dias Custódio ID, Schroeder de Souza J, Mota JF, Gonzalez MC, Rodrigues Vilella P, Anusca Martins K, Paiva Maia YCd, Verde SMML, et al. Impact of Chemotherapy Regimens on Body Composition of Breast Cancer Women: A Multicenter Study across Four Brazilian Regions. Nutrients. 2023; 15(7):1689. https://doi.org/10.3390/nu15071689
Chicago/Turabian StyleGodinho-Mota, Jordana Carolina Marques, Larissa Vaz-Gonçalves, Isis Danyelle Dias Custódio, Jaqueline Schroeder de Souza, João Felipe Mota, Maria Cristina Gonzalez, Priscylla Rodrigues Vilella, Karine Anusca Martins, Yara Cristina de Paiva Maia, Sara Maria Moreira Lima Verde, and et al. 2023. "Impact of Chemotherapy Regimens on Body Composition of Breast Cancer Women: A Multicenter Study across Four Brazilian Regions" Nutrients 15, no. 7: 1689. https://doi.org/10.3390/nu15071689
APA StyleGodinho-Mota, J. C. M., Vaz-Gonçalves, L., Dias Custódio, I. D., Schroeder de Souza, J., Mota, J. F., Gonzalez, M. C., Rodrigues Vilella, P., Anusca Martins, K., Paiva Maia, Y. C. d., Verde, S. M. M. L., Frenzel, A. P., Di Pietro, P. F., Costa Marinho, E. d., & Freitas-Junior, R. (2023). Impact of Chemotherapy Regimens on Body Composition of Breast Cancer Women: A Multicenter Study across Four Brazilian Regions. Nutrients, 15(7), 1689. https://doi.org/10.3390/nu15071689







