A Review on Polyphenols in Salicornia ramosissima with Special Emphasis on Their Beneficial Effects on Brain Ischemia
Abstract
1. Introduction
2. Natural Bioactive Compounds Found in S. ramosissima
3. Bioactive Compounds with Neuroprotective Effect against Brain Ischemia
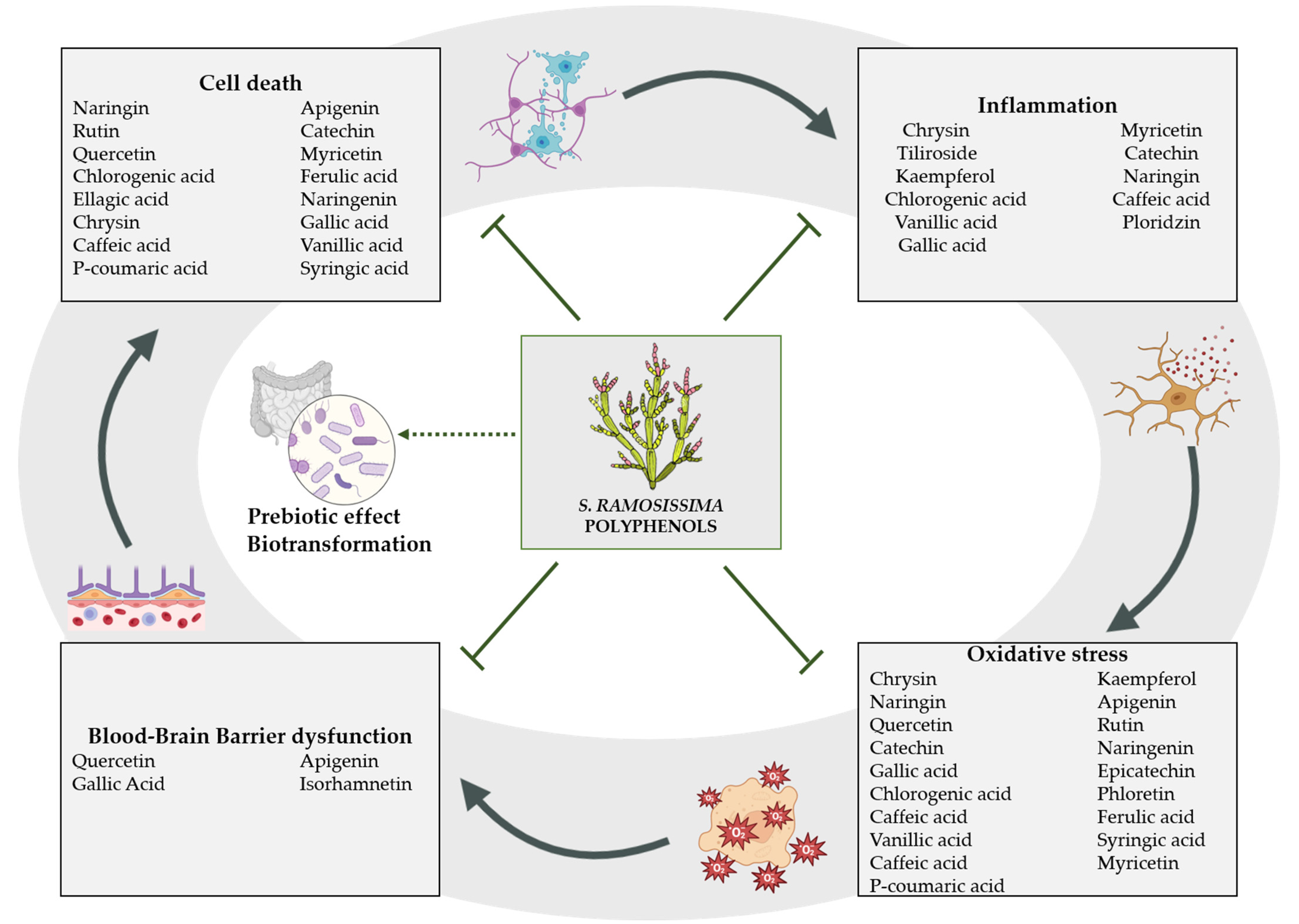
3.1. Polyphenols
3.1.1. Phenolic Acids
3.1.2. Flavonoids
| Polyphenol | Model | Treatment | Observed Effects | Molecular Mechanism | Ref. |
|---|---|---|---|---|---|
| Gallic acid | Male SD rats | 20 min before tMCAO (25, 50 mg/kg; i.v.) | Decreased infarct volume Anti-apoptosis Alleviated mitochondrial dysfunction | ↓ Cyt C ↓ MPTP | [33] |
| Male Wistar rats | Once daily for 10 days before transient 4VO (100 mg/kg; p.o.) | Ameliorated brain oxidative stress Improved the BBB disruption Alleviated anxiety, depression, locomotion behaviors | ↑ SOD ↓ MDA | [34] | |
| Male C57BL/6J mice | 30 min, 1, 12, 24, 48 and 72 h after ischemia in tMCAO (50, 100, 150 mg/kg; i.p.) | Reduced infarct area and edema Improved BBB disruption Anti-inflammatory Improved neurological function Inhibited microglial activation | ↓ IL-1β, TNF-α, IL-6 ↑ IL-10 ↓ MMP-9 ↑ ZO-1, Claudin-5 ↓ Iba-1 | [35] | |
| Caffeic acid | Male SD rats | 30 min before and from 0 h to 5th day after tMCAO (10, 50 mg/kg; i.p.) | Decreased infarct volume and neuron loss Ameliorated neurological dysfunction Attenuated late astrocyte proliferation | ↓ 5-LOX | [36] |
| Male SD rats | 30 min before BCCAO combined with hypotension (10,30, 50 mg/kg; i.p.) | Preserved hippocampal neurons Anti-apoptosis Improved learning and memory function Reduced brain oxidative stress Anti-inflammatory | ↑ SOD ↓ MDA ↓ 5-LOX ↓ NF-κBp65 | [37] | |
| Chlorogenic acid | Male SD rats | For 7 days before BCCAO (20, 100, 500 mg/kg; p.o.) | Reduced infarct volume and hippocampal neuron loss Anti-apoptosis Relieved nerve injury Ameliorated oxidative stress | ↑ BDNF, NGF ↑ SOD, GSH ↓ MDA, ROS ↑ Nrf2/NQO-1/HO-1 | [44] |
| Male SD rats | 2 h after pMCAO (30 mg/kg; i.p.) | Alleviated brain infarction and edema Anti-apoptosis Improved neurobehavioral deficits | ↓ ROS, LPO ↓ Caspase-3, caspase-7 ↓ PARP | [41] | |
| Male SD rats | 2 h after pMCAO (30 mg/kg; i.p.) | Ameliorated oxidative stress Inhibits the activation of astrocytes and microglia Anti-inflammatory | ↓ ROS, LPO ↓ GFAP, Iba-1 ↓ NF-κB ↓ IL-1β, TNF-α | [39] | |
| Ferulic acid | Male SD rats | Pre (2 and 4 h) and post (0,2 and 24 h) tMCAO (100 mg/kg; i.v.) | Alleviated brain infarction Anti-apoptosis Suppressed reactive astrocytosis Improved neurological deficits | ↑ p38 MAPK/p90RSK/CREB/Bcl-2 signaling pathway ↓ GFAP ↓ Mitochondrial Bax ↓ Cyt C, Caspase-3 | [49] |
| Male SD rats | 5 consecutive days after BCCAO (28, 56, 112 mg/kg) | Reduced hippocampal neuron loss Anti-apoptosis Improved memory deficits Anti-oxidative stress | ↑ Bcl-2/Bax ratio ↓ Caspase-3 ↑ SOD, GSH ↓ MDA | [50] | |
| P-coumaric acid | Male SD rats | 5 min after pMCAO (100 mg/kg; i.p.) | Anti-oxidative stress Anti-apoptosis Ameliorated neurological deficits | ↑ Nrf1, SOD ↓ MDA ↓ caspase-3, caspase-9 ↑ ERK, Akt ↓ ASK1 | [52] |
| Male ICR mice | 2 weeks before BCCAO (100 mg/kg; p.o.) | Reduced infarction size Ameliorated brain oxidative stress Anti-apoptosis | ↑ SOD, CAT ↓ MDA ↓ calcium | [54] | |
| Vanillic acid | Male SD rats | Once daily for 14 days before tMCAO (50, 100 mg/kg; p.o.) | Ameliorated cerebral infarct volume Anti-inflammatory Ameliorated oxidative stress Reduce neurological deficits | ↓ NF-κB ↓ IL-1β, IL-6, TNF-α ↓ MDA ↑ CAT, SOD | [55] |
| Male Wistar rats | Once daily for 14 days before BCCAO (100 mg/kg; p.o.) | Reduced hippocampal neuron loss Anti-inflammatory Anti-apoptosis Reversed cognitive deficits | ↑ IL-10, IL-6, TNF-α | [56] | |
| Syringic acid | Male SD rats | 5 min after pMCAO (10 mg/kg; i.p.) | Reduced histopathological changes Anti-oxidative stress Anti-apoptosis | ↑ NRF1, SOD ↓ MDA ↓ Caspase-3, Caspase-9 | [58] |
| Sinapic acid | Male Wistar rats | 0 and 90 min aftertransient 4VO (10 mg/kg; i.p.) | Reduced hippocampal neuronal loss Improved cognitive impairment | [59] | |
| Ellagic acid | Male SD rats | Once daily for 14 days before photothrombotic nerve injury (10, 30 mg/kg; p.o.) | Decreased the volume of infarction Decreased apoptosis Ameliorated neurological deficits | ↑ Bcl-2 | [60] |
| Chrysin | Male Wistar rats | Once daily 3 weeks prior to BCCAO, (10, 30, 100 mg/kg; p.o.) | Anti-apoptosis Attenuated memory impairment and sensorimotor parameters Ameliorated oxidative stress Decreased reactive hyperemia | ↑ GPx ↓ MDA ↓ NO ↓ PGE2 | [68] |
| Male C57/BL6 mice | Once daily for 7 days before tMCAO (75 mg/kg; p.o.) | Reduced infarct volume and neuron loss Anti-inflammatory activity Anti-oxidative effects | ↓ NF-κB, COX-2 ↓ iNOS ↑ SOD ↓ MDA ↓ GFAP, Iba-1 | [69] | |
| Kaempferol | Male SD rats | Once daily for 1 week before tMCAO (1.75, 3.49, 6.99 mM, 1 mL/kg; p.o.) | Decrease infarction volume Improved neurological deficit Anti-inflammatory Anti-oxidative effects | ↑ Nrf2 ↑ Akt ↓ NF-kβ, Gsk3β | [75] |
| Naringin | Male SD rats | Once daily for 7 days before tMCAO (5 mg/kg; i.p.) | Decreased infarction volume Anti-apoptosis | ↓ TNF-α ↓ IL-6 | [79] |
| Male SD rats | Once at reperfusion after tMCAO (80, 120, 160 mg/kg; i.v.) | Decreased infarction volume Reduced neurological damage Anti-apoptosis | ↓ ONOO− | [80] | |
| Phloretin | Male SD rats | Once daily for 14 days prior to tMCAO (20, 40, 80 mg/kg; i.p.) | Reduced infarct volume Anti-oxidative stress Reduced neurological damage | ↑ Nrf2 | [87] |
| Quercetin | Male SD rats | Twice daily for 3 days before BCCAO (25 μmol/kg; i.cv.) | Reduced hippocampal neuron loss Improved neurologic function Reduced brain edema Improved BBB permeability | ↑ Claudin-5, ZO-1 ↓ MMP-9 ↑ Wnt/β-catenin signaling | [72] |
| Epicatechin | Male C57BL/6 mice | 90 min prior to pMCAO (5, 10, 15 mg/kg; p.o.) | Reduced infarct volume and neuron loss Improved motor coordination Anti-oxidative stress | ↑ Nrf2 ↓ Iba-1 | [93] |
| Male C57BL/6 mice | 90 min prior to tMCAO (2.5, 5, 15, 30 mg/kg; p.o.) | Decreased infarction volume Improved neurological score | ↑ Nrf2 | [94] | |
| Apigenin | Male SD rats | Once daily for 7/14 days after tMCAO (25 mg/kg; i.p.) | Reduced infarct volume Anti-apoptosis Improved BBB function Magnification in angiogenesis | ↑ VEGFs ↑ Caveolin-1 | [107] |
| Male SD rats | Once daily for 7 days after tMCAO (25 mg/kg; i.p.) | Decreased infarction volume Improved neurological score | ↓ ROS | [105] | |
| Male SD rats | Once daily for 25 days after tMCAO (20, 40 mg/kg; i.p.) | Decreased infarction volume Improved neuron viability Improve neurological score | ↑ BDNF ↑ Syn-1 | [106] | |
| Myricetin | Male SD rats | Once daily for 7 days prior to pMCAO (1, 5, 25 mg/kg; p.o.) | Decreased infarction volume Anti-inflammatory Anti-apoptosis Decreased oxidative stress | ↓ TNF-α, IL-6, IL-1β ↑ SOD ↓ MDA | [115] |
| Rutin | Male Wistar rats | Pretreatment for 21 days before tMCAO (25 mg/kg; orally) | Decreased oxidative stress Attenuated apoptosis Reduction in infarct size Improved neurobehavioral deficits | ↑ GPx, GR, SOD, CAT, GSH ↓ H2O2, PC ↓ p53 | [100] |
| Catechin | Mongolian gerbils | Once daily for 14 days prior and 7 days post tMCAO (5, 50 mg/kg; solved in drinking water) | Improved hippocampal neuron viability | ↓ iNOS ↓O2− | [97] |
| Male Wistar rats | 5 days prior tMCAO (0.25%, 0.5%; solved in drinking water) | Decreased infarction volume Improve neurological score | ↓ MDA ↓ iNOS ↓ NF-κB | [98] | |
| Naringenin | Male Wistar rats | Once daily for 21 days prior tMCAO (10, 25, 50 mg/kg; p.o.) | Decreased infarction volume Improve neurological score Improved neuron viability | ↑ SOD ↓ iNOS ↓ NF-κB,TNF-α | [84] |
| Phloridzin | Male ddY mice | 0 and 6 h after tMCAO (40, 120, 200 mg/kg; i.p.) (10, 40 µg; i.c.v.) | Decreased infarction volume Improved neurological score Decreased FBG | ↓SGLT | [90] |
| Taxifolin | Male Long-Evans rats | 1 h after pMCAO (0.1, 1 µg/kg; i.v.) | Decreased infarction volume | ↓ iNOS, COX-2 ↓ ICAM-1 ↓ NF-κB | [121] |
| Isorhamnetin | Male ICR mice | 0 h after tMCAO (5 mg/kg; i.p.) | Decreased infarction volume Reduced brain edema Improved BBB function | ↑ Claudin-5, ZO-1, occludin ↓ TNF-α, IL-6, IL-1β ↓ MDA | [126] |
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopes, M.; Sanches-Silva, A.; Castilho, M.; Cavaleiro, C.; Ramos, F. Halophytes as source of bioactive phenolic compounds and their potential applications. Crit. Rev. Food Sci. Nutr. 2021, 2, 1–24. [Google Scholar] [CrossRef]
- Giordano, R.; Saii, Z.; Fredsgaard, M.; Hulkko, L.S.S.; Poulsen, T.B.G.; Thomsen, M.E.; Henneberg, N.; Zucolotto, S.M.; Arendt-Nielsen, L.; Papenbrock, J.; et al. Pharmacological Insights into Halophyte Bioactive Extract Action on Anti-Inflammatory, Pain Relief and Antibiotics-Type Mechanisms. Molecules 2021, 26, 3140. [Google Scholar] [CrossRef]
- Antunes, M.D.; Gago, C.; Guerreiro, A.; Sousa, A.R.; Julião, M.; Miguel, M.G.; Faleiro, M.L.; Panagopoulos, T. Nutritional Characterization and Storage Ability of Salicornia ramosissima and Sarcocornia perennis for Fresh Vegetable Salads. Horticulturae 2021, 7, 6. [Google Scholar] [CrossRef]
- Lopes, M.; Cavaleiro, C.; Ramos, F. Sodium Reduction in Bread: A Role for Glasswort (Salicornia ramosissima J. Woods). Compr. Rev. Food Sci. Food Saf. 2017, 16, 1056–1071. [Google Scholar] [CrossRef]
- Choi, S.C.; Kim, B.J.; Rhee, P.L.; Chang, D.K.; Son, H.J.; Kim, J.J.; Rhee, J.C.; Kim, S.I.; Han, Y.S.; Sim, K.H.; et al. Probiotic Fermented Milk Containing Dietary Fiber Has Additive Effects in IBS with Constipation Compared to Plain Probiotic Fermented Milk. Gut Liver 2011, 5, 22–28. [Google Scholar] [CrossRef]
- Campbell, B.C.V.; De Silva, D.A.; Macleod, M.R.; Coutts, S.B.; Schwamm, L.H.; Davis, S.M.; Donnan, G.A. Ischaemic stroke. Nat. Rev. Dis. Prim. 2019, 5, 70. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) guidelines on intravenous thrombolysis for acute ischaemic stroke. Eur. Stroke J. 2021, 6, I-LXII. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Evora, P.; Castro, E.S.O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Thundyil, J.; Tang, S.C.; Sobey, C.G.; Taylor, S.M.; Arumugam, T.V. athophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011, 6, 11. [Google Scholar] [CrossRef]
- Hankey, G.J. Secondary stroke prevention. Lancet Neurol. 2014, 13, 178–194. [Google Scholar] [CrossRef]
- Hackam, D.G.; Spence, J.D. Combining multiple approaches for the secondary prevention of vascular events after stroke: A quantitative modeling study. Stroke 2007, 38, 1881–1885. [Google Scholar] [CrossRef]
- Garcia-Rodriguez, P.; Ma, F.; Rio, C.D.; Romero-Bernal, M.; Najar, A.M.; Cadiz-Gurrea, M.L.; Leyva-Jimenez, F.J.; Ramiro, L.; Menendez-Valladares, P.; Perez-Sanchez, S.; et al. Diet Supplementation with Polyphenol-Rich Salicornia ramosissima Extracts Protects against Tissue Damage in Experimental Models of Cerebral Ischemia. Nutrients 2022, 14, 5077. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Gavilan, I.; Ramirez, E.; de la Fuente, V. Bioactive Compounds in Salicornia patula Duval-Jouve: A Mediterranean Edible Euhalophyte. Foods 2021, 10, 410. [Google Scholar] [CrossRef] [PubMed]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Hernández-Carlos, B.; Villanueva-Cañongo, C. Shikimic Acid Pathway in Biosynthesis of Phenolic Compounds. In Plant Physiological Aspects of Phenolic Compounds; IntechOpen: London, UK, 2019; pp. 1–15. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Falleh, H.; Trabelsi, N.; Boulaaba, M.; Smaoui, A.; Abdelly, C. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. Comptes Rendus Biol. 2008, 331, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Irfan, M.I.-D.M.; Raghib, F.; Ahmad, B. Role and Regulation of Plants Phenolics in Abiotic Stress Tolerance: An Overview, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. 9. [Google Scholar] [CrossRef]
- Lima, A.R.; Castaneda-Loaiza, V.; Salazar, M.; Nunes, C.; Quintas, C.; Gama, F.; Pestana, M.; Correia, P.J.; Santos, T.; Varela, J.; et al. Influence of cultivation salinity in the nutritional composition, antioxidant capacity and microbial quality of Salicornia ramosissima commercially produced in soilless systems. Food Chem. 2020, 333, 127525. [Google Scholar] [CrossRef]
- Silva, A.M.; Lago, J.P.; Pinto, D.; Moreira, M.M.; Grosso, C.; Cruz Fernandes, V.; Delerue-Matos, C.; Rodrigues, F. Salicornia ramosissima Bioactive Composition and Safety: Eco-Friendly Extractions Approach (Microwave-Assisted Extraction vs. Conventional Maceration). Appl. Sci. 2021, 11, 4744. [Google Scholar] [CrossRef]
- Oliveira-Alves, S.C.; Andrade, F.; Prazeres, I.; Silva, A.B.; Capelo, J.; Duarte, B.; Cacador, I.; Coelho, J.; Serra, A.T.; Bronze, M.R. Impact of Drying Processes on the Nutritional Composition, Volatile Profile, Phytochemical Content and Bioactivity of Salicornia ramosissima J. Woods. Antioxidants 2021, 10, 1312. [Google Scholar] [CrossRef]
- Surget, G.; Stiger-Pouvreau, V.; Le Lann, K.; Kervarec, N.; Couteau, C.; Coiffard, L.J.; Gaillard, F.; Cahier, K.; Guerard, F.; Poupart, N. Structural elucidation, in vitro antioxidant and photoprotective capacities of a purified polyphenolic-enriched fraction from a saltmarsh plant. J. Photochem. Photobiol. B Biol. 2015, 143, 52–60. [Google Scholar] [CrossRef]
- Guerreiro, A.; Rassal, C.; Afonso, C.M.; Galego, L.; Serra, M.; Rodrigues, M.A. Healthy, Tasty and Sustainable Mediterranean Food. UMAMI Taste and Polyphenols of Twiggy Glasswort (Salicornia ramosissima). In International Congress on Engineering and Sustainability in the XXI Century; Springer: Berlin/Heidelberg, Germany, 2017; pp. 191–198. [Google Scholar] [CrossRef]
- Ferreira, D.; Isca, V.M.; Leal, P.; Seca, A.M.; Silva, H.; de Lourdes Pereira, M.; Silva, A.M.; Pinto, D.C. Salicornia ramosissima: Secondary metabolites and protective effect against acute testicular toxicity. Arab. J. Chem. 2018, 11, 70–80. [Google Scholar] [CrossRef]
- Isca, V.M.; Seca, A.M.; Pinto, D.C.; Silva, H.; Silva, A.M. Lipophilic profile of the edible halophyte Salicornia ramosissima. Food Chem. 2014, 165, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Goel, N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019, 24, e00370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cai, P.; Cheng, G.; Zhang, Y. A Brief Review of Phenolic Compounds Identified from Plants: Their Extraction, Analysis, and Biological Activity. Nat. Prod. Commun. 2022, 17, 1934578X211069721. [Google Scholar] [CrossRef]
- Radtke, J.; Linseisen, J.; Wolfram, G. Phenolic acid intake of adults in a Bavarian subgroup of the national food consumption survey. Z. Ernahr. 1998, 37, 190–197. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Medina-Remon, A.; Perez-Jimenez, J.; Martinez-Gonzalez, M.A.; Covas, M.I.; Corella, D.; Salas-Salvado, J.; Gomez-Gracia, E.; Lapetra, J.; Aros, F.; et al. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: The PREDIMED study. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 953–959. [Google Scholar] [CrossRef]
- Scalbert, A.; Williamson, G. Dietary intake and bioavailability of polyphenols. J. Nutr. 2000, 130, 2073S–2085S. [Google Scholar] [CrossRef]
- Gao, J.; Hu, J.; Hu, D.; Yang, X. A Role of Gallic Acid in Oxidative Damage Diseases: A Comprehensive Review. Nat. Prod. Commun. 2019, 14, 1934578X19874174. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.Z.; Ding, Y.H.; Wang, J.; Geng, J.; Yang, H.; Ren, J.; Tang, J.Y.; Gao, J. Neuroprotective effects of gallic acid against hypoxia/reoxygenation-induced mitochondrial dysfunctions in vitro and cerebral ischemia/reperfusion injury in vivo. Brain Res. 2014, 1589, 126–139. [Google Scholar] [CrossRef]
- Jahangiri, H.M.; Sarkaki, A.; Farbood, Y.; Dianat, M.; Goudarzi, G. Gallic acid affects blood-brain barrier permeability, behaviors, hippocampus local EEG, and brain oxidative stress in ischemic rats exposed to dusty particulate matter. Environ. Sci. Pollut. Res. 2020, 27, 5281–5292. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.; Wang, L.; Mao, Y. Gallic acid attenuates cerebral ischemia/re-perfusion-induced blood-brain barrier injury by modifying polarization of microglia. J. Immunotoxicol. 2022, 19, 17–26. [Google Scholar] [CrossRef]
- Zhou, Y.; Fang, S.H.; Ye, Y.L.; Chu, L.S.; Zhang, W.P.; Wang, M.L.; Wei, E.Q. Caffeic acid ameliorates early and delayed brain injuries after focal cerebral ischemia in rats. Acta Pharmacol. Sin. 2006, 27, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Shi, B.; Luo, W.; Yang, J. The protective effect of caffeic acid on global cerebral ischemia-reperfusion injury in rats. Behav. Brain Funct. 2015, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Shah, M.A.; Kang, J.B.; Park, D.J.; Kim, M.O.; Koh, P.O. Chlorogenic acid alleviates cerebral ischemia-induced neuroinflammation via attenuating nuclear factor kappa B activation. Neurosci. Lett. 2022, 773, 136495. [Google Scholar] [CrossRef]
- Huang, S.M.; Chuang, H.C.; Wu, C.H.; Yen, G.C. Cytoprotective effects of phenolic acids on methylglyoxal-induced apoptosis in Neuro-2A cells. Mol. Nutr. Food Res. 2008, 52, 940–949. [Google Scholar] [CrossRef]
- Shah, M.A.; Kang, J.B.; Park, D.J.; Kim, M.O.; Koh, P.O. Chlorogenic acid alleviates neurobehavioral disorders and brain damage in focal ischemia animal models. Neurosci. Lett. 2021, 760, 136085. [Google Scholar] [CrossRef]
- Lafay, S.; Gil-Izquierdo, A.; Manach, C.; Morand, C.; Besson, C.; Scalbert, A. Chlorogenic acid is absorbed in its intact form in the stomach of rats. J. Nutr. 2006, 136, 1192–1197. [Google Scholar] [CrossRef]
- Olthof, M.R.; Hollman, P.C.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef]
- Liu, D.; Wang, H.; Zhang, Y.; Zhang, Z. Protective Effects of Chlorogenic Acid on Cerebral Ischemia/Reperfusion Injury Rats by Regulating Oxidative Stress-Related Nrf2 Pathway. Drug Des. Dev. Ther. 2020, 14, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, L.; Chen, B.; Fang, Y.; Lin, W.; Zhang, T.; Feng, X.; Tao, X.; Wu, Y.; Fu, X.; et al. Chlorogenic acid exerts neuroprotective effect against hypoxia-ischemia brain injury in neonatal rats by activating Sirt1 to regulate the Nrf2-NF-kappaB signaling pathway. Cell Commun. Signal. 2022, 20, 84. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.N.; Wu, W.J.; Sun, C.Z.; Liu, H.F.; Chen, W.B.; Zhan, Q.P.; Lei, Z.G.; Xin, X.; Ma, J.J.; Yao, K.; et al. Antioxidant and Anti-inflammatory Capacity of Ferulic Acid Released from Wheat Bran by Solid-state Fermentation of Aspergillus niger. Biomed. Environ. Sci. 2019, 32, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Tian, L.; Liu, Y.; Liu, J.; Huang, J. Ferulic Acid Protects Endothelial Cells from Hypoxia-Induced Injury by Regulating MicroRNA-92a. Appl. Bionics Biomech. 2022, 2022, 6148361. [Google Scholar] [CrossRef]
- Yogeeta, S.K.; Hanumantra, R.B.R.; Gnanapragasam, A.; Subramanian, S.; Rajakannu, S.; Devaki, T. Attenuation of Abnormalities in the Lipid Metabolism during Experimental Myocardial Infarction Induced by Isoproterenol in Rats: Beneficial Effect of Ferulic Acid and Ascorbic Acid. Basic Clin. Pharmacol. Toxicol. 2006, 98, 467–472. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Tang, N.Y.; Kao, S.T.; Hsieh, C.L. Ferulic Acid Administered at Various Time Points Protects against Cerebral Infarction by Activating p38 MAPK/p90RSK/CREB/Bcl-2 Anti-Apoptotic Signaling in the Subacute Phase of Cerebral Ischemia-Reperfusion Injury in Rats. PLoS ONE 2016, 11, e0155748. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, R.; Li, Y.; Li, Y.; Yang, Z.; Yang, H. Ferulic acid exerts neuroprotective effects against cerebral ischemia/reperfusion-induced injury via antioxidant and anti-apoptotic mechanisms in vitro and in vivo. Int. J. Mol. Med. 2017, 40, 1444–1456. [Google Scholar] [CrossRef]
- Anilkumar, U.; Prehn, J.H. Anti-apoptotic BCL-2 family proteins in acute neural injury. Front. Cell Neurosci. 2014, 8, 281. [Google Scholar] [CrossRef]
- Guven, M.; Aras, A.B.; Akman, T.; Sen, H.M.; Ozkan, A.; Salis, O.; Sehitoglu, I.; Kalkan, Y.; Silan, C.; Deniz, M.; et al. Neuroprotective effect of p-coumaric acid in rat model of embolic cerebral ischemia. Iran. J. Basic Med. Sci. 2015, 18, 356–363. [Google Scholar]
- Konishi, Y.; Hitomi, Y.; Yoshioka, E. Intestinal absorption of p-coumaric and gallic acids in rats after oral administration. J. Agric. Food Chem. 2004, 52, 2527–2532. [Google Scholar] [CrossRef]
- Sakamula, R.; Thong-Asa, W. Neuroprotective effect of p-coumaric acid in mice with cerebral ischemia reperfusion injuries. Metab. Brain Dis. 2018, 33, 765–773. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, Y.; Zhang, S.Y. Vanillic Acid Improve Neural Function after Focal Cerebral Ischemia-reperfusion Rats. Int. J. Pharmacol. 2018, 14, 488–494. [Google Scholar] [CrossRef]
- Khoshnam, S.E.; Sarkaki, A.; Rashno, M.; Farbood, Y. Memory deficits and hippocampal inflammation in cerebral hypoperfusion and reperfusion in male rats: Neuroprotective role of vanillic acid. Life Sci. 2018, 211, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhang, L.; Sun, S.; Yi, Z.; Jiang, X.; Jia, D. Neuroprotective effects of syringic acid against OGD/R-induced injury in cultured hippocampal neuronal cells. Int. J. Mol. Med. 2016, 38, 567–573. [Google Scholar] [CrossRef]
- Guven, M.; Aras, A.B.; Topaloglu, N.; Ozkan, A.; Sen, H.M.; Kalkan, Y.; Okuyucu, A.; Akbal, A.; Gokmen, F.; Cosar, M. The protective effect of syringic acid on ischemia injury in rat brain. Turk. J. Med. Sci. 2015, 45, 233–240. [Google Scholar] [CrossRef]
- Kim, Y.O.; Lee, S.W.; Oh, M.S.; Lee, H.J. Effects of sinapic Acid of 4 vessel occlusion model-induced ischemia and cognitive impairments in the rat. Clin. Psychopharmacol. Neurosci. 2011, 9, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.S.; Deng, R.; Li, S.; Li, X.; Li, K.; Kebaituli, G.; Li, X.; Liu, R. Ellagic acid protects against neuron damage in ischemic stroke through regulating the ratio of Bcl-2/Bax expression. Appl. Physiol. Nutr. Metab. 2017, 42, 855–860. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimaraes, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. NeuroPsychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef]
- Hayakawa, K.; Mishima, K.; Fujiwara, M. Therapeutic Potential of Non-Psychotropic Cannabidiol in Ischemic Stroke. Pharmacology 2010, 3, 2197–2212. [Google Scholar] [CrossRef]
- Pozdnyakov, D.I. 4-Hydroxy-3,5-di-tret-butyl cinnamic acid restores the activity of the hippocampal mitochondria in rats under permanent focal cerebral ischemia. Iran. J. Basic Med. Sci. 2021, 24, 1590–1601. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Campos, H.M.; da Costa, M.; da Silva Moreira, L.K.; da Silva Neri, H.F.; Branco da Silva, C.R.; Pruccoli, L.; Dos Santos, F.C.A.; Costa, E.A.; Tarozzi, A.; Ghedini, P.C. Protective effects of chrysin against the neurotoxicity induced by aluminium: In vitro and in vivo studies. Toxicology 2022, 465, 153033. [Google Scholar] [CrossRef]
- Shooshtari, M.K.; Sarkaki, A.; Mansouri, S.M.T.; Badavi, M.; Khorsandi, L.; Dehcheshmeh, M.G.; Farbood, Y. Protective effects of Chrysin against memory impairment, cerebral hyperemia and oxidative stress after cerebral hypoperfusion and reperfusion in rats. Metab. Brain Dis. 2020, 35, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Chen, L.; Xiao, J.; Wang, C.; Jiang, W.; Zhang, R.; Hao, J. Chrysin protects against focal cerebral ischemia/reperfusion injury in mice through attenuation of oxidative stress and inflammation. Int. J. Mol. Sci. 2014, 15, 20913–20926. [Google Scholar] [CrossRef] [PubMed]
- Michala, A.S.; Pritsa, A. Quercetin: A Molecule of Great Biochemical and Clinical Value and Its Beneficial Effect on Diabetes and Cancer. Diseases 2022, 10, 37. [Google Scholar] [CrossRef]
- Li, M.T.; Ke, J.; Guo, S.F.; Wu, Y.; Bian, Y.F.; Shan, L.L.; Liu, Q.Y.; Huo, Y.J.; Guo, C.; Liu, M.Y.; et al. The Protective Effect of Quercetin on Endothelial Cells Injured by Hypoxia and Reoxygenation. Front. Pharmacol. 2021, 12, 732874. [Google Scholar] [CrossRef]
- Jin, Z.; Ke, J.; Guo, P.; Wang, Y.; Wu, H. Quercetin improves blood-brain barrier dysfunction in rats with cerebral ischemia reperfusion via Wnt signaling pathway. Am J. Transl Res. 2019, 11, 4683–4695. [Google Scholar]
- Kaşıkcı, M.B.; Bağdatlıoğlu, N. Bioavailability of Quercetin. Curr. Res. Nutr. Food Sci. J. 2016, 4, 146–151. [Google Scholar] [CrossRef]
- Wang, J.; Li, T.; Feng, J.; Li, L.; Wang, R.; Cheng, H.; Yuan, Y. Kaempferol protects against gamma radiation-induced mortality and damage via inhibiting oxidative stress and modulating apoptotic molecules in vivo and vitro. Environ. Toxicol. Pharmacol. 2018, 60, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mao, J.; Wang, R.; Li, S.; Wu, B.; Yuan, Y. Kaempferol Protects Against Cerebral Ischemia Reperfusion Injury Through Intervening Oxidative and Inflammatory Stress Induced Apoptosis. Front. Pharmacol. 2020, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Del-Rio, L.; Nag, A.; Gutierrez Casado, E.; Ariza, J.; Awad, A.M.; Joseph, A.I.; Kwon, O.; Verdin, E.; de Cabo, R.; Schneider, C.; et al. Kaempferol increases levels of coenzyme Q in kidney cells and serves as a biosynthetic ring precursor. Free. Radic. Biol. Med. 2017, 110, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, C.; Wang, L.F.; Kuang, X.; Liu, K.; Zhang, H.; Du, J.R. Neuroprotective effect of kaempferol glycosiDes. against brain injury and neuroinflammation by inhibiting the activation of NF-kappaB and STAT3 in transient focal stroke. PLoS ONE 2013, 8, e55839. [Google Scholar] [CrossRef]
- Cao, W.; Feng, S.J.; Kan, M.C. Naringin Targets NFKB1 to Alleviate Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury in PC12 Cells Via Modulating HIF-1alpha/AKT/mTOR-Signaling Pathway. J. Mol. Neurosci. 2021, 71, 101–111. [Google Scholar] [CrossRef]
- Yang, J.; Yuan, L.; Wen, Y.; Zhou, H.; Jiang, W.; Xu, D.; Wang, M. Protective Effects of Naringin in Cerebral Infarction and Its Molecular Mechanism. Med. Sci. Monit. 2020, 26, e918772. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chen, X.; Lu, S.; Li, W.; Yang, D.; Su, W.; Wang, X.; Shen, J. Naringin Attenuates Cerebral Ischemia-Reperfusion Injury Through Inhibiting Peroxynitrite-Mediated Mitophagy Activation. Mol. Neurobiol. 2018, 55, 9029–9042. [Google Scholar] [CrossRef]
- Heidary Moghaddam, R.; Samimi, Z.; Moradi, S.Z.; Little, P.J.; Xu, S.; Farzaei, M.H. Naringenin and naringin in cardiovascular disease prevention: A preclinical review. Eur. J. Pharmacol. 2020, 887, 173535. [Google Scholar] [CrossRef]
- Zeng, W.; Jin, L.; Zhang, F.; Zhang, C.; Liang, W. Naringenin as a potential immunomodulator in therapeutics. Pharmacol. Res. 2018, 135, 122–126. [Google Scholar] [CrossRef]
- Zeng, H.; Shao, B.; Zhuang, J.; Peng, Y.; Chen, H.; Yu, Q.; Xu, C.; Fu, X.; Zhou, H.; Cao, Y.; et al. Naringenin reduces early brain injury in subarachnoid hemorrhage (SAH) mice: The role of the AMPK/SIRT3 signaling pathway. J. Funct. Foods 2020, 72, 104043. [Google Scholar] [CrossRef]
- Raza, S.S.; Khan, M.M.; Ahmad, A.; Ashafaq, M.; Islam, F.; Wagner, A.P.; Safhi, M.M.; Islam, F. Neuroprotective effect of naringenin is mediated through suppression of NF-kappaB signaling pathway in experimental stroke. Neuroscience 2013, 230, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Anunciato Casarini, T.P.; Frank, L.A.; Pohlmann, A.R.; Guterres, S.S. Dermatological applications of the flavonoid phloretin. Eur. J. Pharmacol. 2020, 889, 173593. [Google Scholar] [CrossRef]
- Oldendorf, W.H.; Crane, P.D.; Lawner, P.M.; Braun, L.D. Rapid, transient drop in brain glucose after intravenous phloretin or 3-0-methyl-D-glucose. Stroke 1983, 14, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, L.; Liang, J. Activation of the Nrf2 defense pathway contributes to neuroprotective effects of phloretin on oxidative stress injury after cerebral ischemia/reperfusion in rats. J. Neurol. Sci. 2015, 351, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Dierckx, T.; Haidar, M.; Grajchen, E.; Wouters, E.; Vanherle, S.; Loix, M.; Boeykens, A.; Bylemans, D.; Hardonniere, K.; Kerdine-Romer, S.; et al. Phloretin suppresses neuroinflammation by autophagy-mediated Nrf2 activation in macrophages. J. Neuroinflamm. 2021, 18, 148. [Google Scholar] [CrossRef]
- Betz, A.L.; Drewes, L.R.; Gilboe, D.D. Inhibition of glucose transport into brain by phlorizin, phloretin and glucose analogues. Biochim. Biophys. Acta (BBA)-Biomembr. 1975, 406, 505–515. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Harada, S.; Tokuyama, S. Post-ischemic hyperglycemia exacerbates the development of cerebral ischemic neuronal damage through the cerebral sodium-glucose transporter. Brain Res. 2012, 1489, 113–120. [Google Scholar] [CrossRef]
- Nehlig, A. The neuroprotective effects of cocoa flavanol and its influence on cognitive performance. Br. J. Clin. Pharmacol. 2013, 75, 716–727. [Google Scholar] [CrossRef]
- Schroeter, H.; Heiss, C.; Balzer, J.; Kleinbongard, P.; Keen, C.L.; Hollenberg, N.K.; Sies, H.; Kwik-Uribe, C.; Schmitz, H.H.; Kelm, M. (-)-Epicatechin mediates beneficial effects of flavanol-rich cocoa on vascular function in humans. Proc. Natl. Acad. Sci. USA 2006, 103, 1024–1029. [Google Scholar] [CrossRef]
- Leonardo, C.C.; Agrawal, M.; Singh, N.; Moore, J.R.; Biswal, S.; Dore, S. Oral administration of the flavanol (-)-epicatechin bolsters endogenous protection against focal ischemia through the Nrf2 cytoprotective pathway. Eur. J. Neurosci. 2013, 38, 3659–3668. [Google Scholar] [CrossRef]
- Shah, Z.A.; Li, R.C.; Ahmad, A.S.; Kensler, T.W.; Yamamoto, M.; Biswal, S.; Dore, S. The flavanol (-)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. J. Cereb. Blood Flow Metab. 2010, 30, 1951–1961. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Cho, S.; Wang, J. (-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2 pathways. Ann. Clin. Transl. Neurol. 2014, 1, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Bernatoniene, J.; Kopustinskiene, D.M. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules 2018, 23, 965. [Google Scholar] [CrossRef]
- Inanami, O.; Watanabe, Y.; Syuto, B.; Nakano, M.; Tsuji, M.; Kuwabara, M. Oral administration of (-)catechin protects against ischemia-reperfusion-induced neuronal death in the gerbil. Free. Radic. Res. 1998, 29, 359–365. [Google Scholar] [CrossRef]
- Suzuki, M.; Tabuchi, M.; Ikeda, M.; Umegaki, K.; Tomita, T. Protective effects of green tea catechins on cerebral ischemic damage. Med. Sci. Monit. 2004, 10, BR166–BR174. [Google Scholar] [PubMed]
- Nassiri-Asl, M.; Ghorbani, A.; Salehisar, S.; Asadpour, E.; Sadeghnia, H.R. Effect of rutin on oxidative DNA damage in PC12 neurons cultured in nutrients deprivation condition. Iran. J. Basic Med. Sci. 2020, 23, 390–395. [Google Scholar] [CrossRef]
- Khan, M.M.; Ahmad, A.; Ishrat, T.; Khuwaja, G.; Srivastawa, P.; Khan, M.B.; Raza, S.S.; Javed, H.; Vaibhav, K.; Khan, A.; et al. Rutin protects the neural damage induced by transient focal ischemia in rats. Brain Res. 2009, 1292, 123–135. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, X.; Zhang, Q.; Wang, L.; Li, Y.; Li, Y. Characterization and Evaluation of the Solubility and Oral Bioavailability of Rutin-Ethanolate Solvate. AAPS PharmSciTech 2020, 21, 241. [Google Scholar] [CrossRef]
- Woodman, O.L.; Chan, E. Vascular and anti-oxidant actions of flavonols and flavones. Clin. Exp. Pharmacol. Physiol. 2004, 31, 786–790. [Google Scholar] [CrossRef]
- Olszanecki, R.; Gebska, A.; Kozlovski, V.I.; Gryglewski, R.J. Flavonoids and nitric oxide synthase. J. Physiol. Pharmacol. 2002, 53, 571–584. [Google Scholar]
- Guerrero, J.A.; Lozano, M.L.; Castillo, J.; Benavente-Garcia, O.; Vicente, V.; Rivera, J. Flavonoids inhibit platelet function through binding to the thromboxane A2 receptor. J. Thromb Haemost 2005, 3, 369–376. [Google Scholar] [CrossRef]
- Ling, C.; Lei, C.; Zou, M.; Cai, X.; Xiang, Y.; Xie, Y.; Li, X.; Huang, D.; Wang, Y. Neuroprotective effect of apigenin against cerebral ischemia/reperfusion injury. J. Int. Med. Res. 2020, 48, 300060520945859. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Pang, Q.; Chen, X.; Huang, T.; Liu, M.; Zhai, Q. Angiogenic effects of apigenin on endothelial cells after hypoxia-reoxygenation via the caveolin-1 pathway. Int. J. Mol. Med. 2017, 40, 1639–1648. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Zhao, Y.; Chen, X.; Zhao, K.; Zhai, Q.; Tu, F. Apigenin Protects the Brain against Ischemia/Reperfusion Injury via Caveolin-1/VEGF In Vitro and In Vivo. Oxidative Med. Cell. Longev. 2018, 2018, 7017204. [Google Scholar] [CrossRef]
- Tu, F.; Pang, Q.; Huang, T.; Zhao, Y.; Liu, M.; Chen, X. Apigenin Ameliorates Post-Stroke Cognitive Deficits in Rats Through Histone Acetylation-Mediated Neurochemical Alterations. Med. Sci. Monit. 2017, 23, 4004–4013. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed. Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef]
- Taheri, Y.; Suleria, H.A.R.; Martins, N.; Sytar, O.; Beyatli, A.; Yeskaliyeva, B.; Seitimova, G.; Salehi, B.; Semwal, P.; Painuli, S.; et al. Myricetin bioactive effects: Moving from preclinical evidence to potential clinical applications. BMC Complement Med. Ther. 2020, 20, 241. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Myricetin as a Promising Molecule for the Treatment of Post-Ischemic Brain Neurodegeneration. Nutrients 2021, 13, 241. [Google Scholar] [CrossRef]
- Wu, S.; Yue, Y.; Peng, A.; Zhang, L.; Xiang, J.; Cao, X.; Ding, H.; Yin, S. Myricetin ameliorates brain injury and neurological deficits via Nrf2 activation after experimental stroke in middle-aged rats. Food Funct. 2016, 7, 2624–2634. [Google Scholar] [CrossRef]
- Boriero, D.; Carcereri de Prati, A.; Antonini, L.; Ragno, R.; Sohji, K.; Mariotto, S.; Butturini, E. The anti-STAT1 polyphenol myricetin inhibits M1 microglia activation and counteracts neuronal death. FEBS J. 2021, 288, 2347–2359. [Google Scholar] [CrossRef]
- Zhang, S.; Hu, X.; Guo, S.; Shi, L.; He, Q.; Zhang, P.; Yu, S.; Zhao, R. Myricetin ameliorated ischemia/reperfusion-induced brain endothelial permeability by improvement of eNOS uncoupling and activation eNOS/NO. J. Pharmacol. Sci. 2019, 140, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Xu, P.; Fu, T.; Huang, X.; Song, J.; Chen, M.; Tian, X.; Yin, H.; Han, J. Myricetin against ischemic cerebral injury in rat middle cerebral artery occlusion model. Mol. Med. Rep. 2018, 17, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Sunil, C.; Xu, B. An insight into the health-promoting effects of taxifolin (dihydroquercetin). Phytochemistry 2019, 166, 112066. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Q.; Wang, Y.J.; Yang, G.T.; Gao, Q.L.; Tang, M.X. Taxifolin Inhibits Receptor Activator of NF-kappaB Ligand-Induced Osteoclastogenesis of Human Bone Marrow-Derived Macrophages in vitro and Prevents Lipopolysaccharide-Induced Bone Loss in vivo. Pharmacology 2019, 103, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Yang, Y.; Yang, L.; Jiang, H.; Yu, X.; Wang, Y. Cardioprotective effects of dihydroquercetin against ischemia reperfusion injury by inhibiting oxidative stress and endoplasmic reticulum stress-induced apoptosis via the PI3K/Akt pathway. Food Funct 2019, 10, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Yamamoto, Y.; Maki, T.; Hattori, Y.; Ito, H.; Mizuno, K.; Harada-Shiba, M.; Kalaria, R.N.; Fukushima, M.; Takahashi, R.; et al. Taxifolin inhibits amyloid-beta oligomer formation and fully restores vascular integrity and memory in cerebral amyloid angiopathy. Acta NeuroPathol. Commun. 2017, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Turovskaya, M.V.; Gaidin, S.G.; Mal’tseva, V.N.; Zinchenko, V.P.; Turovsky, E.A. Taxifolin protects neurons against ischemic injury in vitro via the activation of antioxidant systems and signal transduction pathways of GABAergic neurons. Mol. Cell Neurosci. 2019, 96, 10–24. [Google Scholar] [CrossRef]
- Wang, Y.H.; Wang, W.Y.; Chang, C.C.; Liou, K.T.; Sung, Y.J.; Liao, J.F.; Chen, C.F.; Chang, S.; Hou, Y.C.; Chou, Y.C.; et al. Taxifolin ameliorates cerebral ischemia-reperfusion injury in rats through its anti-oxidative effect and modulation of NF-kappa B activation. J. BioMed. Sci. 2006, 13, 127–141. [Google Scholar] [CrossRef]
- Gong, G.; Guan, Y.Y.; Zhang, Z.L.; Rahman, K.; Wang, S.J.; Zhou, S.; Luan, X.; Zhang, H. Isorhamnetin: A review of pharmacological effects. BioMed. Pharmacother. 2020, 128, 110301. [Google Scholar] [CrossRef]
- Zhang, N.; Pei, F.; Wei, H.; Zhang, T.; Yang, C.; Ma, G.; Yang, C. Isorhamnetin protects rat ventricular myocytes from ischemia and reperfusion injury. Exp. Toxicol. Pathol. 2011, 63, 33–38. [Google Scholar] [CrossRef]
- Iida, A.; Usui, T.; Zar Kalai, F.; Han, J.; Isoda, H.; Nagumo, Y. Protective effects of Nitraria retusa extract and its constituent isorhamnetin against amyloid beta-induced cytotoxicity and amyloid beta aggregation. Biosci. Biotechnol. Biochem. 2015, 79, 1548–1551. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fan, L.; Wang, Y.; Ding, J.; Wang, R. Isorhamnetin Alleviates High Glucose-Aggravated Inflammatory Response and Apoptosis in Oxygen-Glucose Deprivation and Reoxygenation-Induced HT22 Hippocampal Neurons Through Akt/SIRT1/Nrf2/HO-1 Signaling Pathway. Inflammation 2021, 44, 1993–2005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.J.; Song, J.Q.; Pan, S.Y.; Wang, K. Treatment with Isorhamnetin Protects the Brain Against Ischemic Injury in Mice. Neurochem Res. 2016, 41, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Lv, Q.; Zhong, C.; Cui, Y.; He, L.; Zhang, C.; Yu, J. Tiliroside Ameliorates Ulcerative Colitis by Restoring the M1/M2 Macrophage Balance via the HIF-1alpha/glycolysis Pathway. Front. Immunol. 2021, 12, 649463. [Google Scholar] [CrossRef]
- Han, R.; Yang, H.; Lu, L.; Lin, L. Tiliroside as a CAXII inhibitor suppresses liver cancer development and modulates E2Fs/Caspase-3 axis. Sci. Rep. 2021, 11, 8626. [Google Scholar] [CrossRef]
- Velagapudi, R.; Aderogba, M.; Olajide, O.A. Tiliroside, a dietary glycosidic flavonoid, inhibits TRAF-6/NF-kappaB/p38-mediated neuroinflammation in activated BV2 microglia. Biochim. Biophys. Acta (BBA) Gen. Subj. 2014, 1840, 3311–3319. [Google Scholar] [CrossRef]
- Di Meo, F.; Valentino, A.; Petillo, O.; Peluso, G.; Filosa, S.; Crispi, S. Bioactive Polyphenols and Neuromodulation: Molecular Mechanisms in Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 8626. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Ren, X.; Zhang, X.; Wu, Z.; Liu, L. The positive correlation of antioxidant activity and prebiotic effect about oat phenolic compounds. Food Chem. 2023, 402, 134231. [Google Scholar] [CrossRef]
- Li, Y.; Qin, C.; Dong, L.; Zhang, X.; Wu, Z.; Liu, L.; Yang, J.; Liu, L. Whole grain benefit: Synergistic effect of oat phenolic compounds and beta-glucan on hyperlipidemia via gut microbiota in high-fat-diet mice. Food Funct 2022, 13, 12686–12696. [Google Scholar] [CrossRef]
- Peh, A.; O’Donnell, J.A.; Broughton, B.R.S.; Marques, F.Z. Gut Microbiota and Their Metabolites in Stroke: A Double-Edged Sword. Stroke 2022, 53, 1788–1801. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef] [PubMed]
- Renouf, M.; Marmet, C.; Giuffrida, F.; Lepage, M.; Barron, D.; Beaumont, M.; Williamson, G.; Dionisi, F. Dose-response plasma appearance of coffee chlorogenic and phenolic acids in adults. Mol. Nutr. Food Res. 2014, 58, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Remesy, C. Absorption and metabolism of polyphenols in the gut and impact on health. BioMed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Kao, Y.C.; Zhou, C.; Sherman, M.; Laughton, C.A.; Chen, S. Molecular basis of the inhibition of human aromatase (estrogen synthetase) by flavone and isoflavone phytoestrogens: A site-directed mutagenesis study. Environ. Health Perspect. 1998, 106, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Thilakarathna, S.H.; Rupasinghe, H.P. Flavonoid bioavailability and attempts for bioavailability enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Han, M.K.; Barreto, T.A.; Martinez, F.J.; Comstock, A.T.; Sajjan, U.S. Randomised clinical trial to determine the safety of quercetin supplementation in patients with chronic obstructive pulmonary disease. BMJ Open Respir Res. 2020, 7, e000392. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, T.; Tanaka, Y.; Kamimaki, I.; Nagao, T.; Tokimitsu, I. Catechin safely improved higher levels of fatness, blood pressure, and cholesterol in children. Obesity 2008, 16, 1338–1348. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef]
- Lee, H.-S. Acute Oral Toxicity of Salicornia herbacea L. Extract in Mice. Biomed. Sci. Lett. 2016, 22, 46–52. [Google Scholar] [CrossRef]
- Ferreira, D.; Pinto, D.; Silva, H.; Girol, A.P.; de Lourdes Pereira, M. Salicornia ramosissima J. Woods seeds affected the normal regenerative function on carbon tetrachloride-induced liver and kidney injury. BioMed. Pharmacother. 2018, 107, 283–291. [Google Scholar] [CrossRef]
- Karthivashan, G.; Park, S.Y.; Kweon, M.H.; Kim, J.; Haque, M.E.; Cho, D.Y.; Kim, I.S.; Cho, E.A.; Ganesan, P.; Choi, D.K. Ameliorative potential of desalted Salicornia europaea L. extract in multifaceted Alzheimer’s-like scopolamine-induced amnesic mice model. Sci. Rep. 2018, 8, 7174. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Karthivashan, G.; Kweon, M.H.; Kim, D.H.; Choi, D.K. The Ameliorative Effects of the Ethyl Acetate Extract of Salicornia europaea L. and Its Bioactive Candidate, Irilin B, on LPS-Induced Microglial Inflammation and MPTP-Intoxicated PD-Like Mouse Model. Oxidative Med. Cell. Longev. 2019, 2019, 6764756. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Ulamek, M.; Jablonski, M. Alzheimer’s mechanisms in ischemic brain degeneration. Anat. Rec. 2009, 292, 1863–1881. [Google Scholar] [CrossRef] [PubMed]
| Polyphenol | Subclass | Compound | Ref. |
|---|---|---|---|
| Flavonoid | Dihydrochalcone | Phloretin | [20] |
| Phloridzin | [20] | ||
| Flavanol | Catechin | [20] | |
| Epicatechin | [20] | ||
| (Epi)gallocatechin | [13] | ||
| Dihydroquercetin (Taxifolin) | [21] | ||
| Flavanone | Naringin | [20] | |
| Naringenin | [20] | ||
| Flavone | Apigenin | [20] | |
| Apigenin-6-arabinosyl-8-glucoside (isoschaftoside) | [21] | ||
| Chrysin | [20] | ||
| Luteolin glucosyllactate | [13] | ||
| Flavonol | Isorhamnetin | [22] | |
| Isorhamnetin 3-glucoside | [22] | ||
| Isorhamnetin-7-O-(6-O-malonyl)-glucoside | [23] | ||
| Isorhamnetin glucopyranoside | [13] | ||
| Kaempferol | [20] | ||
| kaempferol derivative | [21] | ||
| kaempferol-3-O-glucoside | [20] | ||
| kaempferol-3-O-rutinoside | [20] | ||
| Myricetin | [20] | ||
| Quercetin | [20] | ||
| Quercetin-3-O-galactoside | [20] | ||
| Quercetin glucoside | [13] | ||
| Quercetin 3-glucoside (Isoquercitrin) | [21,22,23] | ||
| Quercetin-malonyglucoside | [13,21] | ||
| Quercetin-methyl-ether derivative (isomer 1 and 2) | [21] | ||
| Quercetin-rhamnosyl-hexoside | [13,21] | ||
| Rutin (quercetin 3 -O rhamnosyl glucoside, quercetin rutinoside, vitamin p) | [20] | ||
| Phenolic acids | Hydroxybenzoic acids | Cannabidiolic acid | [13] |
| Salicylic acid derivative | [21] | ||
| Sitostanol | [24] | ||
| Syringic acid | [20] | ||
| Tiliroside | [20] | ||
| Vanillic acid | [20] | ||
| Ellagic acid | [20] | ||
| Gallic acid | [20] | ||
| Gallocatechin | [24] | ||
| Protocatechuic acid | [20] | ||
| Protocatechuic-arabinoside acid | [21] | ||
| Hydroxycinnamic acids | Cinnamic acid | [25] | |
| P-coumaric acid (4-hydroxycinnamic acid) | [13,20,21,23] | ||
| Sinapic acid (3,5-Dimethoxy-4-hydroxycinnamic acid) | [20] | ||
| Ethyl (E)-2-hydroxycinnamate | [24] | ||
| P-coumaric acid benzyl ester derivative | [21] | ||
| Quinic acid | [13,21,23] | ||
| P-coumaroylquinic acid (isomer 1 and 2) | [21] | ||
| Caffeic acid | [20,22] | ||
| Hydrocaffeic acid | [22] | ||
| Caffeic acid-glucuronide-glucoside (isomer 1) | [21] | ||
| Caffeoylquinic acid | [22] | ||
| Chlorogenic acid (3-O-caffeoylquinic acid) | [20,21,23] | ||
| Neochlorogenic acid (5-O-caffeoylquinic acid) | [13,21] | ||
| Dicaffeoylquinic acid (isomer 1, 2, 3 and 4) | [13,22] | ||
| 3,4-Di-O-caffeoylquinic acid | [20,22] | ||
| 3,5-Di-O-caffeoylquinic acid | [20] | ||
| 3,5-Dicaffeoylquinic acid | [21] | ||
| 4,5-Dicaffeoylquinic acid | [21] | ||
| Hydrocaffeoylquinic acid | [13,21,22] | ||
| Dihydrocaffeoyl quinic acid | [22] | ||
| Caffeoyl-hydrocaffeoyl quinic acid | [21,22] | ||
| Tungtungmadic acid (3-Caffeoyl-4-dihydrocaffeoyl quinic acid) (isomer 1 and 2) | [13] | ||
| Ferulic acid | [13,21,23,25] | ||
| Ferulic-glucoside acid | [21] | ||
| Trans-ferulic acid | [20] | ||
| Coumarin | Scopoletin | [13,24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nájar, A.M.; Romero-Bernal, M.; del Río, C.; Montaner, J. A Review on Polyphenols in Salicornia ramosissima with Special Emphasis on Their Beneficial Effects on Brain Ischemia. Nutrients 2023, 15, 793. https://doi.org/10.3390/nu15030793
Nájar AM, Romero-Bernal M, del Río C, Montaner J. A Review on Polyphenols in Salicornia ramosissima with Special Emphasis on Their Beneficial Effects on Brain Ischemia. Nutrients. 2023; 15(3):793. https://doi.org/10.3390/nu15030793
Chicago/Turabian StyleNájar, Ana M., Marina Romero-Bernal, Carmen del Río, and Joan Montaner. 2023. "A Review on Polyphenols in Salicornia ramosissima with Special Emphasis on Their Beneficial Effects on Brain Ischemia" Nutrients 15, no. 3: 793. https://doi.org/10.3390/nu15030793
APA StyleNájar, A. M., Romero-Bernal, M., del Río, C., & Montaner, J. (2023). A Review on Polyphenols in Salicornia ramosissima with Special Emphasis on Their Beneficial Effects on Brain Ischemia. Nutrients, 15(3), 793. https://doi.org/10.3390/nu15030793





