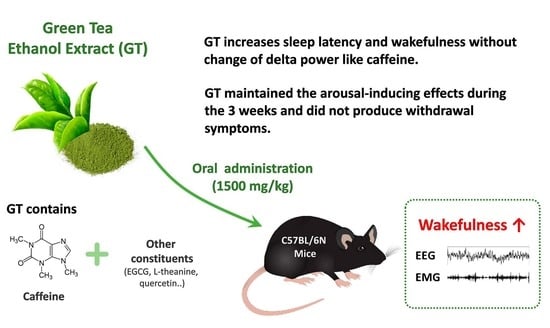
Effects of Chronic Administration of Green Tea Ethanol Extract on Sleep Architecture in Mice: A Comparative Study with a Representative Stimulant Caffeine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of GT
2.3. Animals
2.4. HPLC Analysis Conditions of GT
2.4.1. Preparation of Sample Solution
2.4.2. HPLC Analysis of Caffeine and GT
2.5. Analysis of Sleep Architecture and Sleep–Wake Profile
2.5.1. Surgical Procedure
2.5.2. Pharmacological Treatment
2.5.3. Electroencephalography and Electromyography Recordings
2.5.4. Sleep–Wake State Analysis
2.6. Statistical Analysis
3. Results
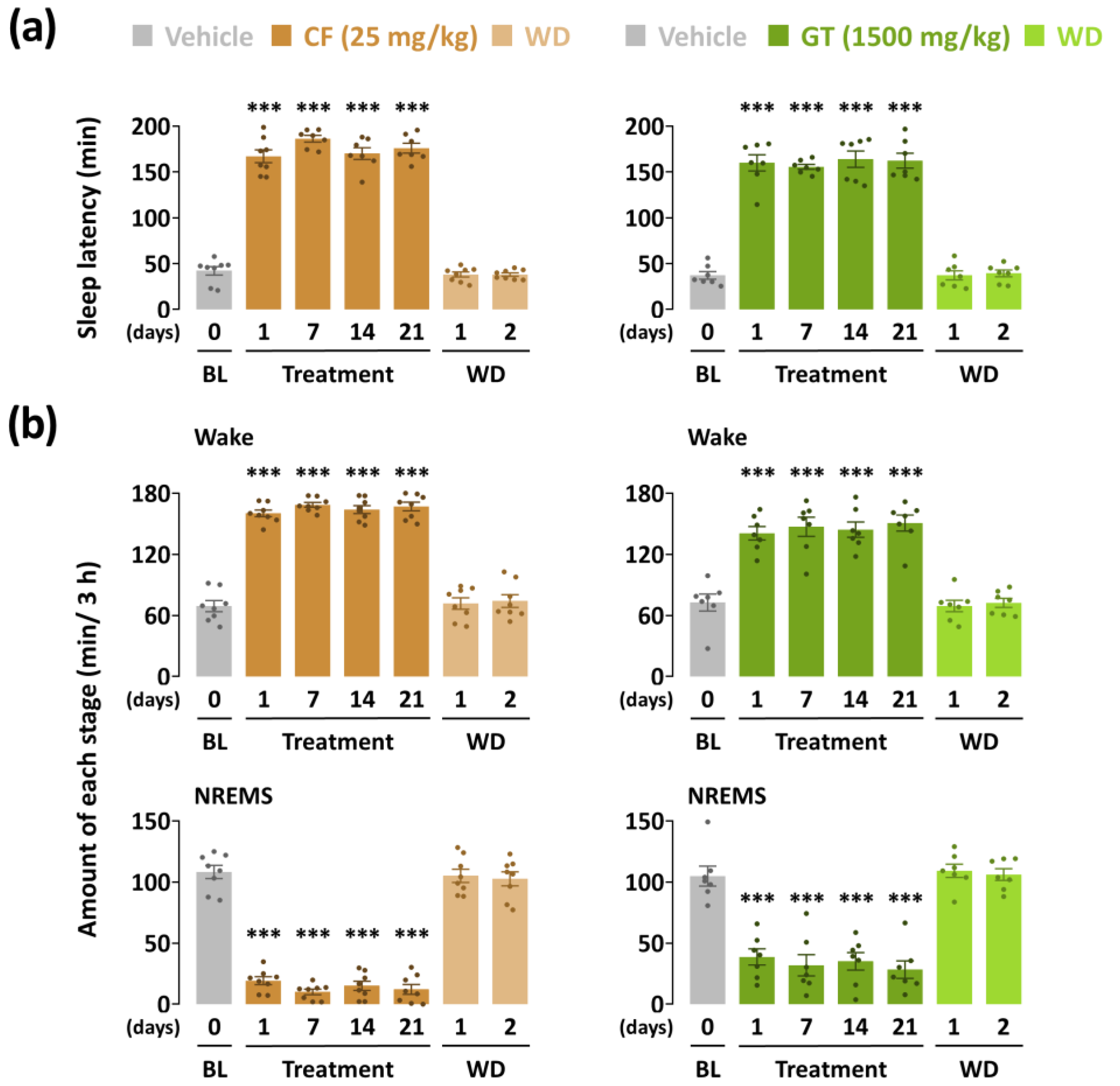
3.1. Effects of Chronic Administration of GT on Sleep Latency and NREMS Amount
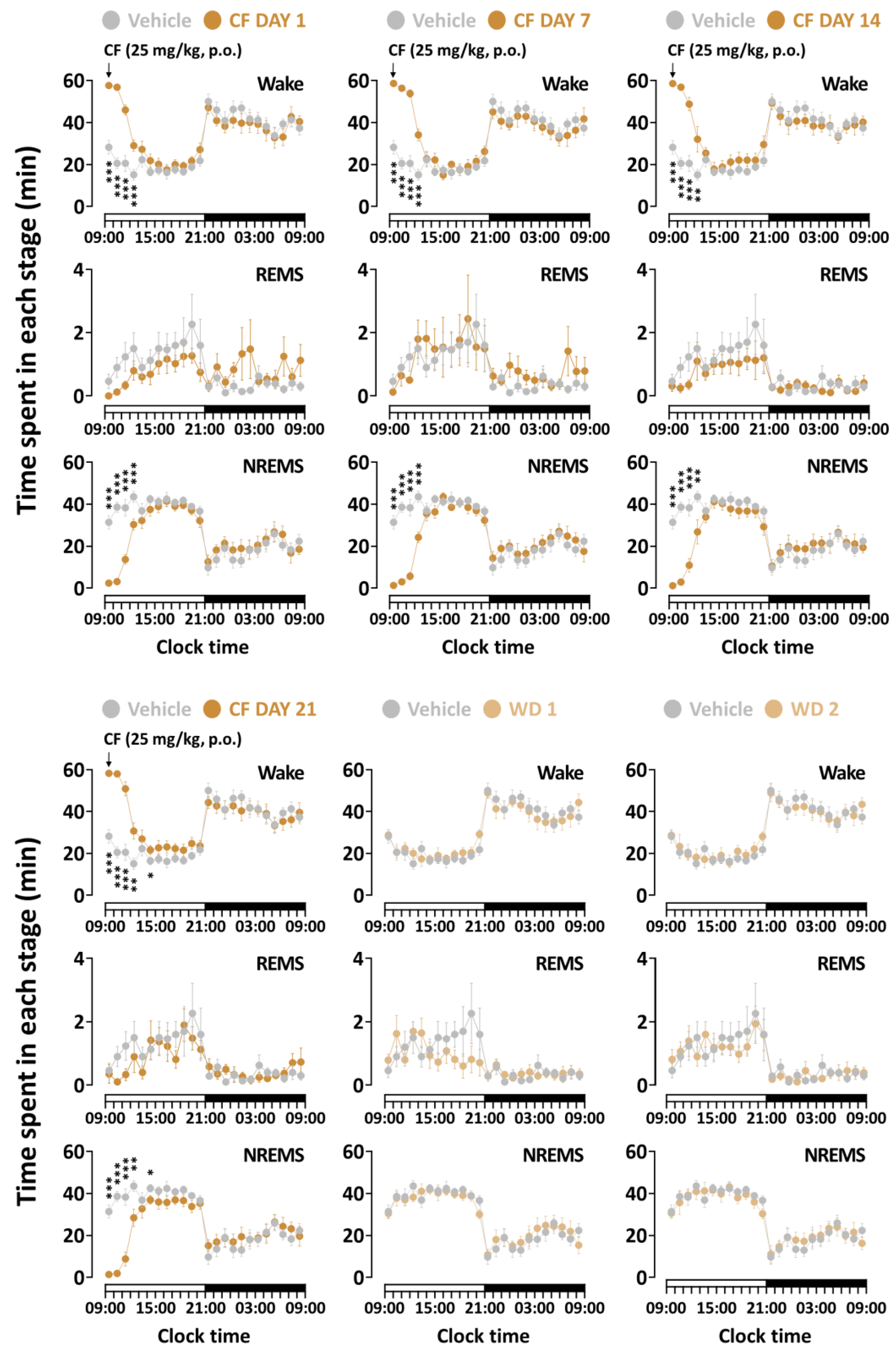
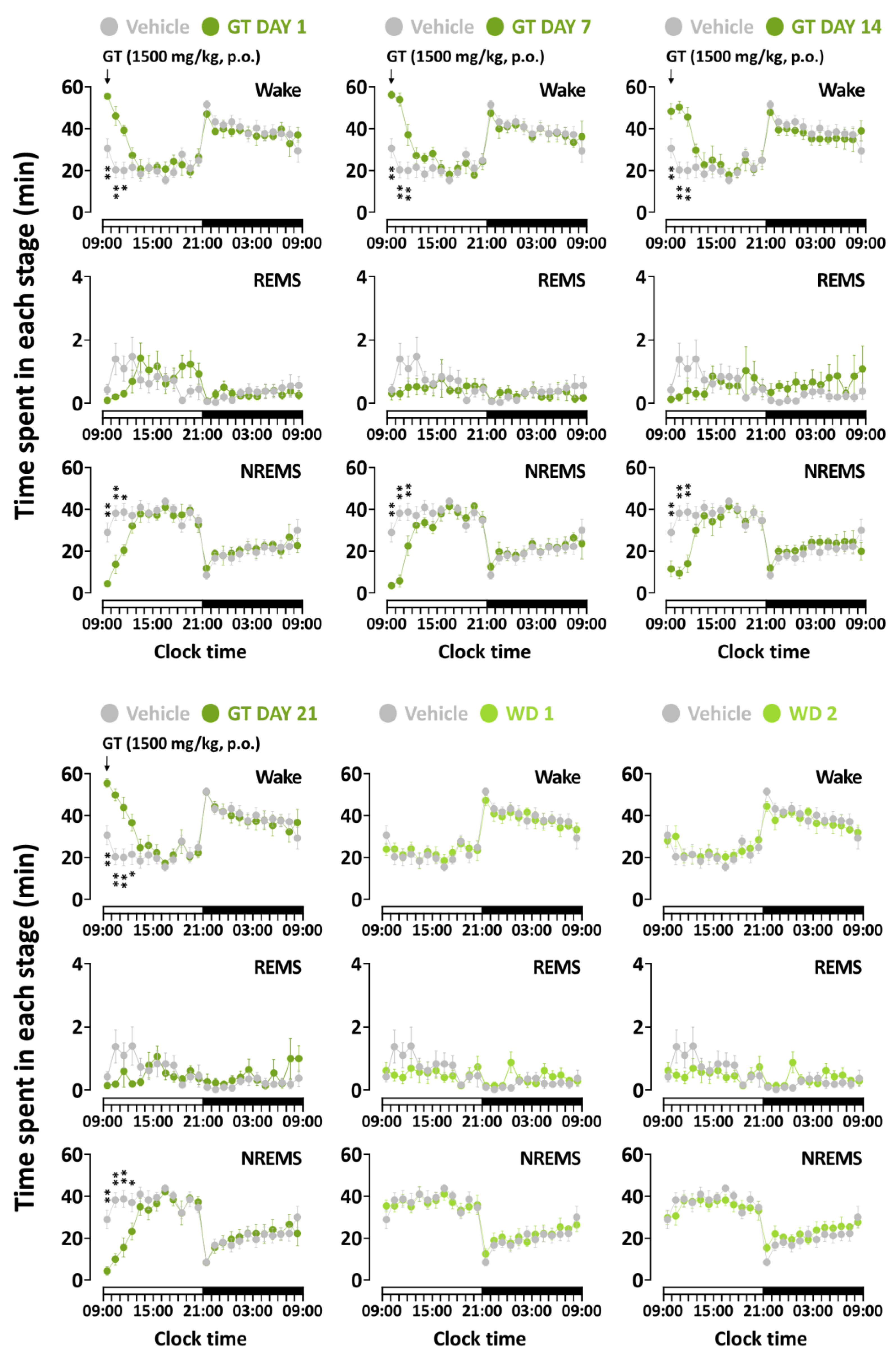
3.2. Effects of Chronic Administration of GT on Time-Course Changes in Sleep–Wake Stages
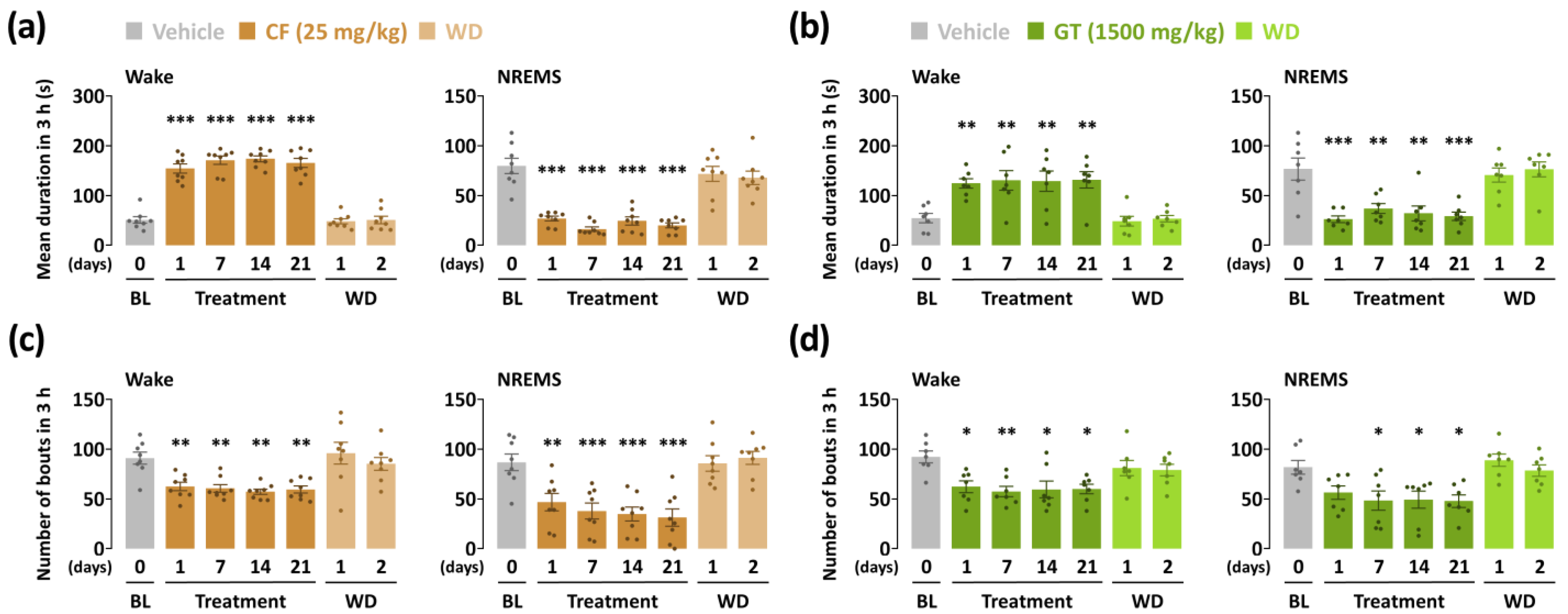
3.3. Effects of Chronic Administration of GT on Characteristics of Sleep–Wake Episodes
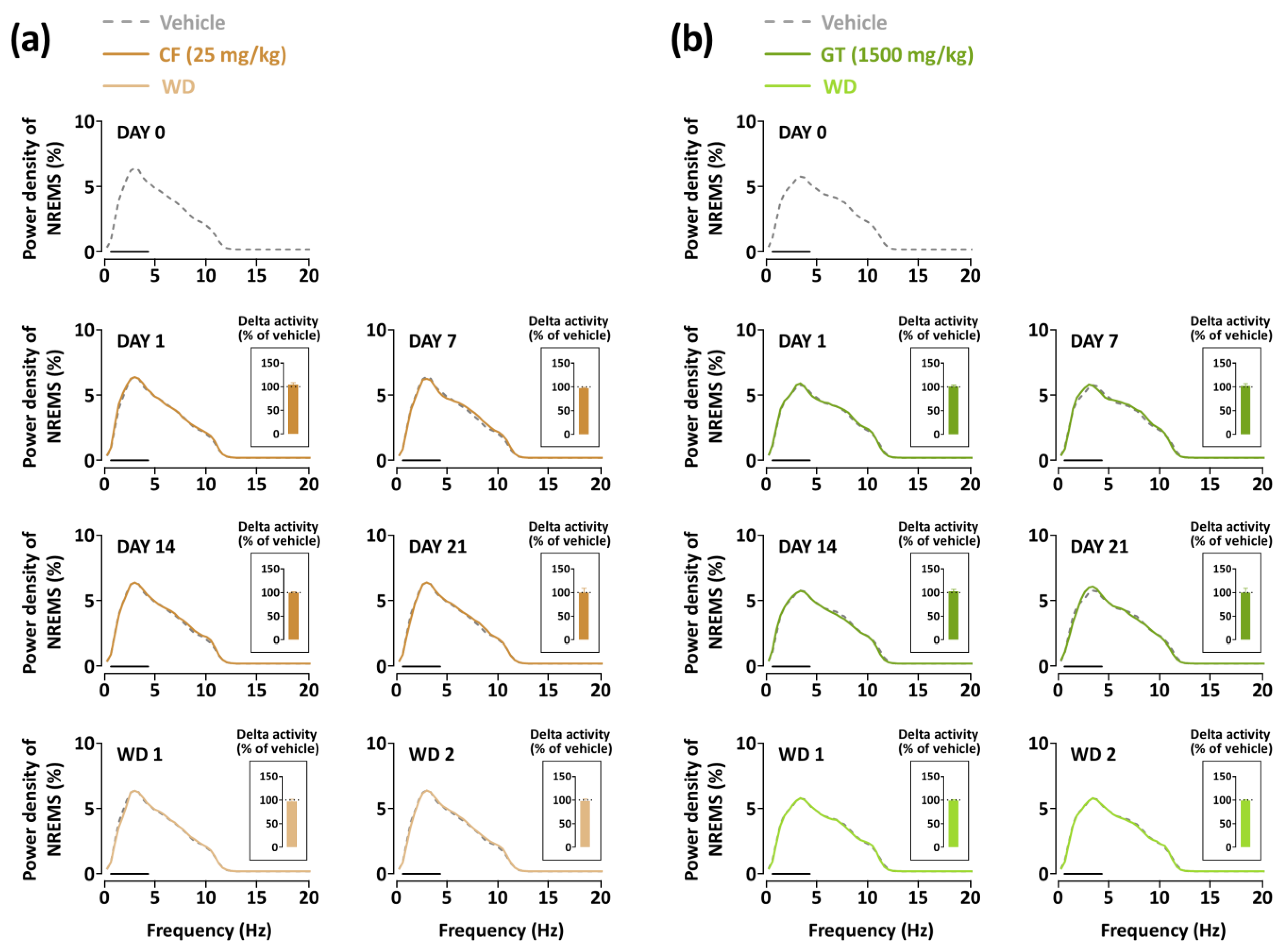
3.4. Effects of Chronic Administration of GT on Delta Power of NREMS
3.5. Caffeine Content of GT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrillon, T.; Windt, J.; Silk, T.; Drummond, S.P.; Bellgrove, M.A.; Tsuchiya, N. Does the mind wander when the brain takes a break? Local sleep in wakefulness, attentional lapses and mind-wandering. Front. Neurosci. 2019, 13, 949. [Google Scholar] [CrossRef]
- Houldin, E.; Fang, Z.; Ray, L.B.; Stojanoski, B.; Owen, A.M.; Fogel, S.M. Reversed and increased functional connectivity in non-REM sleep suggests an altered rather than reduced state of consciousness relative to wake. Sci. Rep. 2021, 11, 11943. [Google Scholar] [CrossRef] [PubMed]
- Szymusiak, R.; McGinty, D. Hypothalamic regulation of sleep and arousal. Ann. N. Y. Acad. Sci. 2008, 1129, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.; Roehrs, T. Insomnia: Epidemiology, characteristics, and consequences. Clin. Cornerstone 2003, 5, 5–15. [Google Scholar] [CrossRef]
- Mitchell, D.C.; Knight, C.A.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Beverage caffeine intakes in the US. Food Chem. Toxicol. 2014, 63, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Thomson, B.M.; Campbell, D.M.; Cressey, P.; Egan, U.; Horn, B. Energy drink consumption and impact on caffeine risk. Food Addit. Contam. 2014, 31, 1476–1488. [Google Scholar] [CrossRef]
- Huang, Z.-L.; Qu, W.-M.; Eguchi, N.; Chen, J.-F.; Schwarzschild, M.A.; Fredholm, B.B.; Urade, Y.; Hayaishi, O. Adenosine A2A, but not A1, receptors mediate the arousal effect of caffeine. Nat. Neurosci. 2005, 8, 858–859. [Google Scholar] [CrossRef]
- Glade, M.J. Caffeine—Not just a stimulant. Nutr. 2010, 26, 932–938. [Google Scholar] [CrossRef]
- Paterson, L.M.; Wilson, S.J.; Nutt, D.J.; Hutson, P.H.; Ivarsson, M. A translational, caffeine-induced model of onset insomnia in rats and healthy volunteers. Psychopharmacology 2007, 191, 943–950. [Google Scholar] [CrossRef]
- Shinomiya, K.; Omichi, J.; Ohnishi, R.; Ito, H.; Yoshida, T.; Kamei, C. Effects of chlorogenic acid and its metabolites on the sleep–wakefulness cycle in rats. Eur. J. Pharmacol. 2004, 504, 185–189. [Google Scholar] [CrossRef]
- Yoon, M.; Jung, J.; Kim, M.; Lee, C.; Cho, S.; Um, M. Effect of Black Pepper (Piper nigrum) Extract on Caffeine-Induced Sleep Disruption and Excitation in Mice. Nutrients 2022, 14, 2249. [Google Scholar] [CrossRef] [PubMed]
- Quiquempoix, M.; Sauvet, F.; Erblang, M.; Van Beers, P.; Guillard, M.; Drogou, C.; Trignol, A.; Vergez, A.; Léger, D.; Chennaoui, M. Effects of Caffeine Intake on Cognitive Performance Related to Total Sleep Deprivation and Time on Task: A Randomized Cross-Over Double-Blind Study. Nat. Sci. Sleep 2022, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. EFSA Panel on Dietetic Products, Nutrition and Allergies. In Scientific Opinion on the Safety of Caffeine; European Food Safety Authority: Parma, Italy, 2015. [Google Scholar]
- Yin, X.; Yang, J.; Li, T.; Song, L.; Han, T.; Yang, M.; Liao, H.; He, J.; Zhong, X. The effect of green tea intake on risk of liver disease: A meta analysis. Int. J. Clin. Exp. Med. 2015, 8, 8339. [Google Scholar] [PubMed]
- Omidi, J.; Abdolmohammadi, S. Green Tea (Camellia sinensis) Ordinary Beverages or Medicinal Beverages: A Review. Int. J. Bioorg. Chem. 2019, 4, 98–100. [Google Scholar] [CrossRef]
- Sung, H.; Nah, J.; Chun, S.; Park, H.; Yang, S.; Min, W. In vivo antioxidant effect of green tea. Eur. J. Clin. Nutr. 2000, 54, 527–529. [Google Scholar] [CrossRef]
- Reygaert, W.C. The antimicrobial possibilities of green tea. Front. Microbiol. 2014, 5, 434. [Google Scholar] [CrossRef]
- Demeule, M.; Michaud-Levesque, J.; Annabi, B.; Gingras, D.; Boivin, D.; Lamy, S.; Bertrand, Y.; Beliveau, R. Green tea catechins as novel antitumor and antiangiogenic compounds. Curr. Med. Chem. Anticancer Agents 2002, 2, 441–463. [Google Scholar] [CrossRef]
- Renzetti, A.; Betts, J.W.; Fukumoto, K.; Rutherford, R.N. Antibacterial green tea catechins from a molecular perspective: Mechanisms of action and structure–activity relationships. Food Funct. 2020, 11, 9370–9396. [Google Scholar] [CrossRef]
- Ding, H.; Li, Y.; Li, W.; Tao, H.; Liu, L.; Zhang, C.; Kong, T.; Feng, S.; Li, J.; Wang, X. Epigallocatechin-3-gallate activates the AMP-activated protein kinase signaling pathway to reduce lipid accumulation in canine hepatocytes. J. Cell Physiol. 2021, 236, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Zhang, H.; Qi, R.; Tsao, R.; Mine, Y. Recent advances in the understanding of the health benefits and molecular mechanisms associated with green tea polyphenols. J. Agric. Food. Chem. 2019, 67, 1029–1043. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ho, C.T.; Zhou, J.; Santos, J.S.; Armstrong, L.; Granato, D. Chemistry and biological activities of processed Camellia sinensis teas: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1474–1495. [Google Scholar] [CrossRef]
- Nian, B.; Chen, L.; Yi, C.; Shi, X.; Jiang, B.; Jiao, W.; Liu, Q.; Lv, C.; Ma, Y.; Zhao, M. A high performance liquid chromatography method for simultaneous detection of 20 bioactive components in tea extracts. Electrophoresis 2019, 40, 2837–2844. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Tsai, Y.-J.; Tsay, J.-S.; Lin, J.-K. Factors affecting the levels of tea polyphenols and caffeine in tea leaves. J. Agric. Food Chem. 2003, 51, 1864–1873. [Google Scholar] [CrossRef]
- Hu, C.-J.; Gao, Y.; Liu, Y.; Zheng, X.-Q.; Ye, J.-H.; Liang, Y.-R.; Lu, J.-L. Studies on the mechanism of efficient extraction of tea components by aqueous ethanol. Food Chem. 2016, 194, 312–318. [Google Scholar] [CrossRef]
- Um, M.Y.; Yoon, M.; Lee, J.; Jung, J.; Cho, S. A Novel Potent Sleep-Promoting Effect of Turmeric: Turmeric Increases Non-Rapid Eye Movement Sleep in Mice Via Histamine H1 Receptor Blockade. Mol. Nutr. Food Res. 2021, 65, 2100100. [Google Scholar] [CrossRef] [PubMed]
- McLellan, T.M.; Caldwell, J.A.; Lieberman, H.R. A review of caffeine’s effects on cognitive, physical and occupational performance. Neurosci. Biobehav. Rev. 2016, 71, 294–312. [Google Scholar] [CrossRef] [PubMed]
- Lara, D.R. Caffeine, mental health, and psychiatric disorders. J. Alzheimer’s Dis. 2010, 20, S239–S248. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.D.; Tola, K. Caffeine: Effects on psychological functioning and performance. In Caffeine; CRC Press: Boca Raton, FL, USA, 2019; pp. 251–299. [Google Scholar]
- Mitler, M.M.; Walsleben, J.; Sangal, R.B.; Hirshkowitz, M. Sleep latency on the maintenance of wakefulness test (MWT) for 530 patients with narcolepsy while free of psychoactive drugs. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Aldosari, M.S.; Olaish, A.H.; Nashwan, S.Z.; Abulmeaty, M.M.; BaHammam, A.S. The effects of caffeine on drowsiness in patients with narcolepsy: A double-blind randomized controlled pilot study. Sleep Breath. 2020, 24, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.-X.; Zhang, Q.-Y.; Cui, S.-Y.; Cui, X.-Y.; Zhang, J.; Zhang, Y.-H.; Bai, Y.-J.; Zhao, Y.-Y. Hypnotic effect of jujubosides from Semen Ziziphi spinosae. J. Ethnopharmacol. 2010, 130, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Juneja, L.R.; Chu, D.; Kim, M. Chemistry and Applications of Green Tea; CRC Press: Boca Raton, FL, USA, 1997; pp. 6–34. [Google Scholar]
- United States Food and Drug Administration. Spilling the Beans: How Much Caffeine Is Too Much? 2018. Available online: https://www.fda.gov/consumers/consumer-updates/spilling-beans-how-much-caffeine-too-much (accessed on 6 February 2023).
- Sinija, V.; Mishra, H.N. Green tea: Health benefits. J. Nutr. Environ. Med. 2008, 17, 232–242. [Google Scholar] [CrossRef]
- Park, K.-S.; Han, J.-Y.; Moon, D.-C.; Hong, J.T.; Oh, K.-W. (–)-Epigallocatechin-3-O-Gallate Augments Pentobarbital-Induced Sleeping Behaviors Through Cl−Channel Activation. J. Med. Food 2011, 14, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Park, K.-S.; Oh, J.H.; Yoo, H.-S.; Lee, Y.-M.; Lee, M.-K.; Hong, J.T.; Oh, K.-W. (−)-Epigallocatechin-3-O-gallate (EGCG) reverses caffeine-induced anxiogenic-like effects. Neurosci. Lett. 2010, 481, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.L.; Everett, J.M.; D’Cunha, N.M.; Sergi, D.; Georgousopoulou, E.N.; Keegan, R.J.; McKune, A.J.; Mellor, D.D.; Anstice, N.; Naumovski, N. The effects of green tea amino acid L-theanine consumption on the ability to manage stress and anxiety levels: A systematic review. Plant Foods Hum. Nutr. 2020, 75, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.-S.; Jung, J.Y.; Jang, I.-S.; Jang, K.-H.; Kim, S.-H.; Ha, J.-H.; Suk, K.; Lee, M.-G. L-theanine partially counteracts caffeine-induced sleep disturbances in rats. Pharmacol. Biochem. Behav. 2012, 101, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Kambe, D.; Kotani, M.; Yoshimoto, M.; Kaku, S.; Chaki, S.; Honda, K. Effects of quercetin on the sleep–wake cycle in rats: Involvement of gamma-aminobutyric acid receptor type A in regulation of rapid eye movement sleep. Brain Res. 2010, 1330, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.-L.; Wu, B.-F.; Shang, J.-H.; Wang, X.-F.; Zhao, Y.-L.; Huang, A.-X. Moringa oleifera seed ethanol extract and its active component kaempferol potentiate pentobarbital-induced sleeping behaviours in mice via a GABAergic mechanism. Pharm. Biol. 2022, 60, 810–824. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Deboer, T. Long-Term Effect of a Single Dose of Caffeine on Sleep, the Sleep EEG and Neuronal Activity in the Peduncular Part of the Lateral Hypothalamus under Constant Dark Conditions. Clocks Sleep 2022, 4, 260–276. [Google Scholar] [CrossRef]
- De Gennaro, L.; Ferrara, M. Sleep spindles: An overview. Sleep Med. Rev. 2003, 7, 423–440. [Google Scholar] [CrossRef]
- Um, M.Y.; Yoon, M.; Kim, M.; Jung, J.; Kim, S.; Kim, D.-O.; Cho, S. Curcuminoids, a major turmeric component, have a sleep-enhancing effect by targeting the histamine H1 receptor. Food Funct. 2022, 13, 12697–12706. [Google Scholar] [CrossRef]
- Kim, D.; Kim, J.; Kim, S.; Yoon, M.; Um, M.; Kim, D.; Kwon, S.; Cho, S. Arousal-Inducing Effect of Garcinia cambogia Peel Extract in Pentobarbital-Induced Sleep Test and Electroencephalographic Analysis. Nutrients 2021, 13, 2845. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.; Kim, S.; Yoon, M.; Um, M.Y.; Cho, S. Effects of Chronic Administration of Green Tea Ethanol Extract on Sleep Architecture in Mice: A Comparative Study with a Representative Stimulant Caffeine. Nutrients 2023, 15, 1042. https://doi.org/10.3390/nu15041042
Kim D, Kim S, Yoon M, Um MY, Cho S. Effects of Chronic Administration of Green Tea Ethanol Extract on Sleep Architecture in Mice: A Comparative Study with a Representative Stimulant Caffeine. Nutrients. 2023; 15(4):1042. https://doi.org/10.3390/nu15041042
Chicago/Turabian StyleKim, Duhyeon, Seonghui Kim, Minseok Yoon, Min Young Um, and Suengmok Cho. 2023. "Effects of Chronic Administration of Green Tea Ethanol Extract on Sleep Architecture in Mice: A Comparative Study with a Representative Stimulant Caffeine" Nutrients 15, no. 4: 1042. https://doi.org/10.3390/nu15041042
APA StyleKim, D., Kim, S., Yoon, M., Um, M. Y., & Cho, S. (2023). Effects of Chronic Administration of Green Tea Ethanol Extract on Sleep Architecture in Mice: A Comparative Study with a Representative Stimulant Caffeine. Nutrients, 15(4), 1042. https://doi.org/10.3390/nu15041042