Secondary School Students and Caffeine: Consumption Habits, Motivations, and Experiences
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Caffeine Consumption Habits Questionnaire (CaffCo)
2.3. Data Analysis
2.3.1. Body Mass Index
2.3.2. Daily Caffeine Intake
2.3.3. Key Motivators for Caffeine Consumption by Source
2.3.4. Non-Consumption of Caffeinated Products
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Consumption of Caffeinated Products by Source
3.2.1. Daily Caffeine Intake
3.2.2. Key Motivators for Caffeine Consumption by Source
3.3. Non-Consumption of Caffeinated Products
3.4. Excess Consumption of Caffeinated Products
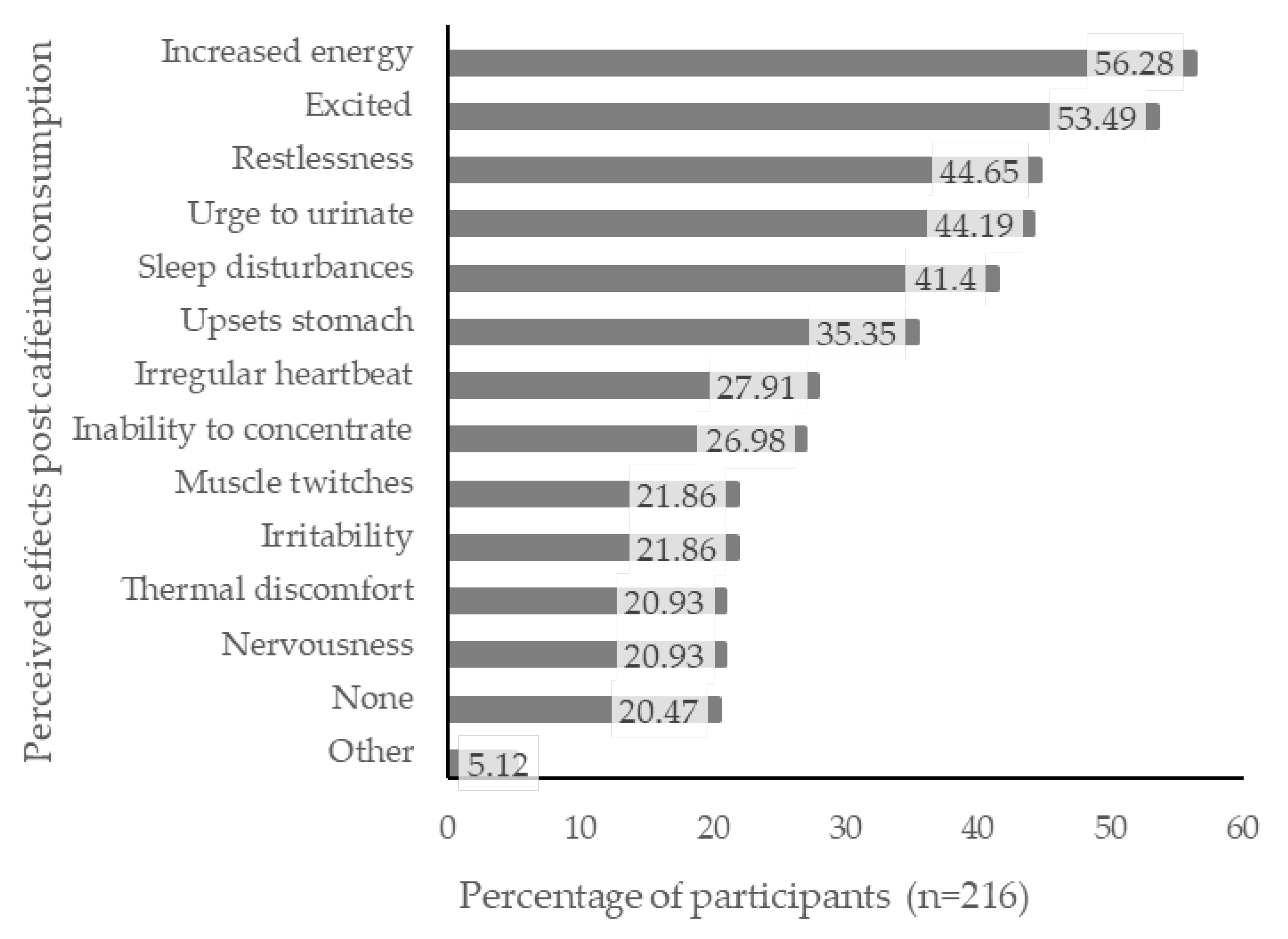
3.5. Perceived Effects from Caffeine Consumption
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fredholm, B.B. Notes on the History of Caffeine Use. In Methylxanthines; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–9. [Google Scholar]
- Gogtay, N.; Giedd, J.N.; Lusk, L.; Hayashi, K.M.; Greenstein, D.; Vaituzis, A.C.; Nugent, T.F.; Herman, D.H.; Clasen, L.S.; Toga, A.W.; et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc. Natl. Acad. Sci. USA 2004, 101, 8174–8179. [Google Scholar] [CrossRef] [PubMed]
- Kristjansson, A.L.; Sigfusdottir, I.D.; Allegrante, J.P.; James, J.E. Adolescent Caffeine Consumption, Daytime Sleepiness, and Anger. J. Caffeine Res. 2011, 1, 75–82. [Google Scholar] [CrossRef]
- Temple, J.L. Caffeine Use in Children: What we know, what we have left to learn, and why we should worry. Neurosci. Biobehav. Rev. 2009, 33, 793–806. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Palmer, A.A.; de Wit, H. Genetics of caffeine consumption and responses to caffeine. Psychopharmacology 2010, 211, 245–257. [Google Scholar] [CrossRef]
- Temple, J.L. Review: Trends, Safety, and Recommendations for Caffeine Use in Children and Adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 36–45. [Google Scholar] [CrossRef]
- Seifert, S.M.; Schaechter, J.L.; Hershorin, E.R.; Lipshultz, S.E. Health Effects of Energy Drinks on Children, Adolescents, and Young Adults. Pediatrics 2011, 127, 511–528. [Google Scholar] [CrossRef]
- Temple, J.L.; Bernard, C.; Lipshultz, S.E.; Czachor, J.D.; Westphal, J.A.; Mestre, M.A. The Safety of Ingested Caffeine: A Comprehensive Review. Front. Psychiatry 2017, 8, 80. [Google Scholar] [CrossRef]
- Temple, J.L.; Dewey, A.M.; Briatico, L.N. Effects of acute caffeine administration on adolescents. Exp. Clin. Psychopharmacol. 2010, 18, 510–520. [Google Scholar] [CrossRef]
- Thomson, B.M.; Campbell, D.M.; Cressey, P.; Egan, U.; Horn, B. Energy drink consumption and impact on caffeine risk. Food Addit. Contam. Part A 2014, 31, 1476–1488. [Google Scholar] [CrossRef]
- Nawrot, P.; Jordan, S.; Eastwood, J.; Rotstein, J.; Hugenholtz, A.; Feeley, M. Effects of caffeine on human health. Food Addit. Contam. 2003, 20, 1–30. [Google Scholar] [CrossRef]
- Seal, A.D.; Bardis, C.N.; Gavrieli, A.; Grigorakis, P.; Adams, J.D.; Arnaoutis, G.; Yannakoulia, M.; Kavouras, S.A. Coffee with High but Not Low Caffeine Content Augments Fluid and Electrolyte Excretion at Rest. Front. Nutr. 2017, 4, 40. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the safety of caffeine. EFSA J. 2015, 13, 4102. [Google Scholar] [CrossRef]
- Bunting, H.; Baggett, A.; Grigor, J. Adolescent and young adult perceptions of caffeinated energy drinks. A qualitative approach. Appetite 2013, 65, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Turton, P.; Piché, L.; Battram, D.S. Adolescent Attitudes and Beliefs Regarding Caffeine and the Consumption of Caffeinated Beverages. J. Nutr. Educ. Behav. 2016, 48, 181–189.e181. [Google Scholar] [CrossRef]
- Rowe, K. Caffeine Intake, Influences and Experiences: The Development of CaffCo: A New Zealand Caffeine Consumption Habits Questionnaire; Massey University: Albany, New Zealand, 2015. [Google Scholar]
- de Onis, M.; Onyango, A.W.; Borghi, E.; Siyam, A.; Nishida, C.; Siekmann, J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007, 85, 660–667. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Valek, M.; Laslavic, B.; Laslavic, Z. Daily caffeine intake among Osijek High School students: Questionnaire study. Croat. Med. J. 2004, 45, 72–75. [Google Scholar]
- Beckford, K.; Grimes, C.A.; Riddell, L.J. Australian children’s consumption of caffeinated, formulated beverages: A cross-sectional analysis. BMC Public Health 2015, 15, 70. [Google Scholar] [CrossRef]
- Tran, N.L.; Barraj, L.M.; Bi, X.; Jack, M.M. Trends and patterns of caffeine consumption among US teenagers and young adults, NHANES 2003–2012. Food Chem. Toxicol. 2016, 94, 227–242. [Google Scholar] [CrossRef]
- Lim, H.S.; Hwang, J.Y.; Choi, J.C.; Kim, M. Assessment of caffeine intake in the Korean population. Food Addit. Contam. Part A 2015, 32, 1786–1798. [Google Scholar] [CrossRef]
- Zucconi, S.; Volpato, C.; Adinolfi, F.; Gandini, E.; Gentile, E.; Loi, A.; Fioriti, L. Gathering consumption data on specific consumer groups of energy drinks. EFSA Supporting Publications 2013, 10, 394E. [Google Scholar] [CrossRef]
- Halldorsson, T.I.; Kristjansson, A.L.; Thorisdottir, I.; Oddsdóttir, C.; Sveinbjörnsson, J.; Benediktsson, R.; Sigfusdottir, I.D.; Jörundsdóttir, H.; H, G. Caffeine exposure from beverages and its association with self-reported sleep duration and quality in a large sample of Icelandic adolescents. Food Chem. Toxicol. 2021, 157, 112549. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk-Bębenek, E.; Jagielski, P.; Schlegel-Zawadzka, M. Caffeine Consumption in a Group of Adolescents from South East Poland—A Cross Sectional Study. Nutrients 2021, 13, 2084. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Wham, C.; Wolber, F.; Dickens, M.; O’Keeffe, K.; Thunders, M.; Thomas, J.; Starck, C.; Rutherfurd-Markwick, K.J. The highs and lows of caffeine intake in New Zealand children. J. Caffeine Adenosine Res. 2018, 8. [Google Scholar] [CrossRef]
- Kwak, H.K.; Sa, J.; Choe, S.; Chaput, J.P.; Chung, J.; Cummings, G.; Lee, J. Prevalence and correlates of highly caffeinated beverage consumption among Korean adolescents. Osong Public Health Res. Perspect. 2021, 12, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Azagba, S.; Langille, D.; Asbridge, M. An emerging adolescent health risk: Caffeinated energy drink consumption patterns among high school students. Prev. Med. 2014, 62, 54–59. [Google Scholar] [CrossRef]
- Faris, M.A.; Epuru, S.; Al-Shimmari, S.; Al-Shammari, E. Alarming high levels of energy drink consumption among school children in Hail, northern of Saudi Arabia. Int. J. Child Health Nutr. 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Terry-McElrath, Y.M.; O’Malley, P.M.; Johnston, L.D. Energy drinks, soft drinks, and substance use among US secondary school students. J. Addict. Med. 2014, 8, 6–13. [Google Scholar] [CrossRef]
- Ludden, A.B.; Wolfson, A.R. Understanding adolescent caffeine use: Connecting use patterns with expectancies, reasons, and sleep. Health Educ. Behav. 2010, 37, 330–342. [Google Scholar] [CrossRef]
- Nowak, D.; Jasionowski, A. Analysis of the Consumption of Caffeinated Energy Drinks among Polish Adolescents. Int. J. Environ. Res. Public Health 2015, 12, 7910–7921. [Google Scholar] [CrossRef]
- Miller, K.E. Wired: Energy drinks, jock identity, masculine norms, and risk taking. J. Am. Coll. Health 2008, 56, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Reissig, C.J.; Strain, E.C.; Griffiths, R.R. Caffeinated Energy Drinks–A Growing Problem. Drug Alcohol Depend. 2009, 99, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, D.D.; Jakovljevic, M.; Scekic, M.; Djordjevic, N. Caffeine consumption patterns and determinants among adolescents in Serbia. Int. J. Adolesc. Med. Health 2016, 30. [Google Scholar] [CrossRef] [PubMed]
- The New Zealand Institute for Plant and Food Research Limited; Ministry of Health. The Concise New Zealand Food Composition Tables; The New Zealand Institute for Plant and Food Research Limited: Albany, New Zealand, 2015; pp. 31–46. [Google Scholar]
- Costa, B.M.; Hayley, A.; Miller, P. Young adolescents’ perceptions, patterns, and contexts of energy drink use. A focus group study. Appetite 2014, 80, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Hammond, D.; Reid, J.L. Exposure and perceptions of marketing for caffeinated energy drinks among young Canadians. Public Health Nutr. 2017, 21, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Braun, H.; Koehler, K.; Geyer, G.; Kleinert, J.; Mester, J.; Schänzer, W. Dietary Supplement Use among Elite Young German Athletes. Int. J. Sport Nutr. Exerc. Metab. 2009, 19, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Arria, A.M.; Caldeira, K.M.; Kasperski, S.J.; O’Grady, K.E.; Vincent, K.B.; Griffiths, R.R.; Wish, E.D. Increased alcohol consumption, nonmedical prescription drug use, and illicit drug use are associated with energy drink consumption among college students. J. Addict. Med. 2010, 4, 74–80. [Google Scholar] [CrossRef]
- Van Batenburg-Eddes, T.; Lee, N.C.; Weeda, W.D.; Krabbendam, L.; Huizinga, M. The potential adverse effect of energy drinks on executive functions in early adolescence. Front. Psychol. 2014, 5, 457. [Google Scholar] [CrossRef]
- James, J.E.; Kristjánsson, Á.L.; Sigfúsdóttir, I.D. Adolescent substance use, sleep, and academic achievement: Evidence of harm due to caffeine. J. Adolesc. 2011, 34, 665–673. [Google Scholar] [CrossRef]
- Pollak, C.P.; Bright, D. Caffeine Consumption and Weekly Sleep Patterns in US Seventh-, Eighth-, and Ninth-Graders. Pediatrics 2003, 111, 42–46. [Google Scholar] [CrossRef]
| Participant Characteristics | Total, n (%) | Boys, n (%) | Girls, n (%) |
|---|---|---|---|
| Gender a | 216 (100) | 75 (35.0) | 139 (65.0) |
| Employment status (n= 192) | |||
| No paid employment | 123 (64.1) | 41 (64.1) | 77 (63.1) |
| Paid employment | 69 (35.9) | 23 (35.9) | 45 (36.9) |
| BMI groups b (n= 133) | |||
| Thin/Underweight | 1 (0.75) | 0 (0.0) | 1 (1.2) |
| Normal | 105 (78.9) | 40 (80.0) | 64 (78.0) |
| Overweight | 24 (18.0) | 9 (18.0) | 15 (18.3) |
| Obese | 3 (2.3) | 1 (2.0) | 2 (2.4) |
| Caffeine Source | All Participants, n (%) (n = 215) a | Boys, n (%) (n = 74) a | Girls, n (%) (n = 139) | Pearson’s Chi-Square Test (ꭕ2) | p-Value b | Odds Ratio | No Paid Employment, n (%) (n = 123) | Paid Employment, n (%) (n = 69) | Pearson’s Chi-Square Test (ꭕ2) | p-Value b | Odds Ratio |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All sources | 204 (94.9) | 69 (93.2) | 133 (95.7) | - | 0.520 c | - | 119 (96.7) | 68 (98.6) | - | 0.665 c | - |
| Tea | 119 (55.3) | 31 (41.9) | 86 (61.9) | 7.786 | 0.005 d | 2.25 | 73 (59.3) | 35 (50.7) | 1.336 | 0.248 | - |
| Coffee | 121 (56.3) | 35 (47.3) | 84 (60.4) | 3.379 | 0.066 | - | 66 (53.7) | 48 (69.6) | 4.637 | 0.031 d | 1.93 |
| Chocolate | 183 (85.1) | 59 (79.7) | 123 (88.5) | 2.980 | 0.084 | - | 111 (90.2) | 62 (89.9) | 0.007 | 0.931 | - |
| Kola drinks | 117 (54.4) | 46 (62.2) | 70 (50.4) | 2.712 | 0.100 | - | 73 (59.3) | 37 (53.6) | 0.592 | 0.441 | - |
| Energy drinks | 68 (31.6) | 32 (43.2) | 36 (25.9) | 6.684 | 0.010 d | 2.18 | 41 (33.3) | 23 (33.3) | 0.000 | 1.000 | - |
| Caffeinated RTD | 47 (21.9) | 18 (24.3) | 28 (20.1) | 0.498 | 0.480 | - | 19 (15.4) | 26 (37.7) | <0.001 d | 3.23 | |
| Sports supplements | 6 (2.8) | 3 (4.1) | 3 (2.2) | - | 0.421 c | - | 3 (2.4) | 3 (4.3) | - | 0.669 c | - |
| Caffeine tablets e | 1 (0.5) | - | - | - | - | - | - | - | - | - | |
| Consumes no caffeine | 11 (5.1) | 5 (6.8) | 6 (4.3) | - | 0.520 c | - | 4 (3.3) | 1 (1.4) | - | 1.000 c | - |
| Caffeine Source | All Participants (mg·day−1) (n = 215) a | Boys (mg·day−1) (n = 74) a | Girls (mg·day−1) (n = 139) | Mann−Whitney Test Statistic (U) | p-Value b | Effect Size ® | No Paid Employment (mg·day−1) (n = 123) | Paid Employment (mg·day−1) (n = 69) | Mann−Whitney Test Statistic (U) | p-Value b | Effect Size ® |
|---|---|---|---|---|---|---|---|---|---|---|---|
| All sources | 68.3 (4.9, 158.9) | 52.3 (18.3, 109) | 67.6 (28.7, 167) | 3986.0 | 0.126 | - | 68.3 (20.4, 134.3) | 67.1 (29.2, 194) | 3722.0 | 0.363 | - |
| Tea | 3.71 (0.00, 25.2) | 0.00 (0.00, 18.3) | 4.33 (0.00, 26.5) | 3702.0 | 0.019 c | 0.165 | 4.64 (0.00, 27.0) | 0.468 (0.00, 23.2) | 3604.5 | 0.197 | - |
| Coffee | 10.7 (0.00, 88.6) | 2.04 (0.00, 55.0) | 17.4 (0.00, 90.9) | 4041.5 | 0.150 | - | 5.87 (0.00, 75.4) | 31.0 (0.00, 116) | 3251.0 | 0.021 c | 0.168 |
| Chocolate | 6.28 (2.58, 11.4) | 5.87 (2.58, 10.0) | 6.72 (2.70, 11.3) | 4088.5 | 0.204 | - | 7.12 (2.70, 11.7) | 5.96 (2.70, 11.8) | 3828.5 | 0.541 | - |
| Kola type drinks | 2.25 (0.00, 9.25) | 5.53 (0.00, 18.2) | 1.06 (0.00, 5.56) | 3466.5 | 0.003 c | 0.209 | 3.62 (0.00, 8.84) | 0.863 (0.00, 11.1) | 3698.5 | 0.312 | - |
| Energy drinks | 0.00 (0.00, 3.18) | 0.00 (0.00, 19.8) | 0.00 (0.00, 1.86) | 3601.0 | 0.003 c | 0.210 | 0.00 (0.00, 5.20) | 0.00 (0.00, 3.18) | 3968.5 | 0.805 | - |
| Caffeinated RTD | 0.00(0.00, 0.00) | 0.00(0.00, 0.71) | 0.00(0.00, 0.00) | 4282.0 | 0.289 | - | 0.00(0.00, 0.00) | 0.00(0.00, 1.54) | 3197.0 | 0.001 c | 0.233 |
| Motivators | Tea (%) | Coffee (%) | Chocolate (%) | Kola Drinks (%) | Energy Drinks (%) | Caffeinated RTDs (%) | Sports Supplements (%) |
|---|---|---|---|---|---|---|---|
| Taste | 90.1 | 87.7 | 97.3 | 91.0 | 72.6 | 74.0 | - |
| Temperature (Warm b /cold c) | 88.4 b | 81.2 b | 71.5 b | 94.2 c | 73.9 c | 60.0 c | - |
| To comfort and relax myself | 88.4 | 52.4 | 64.5 | - | - | 26.0 | - |
| Whenever it is offered to me | 81.0 | 73.7 | 75.8 | 72.7 | 64.4 | 76.0 | - |
| Because it is easily available | 79.3 | 77.1 | 63.4 | 64.4 | 49.3 | - | - |
| To wake up | 34.7 | 87.7 | - | - | 72.6 | - | - |
| To stay awake | - | 85.3 | - | - | 82.2 | - | - |
| For energy | 26.4 | 83.6 | - | 37.2 | 85.0 | - | 61.2 |
| As a treat | - | - | 87.7 | 82.7 | - | - | - |
| With friends | 58.7 | 75.4 | 84.4 | 82.7 | 63.0 | 90.0 | 25.0 |
| For physical energy | - | 67.2 | - | - | 72.6 | 20 | 23.1 |
| For the alcohol content | - | - | - | - | - | 84.0 | - |
| Others are eating/drinking it | 29.7 | 46.7 | 47.3 | 38 | 26.0 | 72.0 | 38.5 |
| Peer pressure | >10 | 12.3 | >10 | >10 | 12.30 | 22.0 | 61.2 |
| As a substitute for illegal drugs | - | - | - | - | - | - | 61.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turner, S.; Ali, A.; Wham, C.; Rutherfurd-Markwick, K. Secondary School Students and Caffeine: Consumption Habits, Motivations, and Experiences. Nutrients 2023, 15, 1011. https://doi.org/10.3390/nu15041011
Turner S, Ali A, Wham C, Rutherfurd-Markwick K. Secondary School Students and Caffeine: Consumption Habits, Motivations, and Experiences. Nutrients. 2023; 15(4):1011. https://doi.org/10.3390/nu15041011
Chicago/Turabian StyleTurner, Sophie, Ajmol Ali, Carol Wham, and Kay Rutherfurd-Markwick. 2023. "Secondary School Students and Caffeine: Consumption Habits, Motivations, and Experiences" Nutrients 15, no. 4: 1011. https://doi.org/10.3390/nu15041011
APA StyleTurner, S., Ali, A., Wham, C., & Rutherfurd-Markwick, K. (2023). Secondary School Students and Caffeine: Consumption Habits, Motivations, and Experiences. Nutrients, 15(4), 1011. https://doi.org/10.3390/nu15041011









