Purine Intake and All-Cause Mortality in Ovarian Cancer: Results from a Prospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Data Collection
2.2.1. Dietary-Exposure Assessment
2.2.2. Immunohistochemistry (IHC)
2.3. Statistical Analysis
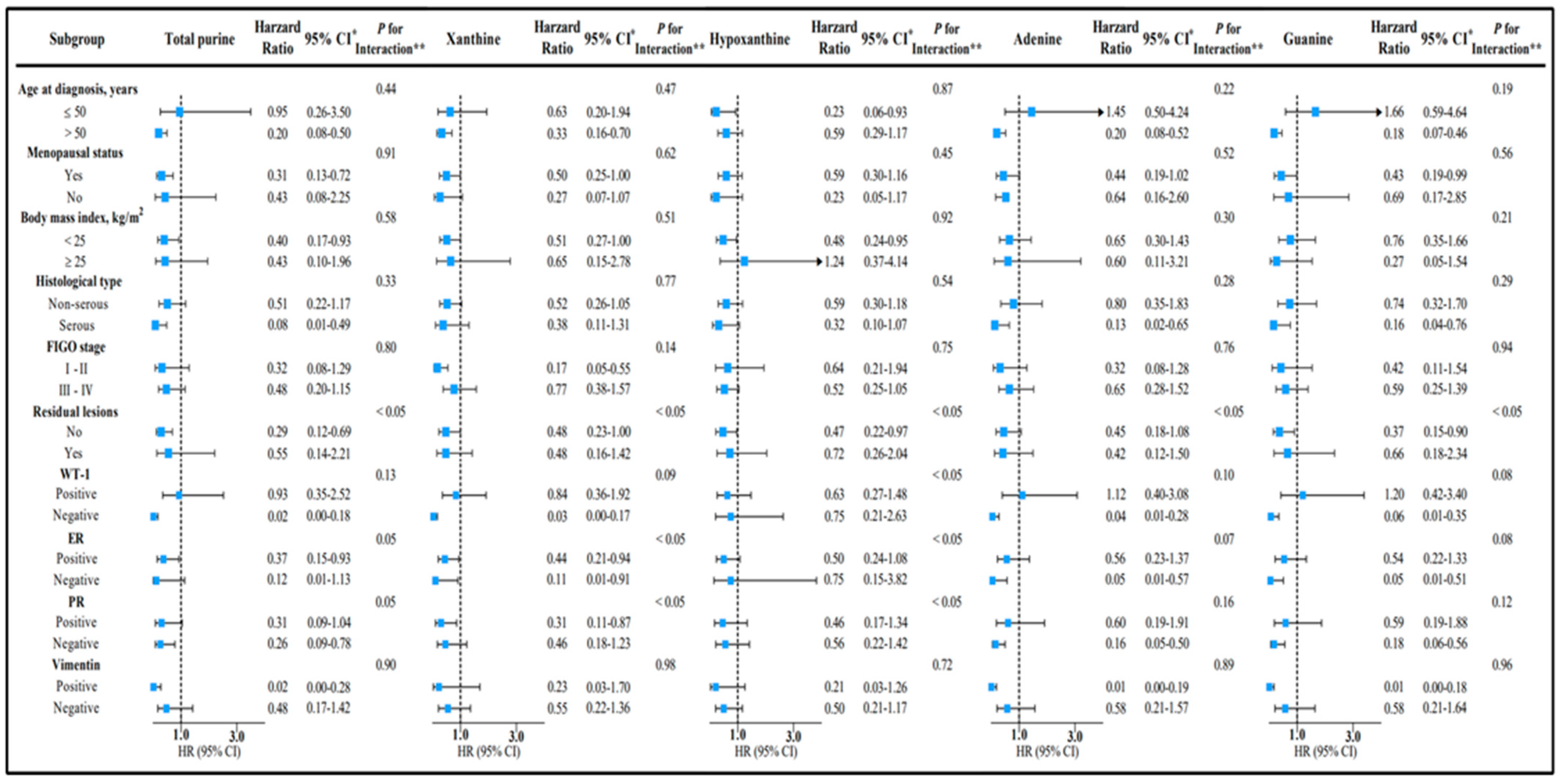
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Doubeni, C.A.; Doubeni, A.R.; Myers, A.E. Diagnosis and Management of Ovarian Cancer. Am. Fam. Physician 2016, 93, 937–944. [Google Scholar] [PubMed]
- Tossetta, G. Metformin Improves Ovarian Cancer Sensitivity to Paclitaxel and Platinum-Based Drugs: A Review of In Vitro Findings. Int. J. Mol. Sci. 2022, 23, 12893. [Google Scholar] [CrossRef] [PubMed]
- Matz, M.; Coleman, M.P.; Carreira, H.; Salmerón, D.; Chirlaque, M.D.; Allemani, C.; Bouzbid, S.; Hamdi-Chérif, M.; Zaidi, Z.; Bah, E.; et al. Worldwide comparison of ovarian cancer survival: Histological group and stage at diagnosis (CONCORD-2). Gynecol. Oncol. 2016, 144, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Yancik, R. Ovarian cancer. Age contrasts in incidence, histology, disease stage at diagnosis, and mortality. Cancer 1993, 71 (Suppl. 2), 517–523. [Google Scholar] [CrossRef] [PubMed]
- Anuradha, S.; Webb, P.M.; Blomfield, P.; Brand, A.H.; Friedlander, M.; Leung, Y.; Obermair, A.; Oehler, M.K.; Quinn, M.; Steer, C.; et al. Survival of Australian women with invasive epithelial ovarian cancer: A population-based study. Med. J. Aust. 2014, 201, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.R.; Shih, I. Ovarian cancer. Annu. Rev. Pathol. 2009, 4, 287–313. [Google Scholar] [CrossRef]
- Kossaï, M.; Leary, A.; Scoazec, J.-Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2017, 85, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Marzioni, D. Natural and synthetic compounds in Ovarian Cancer: A focus on NRF2/KEAP1 pathway. Pharmacol. Res. 2022, 183, 106365. [Google Scholar] [CrossRef] [PubMed]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.-F.; Hao, Y.-Y.; Gao, S.; Li, X.-Q.; Liu, F.-H.; Wen, Z.-Y.; Wang, H.-Y.; Zhang, S.; Yan, S.; Luan, M.; et al. Pre-diagnosis Cruciferous Vegetables and Isothiocyanates Intake and Ovarian Cancer Survival: A Prospective Cohort Study. Front. Nutr. 2021, 8, 778031. [Google Scholar] [CrossRef]
- Jiang, L.; Gong, T.-T.; Gao, S.; Li, X.-Q.; Liu, F.-H.; Wen, Z.-Y.; Wei, Y.-F.; Yan, S.; Hou, R.; Wu, Q.-J. Pre-diagnosis Dairy Product Intake and Ovarian Cancer Mortality: Results From the Ovarian Cancer Follow-Up Study (OOPS). Front. Nutr. 2021, 8, 750801. [Google Scholar] [CrossRef]
- Wen, Z.-Y.; Liu, C.; Liu, F.-H.; Wei, Y.-F.; Xu, H.-L.; Wang, R.; Li, X.-Y.; Li, Y.-Z.; Yan, S.; Qin, X.; et al. Association between pre-diagnostic dietary pattern and survival of ovarian cancer: Evidence from a prospective cohort study. Clin. Nutr. 2021, 41, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Adinolfi, E. Extracellular purines, purinergic receptors and tumor growth. Oncogene 2016, 36, 293–303. [Google Scholar] [CrossRef]
- Peden, D.B.; Hohman, R.; Brown, M.E.; Mason, R.T.; Berkebile, C.; Fales, H.M.; Kaliner, M.A. Uric acid is a major antioxidant in human nasal airway secretions. Proc. Natl. Acad. Sci. USA 1990, 87, 7638–7642. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef] [PubMed]
- Nelson, K.L.; Voruganti, V.S. Purine metabolites and complex diseases: Role of genes and nutrients. Curr. Opin. Clin. Nutr. Metab. Care 2021, 24, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.J.; Gomez-Pinilla, F.; Nagel, M.; Nakagawa, T.; Rodriguez-Iturbe, B.; Sanchez-Lozada, L.G.; Tolan, D.R.; Lanaspa, M.A. Cerebral Fructose Metabolism as a Potential Mechanism Driving Alzheimer’s Disease. Front. Aging Neurosci. 2020, 12, 560865. [Google Scholar] [CrossRef]
- Losenkova, K.; Zuccarini, M.; Karikoski, M.; Laurila, J.; Boison, D.; Jalkanen, S.; Yegutkin, G.G. Compartmentalization of adenosine metabolism in cancer cells and its modulation during acute hypoxia. J. Cell Sci. 2020, 133. [Google Scholar] [CrossRef] [PubMed]
- De Vitto, H.; Arachchige, D.B.; Richardson, B.C.; French, J.B. The Intersection of Purine and Mitochondrial Metabolism in Cancer. Cells 2021, 10, 2603. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wu, Q.; Xia, Y.; Zhang, H.; Gao, S.; Zhang, Y.; Yuan, Y.; Jiang, J.; Qiu, H.; Li, J.; et al. Cohort Profile: The Northeast China Biobank (NEC-Biobank). Leuk. Res. 2022. [Google Scholar] [CrossRef]
- Gong, T.-T.; Liu, F.-H.; Liu, Y.-S.; Yan, S.; Xu, H.-L.; He, X.-H.; Wei, Y.-F.; Qin, X.; Gao, S.; Zhao, Y.-H.; et al. A Follow-Up Study of Ovarian Cancer (OOPS): A Study Protocol. Front. Nutr. 2022, 9, 872773. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, M.; Yuan, C.; Sato, K.; Niedzwiecki, D.; Ye, X.; Saltz, L.; Mayer, R.; Mowat, R.; Whittom, R.; Hantel, A.; et al. Predicted vitamin D status and colon cancer recurrence and mortality in CALGB 89803 (Alliance). Ann. Oncol. 2017, 28, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, Y.; Liu, Y.; Sun, Y.; Ye, K.; Li, W.; Wu, Q.; Chang, Q.; Zhao, Y. Validity and reproducibility of a FFQ for assessing dietary intake among residents of northeast China: Northeast cohort study of China. Br. J. Nutr. 2022, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-X.; Wang, G.-Y.; He, M. China Food Composition, (Standard Edition); Peking University Medical Press: Beijing, China, 2018. [Google Scholar]
- Li, R.; Chambless, L. Test for Additive Interaction in Proportional Hazards Models. Ann. Epidemiol. 2007, 17, 227–236. [Google Scholar] [CrossRef]
- Tomova, G.D.; Arnold, K.F.; Gilthorpe, M.S.; Tennant, P.W.G. Adjustment for energy intake in nutritional research: A causal inference perspective. Am. J. Clin. Nutr. 2021, 115, 189–198. [Google Scholar] [CrossRef]
- Desquilbet, L.; Mariotti, F. Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med. 2010, 29, 1037–1057. [Google Scholar] [CrossRef] [PubMed]
- Ciebiera, M.; Esfandyari, S.; Siblini, H.; Prince, L.; Elkafas, H.; Wojtyła, C.; Al-Hendy, A.; Ali, M. Nutrition in Gynecological Diseases: Current Perspectives. Nutrients 2021, 13, 1178. [Google Scholar] [CrossRef]
- Camandola, S.; Plick, N.; Mattson, M.P. Impact of Coffee and Cacao Purine Metabolites on Neuroplasticity and Neurodegenerative Disease. Neurochem. Res. 2018, 44, 214–227. [Google Scholar] [CrossRef]
- Trudel, D.; Labbé, D.P.; Bairati, I.; Fradet, V.; Bazinet, L.; Têtu, B. Green tea for ovarian cancer prevention and treatment: A systematic review of the in vitro, in vivo and epidemiological studies. Gynecol. Oncol. 2012, 126, 491–498. [Google Scholar] [CrossRef]
- Zhang, M.; Binns, C.W.; Lee, A.H. Tea consumption and ovarian cancer risk: A case-control study in China. Cancer Epidemiol. Biomark. Prev. 2002, 11, 713–718. [Google Scholar]
- Gosvig, C.F.; Kjaer, S.K.; Blaakær, J.; Høgdall, E.; Høgdall, C.; Jensen, A. Coffee, tea, and caffeine consumption and risk of epithelial ovarian cancer and borderline ovarian tumors: Results from a Danish case-control study. Acta Oncol. 2015, 54, 1144–1151. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The enigmatic effects of caffeine in cell cycle and cancer. Cancer Lett. 2007, 247, 26–39. [Google Scholar] [CrossRef]
- Fini, M.A.; Elias, A.; Johnson, R.J.; Wright, R.M. Contribution of uric acid to cancer risk, recurrence, and mortality. Clin. Transl. Med. 2012, 1, 16. [Google Scholar] [CrossRef]
- Di Virgilio, F. Purines, purinergic receptors, and cancer. Cancer Res. 2012, 72, 5441–5447. [Google Scholar] [CrossRef]
- Fredholm, B.B. Adenosine, an endogenous distress signal, modulates tissue damage and repair. Cell Death Differ. 2007, 14, 1315–1323. [Google Scholar] [CrossRef]
- Junger, W.G. Immune cell regulation by autocrine purinergic signalling. Nat. Rev. Immunol. 2011, 11, 201–212. [Google Scholar] [CrossRef]
- Lu, J.-J.; Abudukeyoumu, A.; Zhang, X.; Liu, L.-B.; Li, M.-Q.; Xie, F. Heme oxygenase 1: A novel oncogene in multiple gynecological cancers. Int. J. Biol. Sci. 2021, 17, 2252–2261. [Google Scholar] [CrossRef] [PubMed]
- Strasak, A.M.; Rapp, K.; Hilbe, W.; Oberaigner, W.; Ruttmann, E.; Concin, H.; Diem, G.; Pfeiffer, K.P.; Ulmer, H. The role of serum uric acid as an antioxidant protecting against cancer: Prospective study in more than 28 000 older Austrian women. Ann. Oncol. 2007, 18, 1893–1897. [Google Scholar] [CrossRef] [PubMed]
- Wayne, S.J.; Lopez, S.T.; Butler, L.M.; Baumgartner, K.B.; Baumgartner, R.N.; Ballard-Barbash, R. Changes in dietary intake after diagnosis of breast cancer. J. Am. Diet. Assoc. 2004, 104, 1561–1568. [Google Scholar] [CrossRef] [PubMed]
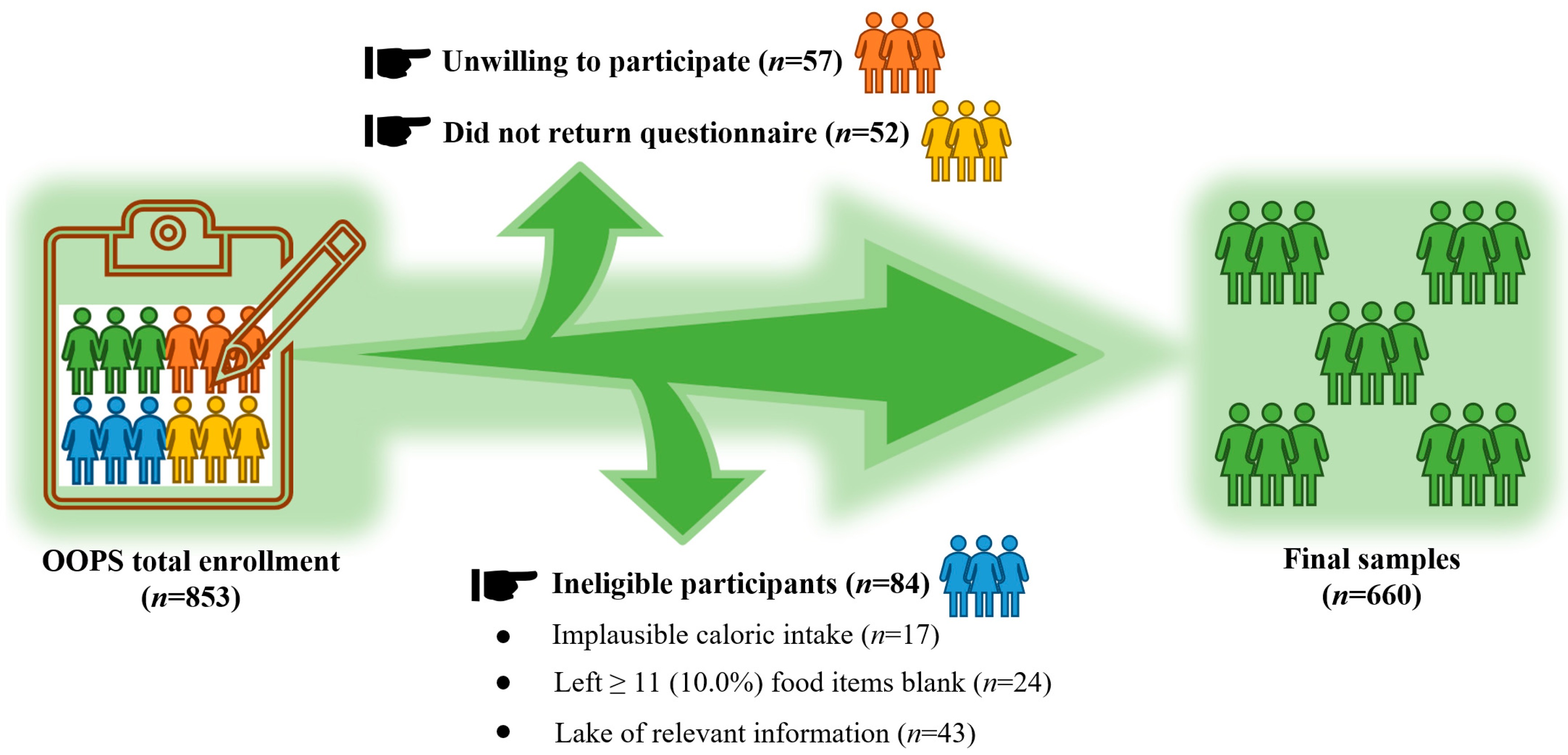
| Characteristics | All Patients | Terciles of Total Purine Intake | p Value | ||
| I (n = 234) | II (n = 234) | III (n = 235) | |||
| Range (mg/day) | <179.48 | 179.48–<189.05 | ≥189.05 | ||
| Median (IQR) age at diagnosis (years) | 53.00 (12.00) | 53.00 (12.00) | 53.00 (12.00) | 54.00 (13.00) | 0.98 |
| Median (IQR) follow-up time (months) | 31.20 (26.84) | 31.28 (24.73) | 29.90 (26.00) | 33.67 (27.67) | 0.14 |
| Median (IQR) body-mass index (kg/m2) | 23.30 (4.20) | 23.30 (3.60) | 22.90 (4.20) | 23.30 (5.00) | 0.19 |
| Median (IQR) physical activity (MET h/d) | 14.10 (15.70) | 14.60 (15.50) | 12.55 (15.40) | 14.70 (15.70) | 0.33 |
| Ever smoked cigarettes | 68 (9.67) | 24 (10.26) | 23 (9.83) | 21 (8.94) | 0.89 |
| Ever consumed alcohol | 149 (21.19) | 38 (16.24) | 56 (23.93) | 55 (23.40) | 0.08 |
| Ever experienced menopause | 508 (72.26) | 167 (71.37) | 168 (71.79) | 173 (73.62) | 0.85 |
| Parity | <0.05 | ||||
| ≤1 | 505 (71.83) | 154 (65.81) | 175 (74.79) | 176 (74.89) | |
| ≥2 | 198 (28.17) | 80 (34.19) | 59 (25.21) | 59 (25.11) | |
| Educational level | 0.11 | ||||
| Junior secondary or below | 375 (53.34) | 138 (58.97) | 120 (51.28) | 117 (49.79) | |
| Senior high school/technical secondary school | 147 (20.91) | 44 (18.81) | 57 (24.36) | 46 (19.57) | |
| Junior college/university or above | 181 (25.75) | 52 (22.22) | 57 (24.36) | 72 (30.64) | |
| Income per month (CNY) | 0.06 | ||||
| <5000 | 421 (59.89) | 157 (67.09) | 133 (56.84) | 131 (55.74) | |
| 5000 to <10000 | 194 (27.60) | 49 (20.94) | 70 (29.91) | 75 (31.92) | |
| ≥10000 | 88 (12.51) | 28 (11.97) | 31 (13.25) | 29 (12.34) | |
| Ever changed diet | 0.12 | ||||
| No | 535 (76.10) | 189 (80.77) | 172 (73.50) | 174 (74.04) | |
| Yes | 168 (23.90) | 45 (19.23) | 62 (26.50) | 61 (25.96) | |
| Mean (SD) total energy intake (kcal/d) | 1455.75 (552.64) | 1028.14 (282.69) | 1368.28 (332.36) | 1968.65 (521.53) | <0.05 |
| Mean (SD) total fatty-acid intake (g/d) | 23.81 (13.94) | 12.78 (5.23) | 21.69 (7.78) | 36.90 (14.05) | <0.05 |
| Mean (SD) cholesterol intake (mg/d) | 355.75 (217.67) | 215.69 (158.40) | 330.71 (164.44) | 520.15 (206.99) | <0.05 |
| Mean (SD) total purine intake (mg/d) | 260.26 (140.67) | 129.83 (34.66) | 231.54 (31.34) | 418.73 (118.87) | <0.05 |
| Mean (SD) xanthine intake (mg/d) | 14.77 (8.49) | 7.65 (3.04) | 13.68 (4.35) | 22.97 (8.30) | <0.05 |
| Mean (SD) hypoxanthine (mg/d) | 62.87 (42.80) | 29.22 (14.62) | 56.05 (21.53) | 103.19 (44.82) | <0.05 |
| Mean (SD) adenine intake (mg/d) | 90.42 (49.63) | 46.19 (13.38) | 80.33 (15.47) | 144.53 (44.43) | <0.05 |
| Mean (SD) guanine intake (mg/d) | 92.13 (50.92) | 46.75 (13.86) | 81.45 (14.56) | 147.94 (45.56) | <0.05 |
| Characteristics | Deaths (% of Total) | Model 1 | Model 2 | Model 3 | |
|---|---|---|---|---|---|
| Total Purine (mg/day) | T1 (<179.78) | 47 (36.15) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (179.78–<289.05) | 51 (39.23) | 1.08 (0.73–1.61) | 1.03 (0.68–1.56) | 0.88 (0.56–1.37) | |
| T3 (≥289.05) | 32 (24.62) | 0.63 (0.40–0.99) | 0.67 (0.42–1.08) | 0.39 (0.19–0.80) | |
| Continuous (per SD) | 130 (100.00) | 0.89 (0.75–1.07) | 0.90 (0.75–1.08) | 0.60 (0.41–0.88) | |
| p for trend ** | <0.05 | 0.08 | <0.05 | ||
| Xanthine (mg/day) | T1 (<9.69) | 51 (39.23) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (9.69–<16.82) | 41 (31.54) | 0.75 (0.50–1.13) | 0.84 (0.55–1.28) | 0.80 (0.51–1.25) | |
| T3 (≥16.82) | 38 (29.23) | 0.67 (0.44–1.02) | 0.68 (0.44–1.05) | 0.52 (0.29–0.94) | |
| Continuous (per SD) | 130 (100.00) | 0.91 (0.76–1.09) | 0.91 (0.76–1.09) | 0.80 (0.60–1.06) | |
| p for trend ** | 0.07 | 0.09 | <0.05 | ||
| Hypoxanthine (mg/day) | T1 (<38.35) | 49 (37.69) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (38.35–<71.43) | 45 (34.62) | 0.88 (0.59–1.33) | 0.94 (0.62–1.42) | 0.90 (0.58–1.39) | |
| T3 (≥71.43) | 36 (27.69) | 0.67 (0.43–1.03) | 0.71 (0.45–1.10) | 0.59 (0.33–1.06) | |
| Continuous (per SD) | 130 (100.00) | 0.87 (0.73–1.05) | 0.89 (0.74–1.06) | 0.82 (0.63–1.07) | |
| p for trend ** | 0.06 | 0.12 | 0.07 | ||
| Adenine (mg/day) | T1 (<61.60) | 45 (34.62) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (61.60–<100.11) | 50 (38.46) | 1.11 (0.74–1.67) | 1.16 (0.77–1.75) | 1.08 (0.69–1.69) | |
| T3 (≥100.11) | 35 (26.92) | 0.76 (0.49–1.18) | 0.80 (0.51–1.27) | 0.54 (0.27–1.06) | |
| Continuous (per SD) | 130 (100.00) | 0.91 (0.76–1.09) | 0.92 (0.77–1.10) | 0.60 (0.41–0.87) | |
| p for trend ** | 0.17 | 0.27 | 0.06 | ||
| Guanine (mg/day) | T1 (<63.59) | 44 (33.85) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| T2 (63.59–<101.59) | 51 (39.23) | 1.16 (0.78–1.74) | 1.13 (0.75–1.71) | 1.06 (0.67–1.65) | |
| T3 (≥101.59) | 35 (26.92) | 0.77 (0.49–1.19) | 0.79 (0.50–1.25) | 0.54 (0.27–1.08) | |
| Continuous (per SD) | 130 (100.00) | 0.91 (0.76–1.09) | 0.92 (0.77–1.11) | 0.64 (0.44–0.92) | |
| p for trend ** | 0.18 | 0.26 | 0.08 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Z.; Gong, T.; Wei, Y.; Zheng, G.; Zhao, J.; Zou, B.; Qin, X.; Yan, S.; Liu, F.; Xiao, Q.; et al. Purine Intake and All-Cause Mortality in Ovarian Cancer: Results from a Prospective Cohort Study. Nutrients 2023, 15, 931. https://doi.org/10.3390/nu15040931
Du Z, Gong T, Wei Y, Zheng G, Zhao J, Zou B, Qin X, Yan S, Liu F, Xiao Q, et al. Purine Intake and All-Cause Mortality in Ovarian Cancer: Results from a Prospective Cohort Study. Nutrients. 2023; 15(4):931. https://doi.org/10.3390/nu15040931
Chicago/Turabian StyleDu, Zongda, Tingting Gong, Yifan Wei, Gang Zheng, Junqi Zhao, Bingjie Zou, Xue Qin, Shi Yan, Fanghua Liu, Qian Xiao, and et al. 2023. "Purine Intake and All-Cause Mortality in Ovarian Cancer: Results from a Prospective Cohort Study" Nutrients 15, no. 4: 931. https://doi.org/10.3390/nu15040931
APA StyleDu, Z., Gong, T., Wei, Y., Zheng, G., Zhao, J., Zou, B., Qin, X., Yan, S., Liu, F., Xiao, Q., Wu, Q., Gao, S., & Zhao, Y. (2023). Purine Intake and All-Cause Mortality in Ovarian Cancer: Results from a Prospective Cohort Study. Nutrients, 15(4), 931. https://doi.org/10.3390/nu15040931








