Abstract
Diet can be a complementary treatment for Hashimoto’s disease by affecting thyroid function and anti-inflammatory properties. It is still unclear which dietary strategy would be the most beneficial. The aim of this systematic review is to examine all the data currently available in the literature on the effects of nutritional intervention on biochemical parameters (anti-thyroid antibody and thyroid hormones levels) and characteristic symptoms in the course of Hashimoto’s thyroiditis. This systematic review was prepared based on PRISMA guidelines. Articles in PubMed and Scopus databases published up to November 2022 were searched. As a result of the selection, out of 1350 publications, 9 were included for further analysis. The nutritional interventions included the following: elimination of gluten (3 articles) or lactose (1 article), energy restriction with or without excluding selected foods (n = 2), consumption of Nigella sativa (n = 2), or dietary iodine restriction (n = 1). The intervention duration ranged from 21 days to 12 months and included individuals with various thyroid function. Of the nine studies, three studies were female only. An improvement was observed during an energy deficit and after the elimination of selected ingredients (e.g., gluten, lactose, or goitrogens), as well as after the intervention of Nigella sativa. These interventions improved antibody levels against peroxidase (anti-TPO), (thyrotropin) TSH, and free thyroxine (fT4). No improvement was seen on the iodine-restricted diet. Varied outcomes of analyzed dietary interventions may be due to the heterogeneous thyroid condition, high variability between patients, and differences in habitual intake of critical nutrients (e.g., iodine, selenium, and iron) in different populations. Therefore, there is a great need for further experimental studies to determine whether any nutritional interventions are beneficial in Hashimoto’s disease.
1. Introduction
Hashimoto’s thyroiditis (HT), also known as chronic lymphocytic thyroiditis, is one of the autoimmune disorders of the thyroid gland. This disease is more often diagnosed in women than in men (350 vs. 80 per 100 thousand people a year) [1]. In the development of Hashimoto’s disease, genetic factors account for 70–80% of the risk and environmental factors for 20–30% [2]. HT is diagnosed on the basis of the presence of antibodies in the thyroid gland (anti-TPO, i.e., antibodies against thyroid peroxidase and anti-TG, i.e., antibodies against thyroglobulin) [3]. HT is also characterized by the infiltration of lymphocytes into thyroid tissue, which can be observed on ultrasound. Other diagnostic markers recommended for blood tests are TSH (thyrotropic hormone) and fT4 (free thyroxine) [2,3]. Hashimoto’s disease worsens the functioning of the thyroid gland, the hormones of which have multiple effects on other organs and tissues. The symptoms of Hashimoto’s disease include, among others, chronic fatigue, nervousness, mood swings, and gastrointestinal or cardiovascular problems [3]. Treatment includes replacement of thyroid hormones for hypothyroidism associated with HT. The basic pharmacotherapy is levothyroxine [4]. Despite the achievement of euthyroidism (state of normal thyroid hormone levels), the quality of life of some patients is unsatisfactory. This may be because of the impact of autoimmunity, although the results are inconclusive [5,6,7].
The implementation of a diet is a non-invasive method that can provide measurable benefits. The literature emphasizes the fundamental influence of various nutrients. Anti-inflammatory nutrients, such as vitamin D, antioxidants, monounsaturated and polyunsaturated fatty acids, magnesium, and zinc, are important to reduce thyroid inflammation. [8]. Iodine and selenium are involved in the synthesis and metabolism of thyroid hormones [9]. An adequate supply of iron, folic acid, and vitamin B12 is also important as a result of frequent anemia and cardiovascular diseases in this group of patients with Hashimoto’s thyroiditis [3]. According to current knowledge, gluten or lactose should be eliminated in the presence of food intolerances or diseases such as celiac disease [9,10,11]. One of the principles of a properly balanced diet is to limit saturated fatty acids, sugars, and refined carbohydrates, which have a pro-inflammatory effect [8,10]. Such an anti-inflammatory diet is represented, among others, by model of the Mediterranean diet. In a study by Ruggeri et al. [12], Hashimoto’s patients who adhered to the principles of the Mediterranean diet had lower oxidative stress parameters, which can have an impact on reducing the inflammatory process in the thyroid.
To the best of our knowledge, the reviews or meta-analyses conducted so far have focused on the importance of selected nutrients in Hashimoto’s disease, i.e., selenium, vitamin D, iodine, gluten, zinc, iron, and goitrogens [8,10,11,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27]. However, current clinical evidence of the effect of the dietary factors on HT is still scanty and inconclusive. Therefore, the aim of this systematic review is to examine all the data available in the literature about the effects of nutritional/dietary intervention on the course of Hashimoto’s thyroiditis measured by hormone and anti-body levels and body weight status normalization.
2. Materials and Methods
2.1. Data Sources and Search Strategy
In this systematic review, international electronic databases: Scopus and PubMed were used. The articles were searched by title or abstract or controlled keywords (MeSH): “Hashimoto disease”, “minerals”, “vitamins”, “diet”, “nutrition therapy”, and “nutrients”. An asterisk (*) was applied to the keywords to denote a wildcard term. MeSH words were verified against the MeSH 2023 terms index available at [28]. To search for MeSH terms, the following filters were used: “FullWord”, “Exact Match”, and “All Terms” (Main Heading (Descriptor) Terms + Qualifier Terms + Supplementary Concept Record Fields). Finally, articles were searched using the formula presented in Table 1. Studies potentially eligible for inclusion and available as of 30 November 2022 were identified.

Table 1.
Strategy for the primary literature search conducted in PubMed and Scopus databases.
2.2. Inclusion and Exclusion Criteria
The following inclusion criteria were used:
- original articles focusing on nutritional/dietary interventions (or nutritional/dietary interventions + supplementation) in patients diagnosed with Hashimoto’s disease;
- articles in English;
- articles to which full access has been granted.
The exclusion criteria included the following:
- articles other than original (case study, review, meta-analysis, commentary, book chapter, post-conference materials, and so on);
- articles that do not include dietary interventions;
- nutritional interventions without a control group;
- non-human studies;
- articles not focused on Hashimoto’s disease;
- articles including patients with thyroidectomy;
- supplementation-only interventions;
- interventions that were designed to induce Hashimoto’s disease;
- articles to which full access has not been granted despite attempts to contact a corresponding author.
2.3. Construction of the Review
This systematic review was prepared according to the PRISMA checklist [29] and the PICO model (P—population/patient; I—intervention/indicator; C—comparative/control; O—outcome):
- Population: Patients diagnosed with Hashimoto’s disease at any stage of life and regardless of gender and levothyroxine treatment status.
- Intervention: Nutritional/dietary interventions (healthy eating, elimination diets) or nutrition + supplementation.
- Comparison: The control group with/no nutritional/dietary intervention and with/no supplementation.
- Outcome: Parameters’ evaluation including at least one marker from the following: thyroid hormones (fT3, fT4); TSH; antibodies: anti-TPO and anti-TG; ultrasound examination of the thyroid gland; quality of life; anthropometric measurements (body weight, waist circumference, hip circumference); or body composition analysis [30].
All data were obtained from article texts, tables, and figures. The following information was searched for and extracted from the included articles: authors, year of publication, number of patients, sex of patients, Hashimoto’s disease diagnosis before inclusion to the intervention, the number of dropouts during the study, thyroid function (e.g., hypothyroidism, euthyrosis), characteristics of patients, criteria for inclusion and exclusion from the study, frequency of contacts during the intervention, characteristics and duration of nutritional intervention, markers in baseline and after the regiment, and type of study.
3. Results
3.1. Characteristics of Selected Articles
A total of 1350 studies were identified through two electronic database searches. After removing duplicates (n = 311), articles were reviewed by title or abstract by two independent researchers. Based on the inclusion and exclusion criteria, 18 articles were pre-qualified for the full text reading. Subsequent papers were excluded at this stage for reasons such as non-original article; the correspondence author did not grant access to the full text; effect of supplementation/drug on HT/AITD; the influence of lifestyle and the lack of a control group; and non-human research. During the selection of articles, the researchers discussed any ambiguous situations with each other and the classification of articles based on the inclusion and exclusion criteria. Finally, nine publications met the inclusion criteria; the detailed procedure is presented in Figure 1.
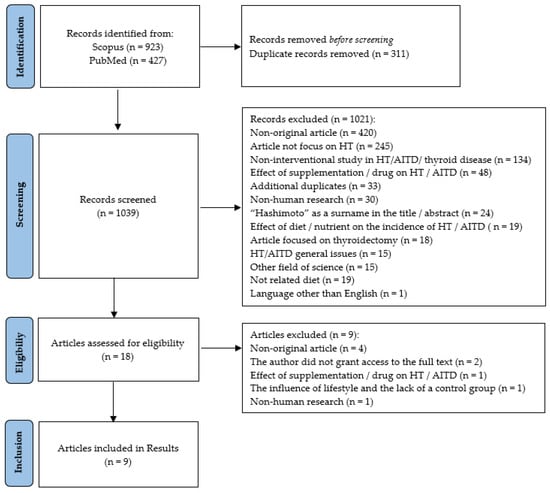
Figure 1.
PRISMA flow diagram of the selected and included articles. HT—Hashimoto’s thyroiditis; AITD—autoimmune thyroid disease.
The included articles were published mainly during the last eight years [31,32,33,34,35,36,37,38], except for the one published in 2003 [39]. Four out of nine studies were conducted in Poland [31,32,33,36]. The other four studies were conducted in Turkey [38], Iran [34,35], Italy [37], and South Korea [39]. Patients were mainly recruited from clinics [32,34,35,38]. In the case of four studies [31,36,37,39], this information was not provided by the authors.
Four out of nine studies involved women only [31,32,33,36]. Hashimoto’s thyroiditis was diagnosed mostly on the basis of characteristic ultrasound features [31,32,33,36,38] and increased levels of any of the thyroid antibodies [31,32,33,36,37,38,39]. In two studies, the authors stated that HT was diagnosed by a licensed physician [34,35].
The main characteristics of the included studies and clinicopathologic features are presented in Table 2.

Table 2.
The characteristics of the included studies, summary of dietary interventions, and clinicopathologic features.
3.2. Characteristics of the Nutritional Intervention
The details of nutritional interventions are described in Table 3.

Table 3.
Description of the implemented nutritional/dietary interventions.
Three studies involved a gluten-free diet [31,33,36] and two studies involved a restriction of selected food products [32,37]. Other interventions included the following: lactose elimination [38], iodine restriction [39], and black cumin consumption [34,35]. Intervention time ranged from 3 weeks [37] to 12 months [31,33]. The size of the experimental groups ranged from 16 [36] to 108 patients [37], while the control groups consisted of 12 [38] up to 72 individuals [37].
The lactose-free diet intervention included lactose-intolerant patients (euthyroid and with subclinical hypothyroidism) as an experimental group and euthyroid patients without lactose intolerance as controls [38]. In three studies, participants had normal thyroid function [31,33,36], whereas in the dietary iodine restriction intervention, participants were hypothyroid [39]. In four studies, this information was missing [32,34,35,37].
Patients were treated with levothyroxine in five out of nine studies [32,33,34,35,38]. In three studies, there was no such pharmacological treatment [31,36,39] and, in one study, information on pharmacological treatment was not provided [37].
The included studies differed in terms of dropout rates: 37% on lactose-free diet [38]; 33% on gluten-free diet [31]; and 15% on Nigella stativa intervention [34,35] and the reduction diet with/without the IgG test, including zinc and selenium supplementation [32]. In the case of the 6-month gluten-free intervention [36] and iodine restriction [39], all participants completed the study. Information on dropout rates is missing in the low-carbohydrate diet intervention involving 180 patients [37].
The study participants were contacted by the researchers weekly [34,35,37], every two months [31,32,36], or every three months [33]. In two studies, the evaluation was conducted at the baseline and repeated at the end of the intervention [38,39].
3.3. Main Outcomes
Krysiak et al. [31] examined the effect of a gluten-free diet on the concentration of vitamin D in women with Hashimoto’s disease. The experimental group was also diagnosed with non-celiac gluten sensitivity and recommended a gluten-free diet. The control group, without non-celiac gluten sensitivity, followed a regular diet. Both groups received the same dose of vitamin D (supplementation). In the control group, there was a significant decrease in anti-TPO (p = 0.0017) and anti-TG (p = 0.0056) levels and an increase in vitamin D concentration (p = 0.0006) compared with the experimental group. Although the typical diet, compared with the gluten-free diet, showed better results in improving the concentration of anti-TPO, anti-TG and vitamin D, in both groups, a significant improvement in these parameters was observed. However, no changes were observed in the case of TSH, fT3, and fT4 in the groups and between them. According to the authors, a gluten-free diet may hinder the absorption of vitamin D into the blood and thus hinder therapeutic support in lowering thyroid antibodies.
Ostrowska et al. [32] observed that body weight, BMI, and % body fat decreased significantly in both the experimental group (reduction diet + elimination products based on IgG test) (all p < 0.001) and the control group (reduction diet) (all p < 0.001). The experimental group showed a significantly better improvement in the aforementioned parameters than the control groups (p < 0.001, p < 0.002, and p < 0.026, respectively). TSH, fT3, fT4, anti-TG, and anti-TPO levels decreased significantly in both the experimental group (all p < 0.001) and the control group (all p < 0.001). However, the experimental group had a significantly better improvement in the previously mentioned parameters compared with the control groups (p < 0.001, p < 0.001, p < 0.001, p < 0.048, and p < 0.001, respectively). Additionally, the authors noticed that a decrease in BMI correlated with an increase in fT3 (p < 0.001) and fT4 (p = 0.003). The reduction in adipose tissue correlated with an increase in fT3 (p < 0.001) and fT4 (p = 0.035). The change in body weight, BMI, and adipose tissue content was positively correlated with anti-TG (p = 0.001, p < 0.001, p = 0.003 respectively). BMI change positive correlation with anti-TPO titer (p = 0.023).
A 12-month study by Pobłocki et al. [33] found that, in LT4-treated patients, a gluten-free diet decreased TSH (p < 0.044), but not thyroid hormones or anti-TPO and anti-TG antibodies. However, after analyzing the changes in the median concentration of the tested blood indices, a significance was noticed in TSH (p = 0.039) and fT4 (p = 0.022). An analysis of changes in the concentration of the studied parameters after logarithmic transformation was also performed, which showed the improvement in anti-TG, TSH, and fT4 at 3, 6, and 12 months of the intervention. A reduction in anti-TPO was also observed, but only in the third month of the trial.
During the consumption of 2 g Nigella sativa [34,35], a decrease in TSH (p = 0.03), weight (p = 0.004), BMI (p = 0.002), waist circumference (p = 0.006), and hip circumference (p = 0.001) was observed in levothyroxine-treated patients. In the experimental group, there was also an increase in T3 (p = 0.008) and improvement in some markers of oxidative stress and endothelial dysfunction. The concentration of anti-TPO, T4, nesfatin-1, and vascular endothelial growth factor (VEGF) remained unchanged. No significant changes were noticed in the control group. After the intervention, there was a significant improvement in the study group compared with the control group in terms of TSH (p = 0.02), T4 (p = 0.04), and anti-TPO (p = 0.01). In the experimental group, there was also an improvement in HDL cholesterol (p = 0.046), LDL (p = 0.002), and TG (p = 0.02), but not total cholesterol. However, there was a significant difference in HDL levels (p = 0.027) between the groups after the intervention. However, after the intervention, there was a significant improvement in HDL levels (p = 0.027) in the study group compared with the control group.
Krysiak et al. [36] investigated the effect of a 6-month gluten elimination diet on euthyroid untreated patients with Hashimoto’s disease. Patients also had positive anti-tissue transglutaminase antibodies (anti-tTG), but no clinical symptoms of celiac disease or diagnosed celiac disease. The gluten-free diet significantly reduced the anti-TPO and anti-TG antibodies in the experimental group compared with the control group (without a gluten-free diet). There was also an improvement in vitamin D level, which, according to the authors, could also have had an impact on the improvement in antibody titers.
The study by Esposito et al. [37] based on a low-carbohydrate diet with the exclusion of several products did not affect TSH, fT3, and fT4. On the other hand, it resulted in a decrease in BMI (p < 0.000), lean mass (p < 0.000), body weight (p < 0.000), fat mass (p < 0.05), anti-TG (p < 0.013), anti-microsomal (p < 0.000), and anti-TPO (p < 0.029). However, this intervention was much shorter than those used in other studies (3 weeks), making it difficult to compare results.
Asik et al. [38] examined the effect of an 8-week lactose-free diet on the course in levothyroxine-treated patients with Hashimoto’s disease. They observed that, after the intervention in the group of patients with HT and lactose intolerance, the TSH value decreased significantly (p < 0.05) compared with the results before the intervention. However, in the group of lactose-tolerant patients with HT, the results before and after were similar (p > 0.05). No significant change in fT4 was observed in any of the groups, which the authors explained with a too short intervention to draw robust conclusions. The authors also suggested that, in the case of a high dose of levothyroxine used by patients or resistance to its treatment and difficulty in regulating TSH, it is worth considering testing for lactose intolerance.
In the study by Yoon et al. [39], 18 of 23 patients (78.3%) in the experimental group had returned to normal thyroid function and 17.3% of patients had exacerbated hypothyroidism after 3 months. There was an improvement in fT4 and TSH (all p < 0.05) but not in T3 and T4 levels. In the control group, 10 out of 22 patients (45.5%) recovered to euthyroidism, 2 patients had hypothyroidism, and another 10 patients had an exacerbation of hypothyroidism. There was an improvement in fT4 (all p < 0.05) but not in T3, T4, and TSH levels. It is worth noting that this was a region with excessive iodine intake.
4. Discussion
Despite the small number of studies, the collected data demonstrate the potential positive effect of the nutritional strategies on the course of Hashimoto’s thyroiditis. Most often, nutritional intervention included the elimination of components such as gluten, lactose, or selected food products [32,33,36,37,38]. Among 83 patients, almost 76% of HT patients taking levothyroxine (LT4) were lactose-intolerant [38]. Lactose is a common component of levothyroxine formulations, which can lead to impaired LT4 efficacy in sensitive individuals [40]. Lactose intolerance is associated, among others, with bacterial overgrowth, malabsorption, and damage to the intestinal villi, which results in a greater demand for LT4. In the case of a need for a high dose of levothyroxine or resistance to treatment and difficulty in regulating TSH, it is worth considering testing patients for lactose intolerance [38]. The need for a higher dose of levothyroxine in lactose intolerant patients was reported by other authors [41]. However, Marabotto et al. [42] did not observe any differences in cumulative LT4 dose requirements in Hashimoto’s disease patients with or without lactose intolerance. To the best of our knowledge, studies to date have shown no benefit from lactose restriction in people with Hashimoto’s disease who have not been treated with levothyroxine.
The elimination of gluten from a diet is a treatment for diseases such as celiac disease, Duhring’s disease, wheat allergy, or non-celiac gluten sensitivity (NCGS) [43]. Among patients with Hashimoto’s disease, celiac disease is more prevalent [44]. This may result in a higher levothyroxine requirement owing to the lower absorption capacity of LT4 in the gastrointestinal tract [45]. Patients with Hashimoto’s thyroiditis may develop NCGS, which may be immune-related. It is not associated with celiac disease or allergies to wheat or gluten. It causes non-specific symptoms after eating foods containing gluten, such as headaches, joint and muscle aches, or the so-called “foggy mind”. Such symptoms often accompany Hashimoto’s disease [46]. According to a systematic review of a gluten-free diet on parameters in the course of Hashimoto’s thyroiditis, among the six included studies, no significant change in TSH was noted at any time [47]. In the case of anti-TPO and anti-TG, Krysiak et al. [36] observed a lowering of the antibody level and, in study by Valentino [48], one patient had a significantly reduced anti-TPO after 18 months (p < 0.001). According to the experts of the Polish Society of Parenteral Nutrition, Enteral Nutrition, and Metabolism (POLSPEN), the elimination of gluten from the diet in Hashimoto’s thyroiditis is unjustified, if there are no medical indications [49].
The meta-analysis of Song et al. [50] showed that obesity was correlated with Hashimoto’s disease (p = 0.022). A significant association was also observed between elevated anti-TPO (p = 0.001) and obesity, but no relationship was found between a positive anti-TG result and obesity. According to three meta-analyses, selenium supplementation leads to a reduction in antibodies, although, as the authors indicate, the quality of the included evidence is low [13,14,21]. According to the meta-analyses, Nigella sativa reduces body weight [51,52]. A systematic review by Khabbazi [53] suggested that Nigella sativa might be effective in the treatment of rheumatoid arthritis, especially in animal and in vitro studies, as well as in humans (a decrease in the disease activity score 28 (DAS28) was especially noted). It is also supposed to alleviate the course of other autoimmune diseases, such as autoimmune encephalomyelitis [54], ulcerative colitis [55], and psoriasis [56].
Iodine deficiency is a known factor in causing the goiter of the thyroid gland. To prevent iodine deficiency, fortifications have been introduced, e.g., with iodized salt. On the other hand, of consumption >1100 mcg/day can also lead to a thyroid dysfunction. Excess iodine is toxic to the thyroid cells because it causes the inflammation that leads to the development of Hashimoto’s thyroiditis [18]. Excess iodine may lead to the Wolff–Chaikoff effect, i.e., a temporary or permanent decrease in the synthesis of thyroid hormones and indirect inhibition of thyroid peroxidase activity [57]. However, the data from Denmark do not confirm the adverse effect of iodine fortification of food on the course of thyroid diseases [58].
Strengths and Limitations
In the literature, there are still insufficient data indicating which nutritional/dietary interventions in patients with Hashimoto’s disease contribute to success in the context of metabolic parameters and body weight management. What sets this review apart is that it summarizes the results of nutritional interventions with a control group among patients with Hashimoto’s disease. Observational studies (cross-sectional studies, case reports, and animal studies) were excluded in order to obtain more reliable results. There was no time limit for searching for articles. The strength is the analysis of the results for an age homogeneous group—in this review, only adults were included. However, the studied group was not homogeneous with respect to the levothyroxine treatment, thyroid status, and outcomes evaluated.
However, this review is not without limitations. It is difficult to unequivocally deduce the role of nutritional intervention on the described parameters in Hashimoto’s disease for several reasons. First of all, the number of studies is small, the interventions are diverse, and other factors may have influenced the results (e.g., vitamin D). Secondly, the disease is not a homogeneous condition, and variability between patients is high. The basic supply of critical nutrients such as iodine, selenium, or iron has not been addressed in the studies and may differ between countries and individuals. For example, adequate iodine intake is reported for Poland, Turkey, Iran, and Italy, as well as excessive iodine intake for South Korea [59]. For this reason, it is likely that different populations will respond differently to the nutritional interventions analyzed, and recommendations may need to be given differently to patients residing in different areas of the world, where the baseline supply differs. Additionally, articles available only in two online databases and only published in English were analyzed. This search strategy eliminates publications in local languages as well as publications in paper-only journals.
5. Conclusions
Based on this systematic review, it is difficult to present unequivocal conclusions owing to the variety of nutritional support implemented, its duration, and the size of the groups. Previous studies have had a positive or neutral impact on biochemical parameters or symptoms in the course of Hashimoto’s thyroiditis to varying degrees. However, there is a great need for further research to clearly determine which type of nutritional intervention would be the most beneficial for patients with Hashimoto’s thyroiditis.
Author Contributions
Conceptualization, K.O. and J.M.-R.; methodology, K.O.; investigation, K.O.; resources, K.O.; data curation, K.O. and J.M.-R.; writing—original draft preparation, K.O.; writing—review and editing, K.O. and J.M.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Attard, C.C.; Vella, S. Aetiology of thyroid autoimmunity. Malta Med. J. 2018, 30, 26–31. [Google Scholar]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef] [PubMed]
- Caturegli, P.; De Remigis, A.; Rose, N.R. Hashimoto thyroiditis: Clinical and diagnostic criteria. Autoimmun. Rev. 2014, 13, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Ralli, M.; Angeletti, D.; Fiore, M.; D’Aguanno, V.; Lambiase, A.; Artico, M.; de Vincentiis, M.; Greco, A. Hashimoto’s thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun. Rev. 2020, 19, 102649. [Google Scholar] [CrossRef]
- Uysal, H.B.; Ayhan, M. Autoimmunity affects health-related quality of life in patients with Hashimoto’s thyroiditis. Kaohsiung J. Med. Sci. 2016, 32, 427–433. [Google Scholar] [CrossRef]
- Patti, M.; Christian, R.; Palokas, M. Association between anti-thyroid antibodies and quality of life in patients with Hashimoto thyroiditis: A systematic review and meta-analysis. JBI Evid. Synth. 2021, 19, 2307–2338. [Google Scholar] [CrossRef]
- Groenewegen, K.L.; Mooij, C.F.; van Trotsenburg, A.S.P. Persisting symptoms in patients with Hashimoto’s disease despite normal thyroid hormone levels: Does thyroid autoimmunity play a role? A systematic review. J. Transl. Autoimmun. 2021, 4, 100101. [Google Scholar] [CrossRef]
- Danailova, Y.; Velikova, T.; Nikolaev, G.; Mitova, Z.; Shinkov, A.; Gagov, H.; Konakchieva, R. Nutritional Management of Thyroiditis of Hashimoto. Int. J. Mol. Sci. 2022, 23, 5144. [Google Scholar] [CrossRef]
- Hu, S.; Rayman, M.P. Multiple Nutritional Factors and the Risk of Hashimoto’s Thyroiditis. Thyroid 2017, 27, 597–610. [Google Scholar] [CrossRef]
- Ihnatowicz, P.; Drywien, M.; Wator, P.; Wojsiat, J. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Annals Agric. Environ. Med. 2020, 27, 184–193. [Google Scholar] [CrossRef]
- Szczuko, M.; Syrenicz, A.; Szymkowiak, K.; Przybylska, A.; Szczuko, U.; Pobłocki, J.; Kulpa, D. Doubtful Justification of the Gluten-Free Diet in the Course of Hashimoto’s Disease. Nutrients 2022, 14, 1727. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, R.M.; Giovinazzo, S.; Barbalace, M.C.; Cristani, M.; Alibrandi, A.; Vicchio, T.M.; Giuffrida, G.; Aguennouz, M.H.; Malaguti, M.; Angeloni, C.; et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid 2021, 31, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Toulis, K.A.; Anastasilakis, A.D.; Tzellos, T.G.; Goulis, D.G.; Kouvelas, D. Selenium supplementation in the treatment of Hashimoto’s thyroiditis: A systematic review and a meta-analysis. Thyroid 2010, 20, 1163–1173. [Google Scholar] [CrossRef] [PubMed]
- Wichman, J.; Winther, K.H.; Bonnema, S.J.; Hegedüs, L. Selenium Supplementation Significantly Reduces Thyroid Autoantibody Levels in Patients with Chronic Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. Thyroid 2016, 26, 1681–1692. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lv, S.; Chen, G.; Gao, C.; He, J.; Zhong, H.; Xu, Y. Meta-Analysis of the Association between Vitamin D and Autoimmune Thyroid Disease. Nutrients 2015, 7, 2485–2498. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Multiple nutritional factors and thyroid disease, with particular reference to autoimmune thyroid disease. Proc. Nutr. Soc. 2019, 78, 34–44. [Google Scholar] [CrossRef]
- Mandecka, A.; Regulska-Ilow, B. Metabolic disorders and nutritional status in autoimmune thyroid diseases. Postepy Hig. Med. Dosw. 2015, 69, 80–90. [Google Scholar]
- Ihnatowicz, P.; Wątor, P.; Drywień, M.E. The importance of gluten exclusion in the management of Hashimoto’s thyroiditis. Annals Agric. Environ. Med. 2021, 28, 558–568. [Google Scholar] [CrossRef]
- Liontiris, M.I.; Mazokopakis, E.E. A concise review of Hashimoto thyroiditis (HT) and the importance of iodine, selenium, vitamin D and gluten on the autoimmunity and dietary management of HT patients. Points that need more investigation. Hell. J. Nucl. Med. 2017, 20, 51–56. [Google Scholar]
- Jiang, H.; Chen, X.; Qian, X.; Shao, S. Effects of vitamin D treatment on thyroid function and autoimmunity markers in patients with Hashimoto’s thyroiditis—A meta-analysis of randomized controlled trials. J. Clin. Pharm. Ther. 2022, 47, 767–775. [Google Scholar] [CrossRef]
- Winther, K.H.; Wichman, J.E.M.; Bonnema, S.J.; Hegedüs, L. Insufficient documentation for clinical efficacy of selenium supplementation in chronic autoimmune thyroiditis, based on a systematic review and meta-analysis. Endocrine 2017, 55, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, Y.; Li, H.; Li, H. Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: Systematic review and meta-analysis. J. Int. Med. Res. 2021, 49, 3000605211060675. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Muscogiuri, G.; Barrea, L.; Mathieu, C.; Vallone, C.V.; Mascitelli, L.; Bizzaro, G.; Altieri, V.M.; Tirabassi, G.; Balercia, G.; et al. Does vitamin D play a role in autoimmune endocrine disorders? A proof of concept. Rev. Endocr. Metab. Disord. 2017, 18, 335–346. [Google Scholar] [CrossRef]
- Štefanić, M.; Tokić, S. Serum 25-hydoxyvitamin D concentrations in relation to Hashimoto’s thyroiditis: A systematic review, meta-analysis and meta-regression of observational studies. Eur. J. Nutr. 2020, 59, 859–872. [Google Scholar] [CrossRef] [PubMed]
- Taheriniya, S.; Arab, A.; Hadi, A.; Fadel, A.; Askari, G. Vitamin D and thyroid disorders: A systematic review and Meta-analysis of observational studies. BMC Endocr. Disord. 2021, 21, 171. [Google Scholar] [CrossRef] [PubMed]
- van Zuuren, E.J.; Albusta, A.Y.; Fedorowicz, Z.; Carter, B.; Pijl, H. Selenium supplementation for Hashimoto’s thyroiditis. Cochrane Database Syst. Rev. 2013, 3, CD010223. [Google Scholar] [CrossRef] [PubMed]
- Benvenga, S.; Ferrari, S.M.; Elia, G.; Ragusa, F.; Patrizio, A.; Paparo, S.R.; Camastra, S.; Bonofiglio, D.; Antonelli, A.; Fallahi, P. Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies. Nutrients 2020, 12, 1337. [Google Scholar] [CrossRef]
- Medical Subject Headings 2023. Available online: https://meshb.nlm.nih.gov/search (accessed on 10 September 2022).
- Page, M.J.; Moher, D.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (PICO) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Krysiak, R.; Kowalcze, K.; Okopień, B. Gluten-free diet attenuates the impact of exogenous vitamin D on thyroid autoimmunity in young women with autoimmune thyroiditis: A pilot study. Scand. J. Clin. Lab. Investig. 2022, 82, 518–524. [Google Scholar] [CrossRef]
- Ostrowska, L.; Gier, D.; Zyśk, B. The Influence of Reducing Diets on Changes in Thyroid Parameters in Women Suffering from Obesity and Hashimoto’s Disease. Nutrients 2021, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Pobłocki, J.; Pańka, T.; Szczuko, M.; Telesiński, A.; Syrenicz, A. Whether a Gluten-Free Diet Should Be Recommended in Chronic Autoimmune Thyroiditis or Not?—A 12-Month Follow-Up. J. Clin. Med. 2021, 10, 3240. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Tajmiri, S. The effects of powdered black cumin seeds on markers of oxidative stress, intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in patients with Hashimoto’s thyroiditis. Clin. Nutr. ESPEN 2020, 37, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Farhangi, M.A.; Dehghan, P.; Tajmiri, S.; Abbasi, M.M. The effects of Nigella sativa on thyroid function, serum Vascular Endothelial Growth Factor (VEGF)-1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: A randomized controlled trial. BMC Complement. Altern. Med. 2016, 16, 471. [Google Scholar] [CrossRef]
- Krysiak, R.; Szkróbka, W.; Okopień, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naïve Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp. Clin. Endocrinol. Diabetes 2019, 127, 417–422. [Google Scholar] [CrossRef]
- Esposito, T.; Lobaccaro, J.M.; Esposito, M.G.; Monda, V.; Messina, A.; Paolisso, G.; Varriale, B.; Monda, M.; Messina, G. Effects of low-carbohydrate diet therapy in overweight subject with autoimmune thyroiditis: Possible synergism with ChREBP. Drug Des. Devel. Ther. 2016, 10, 2939–2946. [Google Scholar]
- Asik, M.; Gunes, F.; Binnetoglu, E.; Eroglu, M.; Bozkurt, N.; Sen, H.; Akbal, E.; Bakar, C.; Beyazit, Y.; Ukinc, K. Decrease in TSH levels after lactose restriction in Hashimoto’s thyroiditis patients with lactose intolerance. Endocrine 2014, 46, 279–284. [Google Scholar] [CrossRef]
- Yoon, S.J.; Choi, S.R.; Kim, D.M.; Kim, J.U.; Kim, K.W.; Ahn, C.W.; Cha, B.S.; Lim, S.K.; Kim, K.R.; Lee, H.C.; et al. The Effect of Iodine Restriction on Thyroid Function in Patients with Hypothyroidism Due to Hashimoto’s Thyroiditis. Yonsei Med. J. 2003, 44, 227–235. [Google Scholar] [CrossRef]
- Ruchała, M.; Szczepanek-Parulska, E.; Zybek, A. The influence of lactose intolerance and other gastro-intestinal tract disorders on L-thyroxine absorption. Endokrynol. Pol. 2012, 63, 318–323. [Google Scholar]
- Cellini, M.; Santaguida, M.G.; Gatto, I.; Virili, C.; Del Duca, S.C.; Brusca, N.; Capriello, S.; Gargano, L.; Centanni, M. Systematic appraisal of lactose intolerance as cause of increased need for oral thyroxine. J. Clin. Endocrin. Metab. 2014, 99, E1454–E1458. [Google Scholar] [CrossRef]
- Marabotto, E.; Ferone, D.; Sheijani, A.D.; Vera, L.; Ziola, S.; Savarino, E.; Bodini, G.; Furnari, M.; Zentilin, P.; Savarino, V.; et al. Prevalence of Lactose Intolerance in Patients with Hashimoto Thyroiditis and Impact on LT4 Replacement Dose. Nutrients 2022, 14, 3017. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Dore, M.P.; Fanciulli, G.; Rouatbi, M.; Mereu, S.; Pes, G.M. Autoimmune Thyroid Disorders Are More Prevalent in Patients with Celiac Disease: A Retrospective Case-Control Study. J. Clin. Med. 2022, 11, 6027. [Google Scholar] [CrossRef]
- Virili, C.; Bassotti, G.; Santaguida, M.G.; Iuorio, R.; Del Duca, S.C.; Mercuri, V.; Picarelli, A.; Gargiulo, P.; Gargano, L.; Centanni, M. Atypical celiac disease as cause of increased need for thyroxine: A systematic study. J. Clin. Endocrin. Metab. 2012, 97, E419–E422. [Google Scholar] [CrossRef] [PubMed]
- Losurdo, G.; Principi, M.; Iannone, A.; Amoruso, A.; Ierardi, E.; Di Leo, A.; Barone, M. Extra-intestinal manifestations of non-celiac gluten sensitivity: An expanding paradigm. World J. Gastroenterol. 2018, 24, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Malandrini, S.; Trimboli, P.; Guzzaloni, G.; Virili, C.; Lucchini, B. What about TSH and Anti-Thyroid Antibodies in Patients with Autoimmune Thyroiditis and Celiac Disease Using a Gluten-Free Diet? A Systematic Review. Nutrients 2022, 14, 1681. [Google Scholar] [CrossRef] [PubMed]
- Valentino, R.; Savastano, S.; Tommaselli, A.P.; Dorato, M.; Scarpitta, M.T.; Gigante, M.; Micillo, M.; Paparo, F.; Petrone, E.; Lombardi, G.; et al. Prevalence of coeliac disease in patients with thyroid autoimmunity. Horm. Res. 1999, 51, 124–127. [Google Scholar] [CrossRef]
- Szostak-Wegierek, D.; Bednarczuk, T.; Respondek, W.; Traczyk, I.; Cukrowska, B.; Ostrowska, L.; Włodarek, D.; Jeznach-Steinhagen, A.; Bierła, J.; Lange, E.; et al. The validity of gluten-free diet in Hashimoto’s thyroiditis: Statement of the Expert Committee of the Section of Medical Dietetics of the Polish Society for Parenteral, Enteral Nutrition and Metabolism (POLSPEN). Adv. Clin. Nutr. 2018, 47, 33–47. [Google Scholar]
- Song, R.H.; Wang, B.; Yao, Q.M.; Li, Q.; Jia, X.; Zhang, J.A. The Impact of Obesity on Thyroid Autoimmunity and Dysfunction: A Systematic Review and Meta-Analysis. Front. Immunol. 2019, 10, 2349. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Sheikhi, A.; Varkaneh, H.K.; Zarezadeh, M.; Rahmani, J.; Milajerdi, A. Effect of Nigella sativa supplementation on obesity indices: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2018, 38, 48–57. [Google Scholar] [CrossRef]
- Namazi, N.; Larijani, B.; Ayati, M.H.; Abdollahi, M. The effects of Nigella sativa L. on obesity: A systematic review and meta-analysis. J. Ethnopharmacol. 2018, 219, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Khabbazi, A.; Javadivala, Z.; Seyedsadjadi, N.; Malek Mahdavi, A. A Systematic Review of the Potential Effects of Nigella sativa on Rheumatoid Arthritis. Planta Med. 2020, 86, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Noor, N.A.; Fahmy, H.M.; Mohammed, F.F.; Elsayed, A.A.; Radwan, N.M. Nigella sativa amliorates inflammation and demyelination in the experimental autoimmune encephalomyelitis-induced Wistar rats. Int. J. Clin. Exp. Pathol. 2015, 8, 6269–6286. [Google Scholar] [PubMed]
- Nikkhah-Bodaghi, M.; Darabi, Z.; Agah, S.; Hekmatdoost, A. The effects of Nigella sativa on quality of life, disease activity index, and some of inflammatory and oxidative stress factors in patients with ulcerative colitis. Phytother. Res. 2019, 33, 1027–1032. [Google Scholar] [CrossRef]
- Ahmed, J.H.; Kadhim, S.N.; Al-Hamdi, K.I. The effectiveness of Nigella sativa, methotrexate and their combination in the treatment of moderate to severe psoriasis. J. Clin. Exp. Investig. 2014, 5, 521–528. [Google Scholar] [CrossRef]
- Leung, A.M.; Braverman, L.E. Consequences of excess iodine. Nat. Rev. Endocrinol. 2014, 10, 136–142. [Google Scholar] [CrossRef]
- Møllehave, L.T.; Linneberg, A.; Skaaby, T.; Knudsen, N.; Jørgensen, T.; Thuesen, B.H. Trends in treatments of thyroid disease following iodine fortification in Denmark: A nationwide register-based study. Clin. Epidemiol. 2018, 10, 763–770. [Google Scholar] [CrossRef]
- Zimmermann, M.B.; Andersson, M. Global Endocrinology: Global perspectives in endocrinology: Coverage of iodized salt programs and iodine status in 2020. Eur. J. Endocrinol. 2021, 185, 13–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).