What We Know about Euterpe Genus and Neuroprotection: A Scoping Review
Abstract
1. Introduction
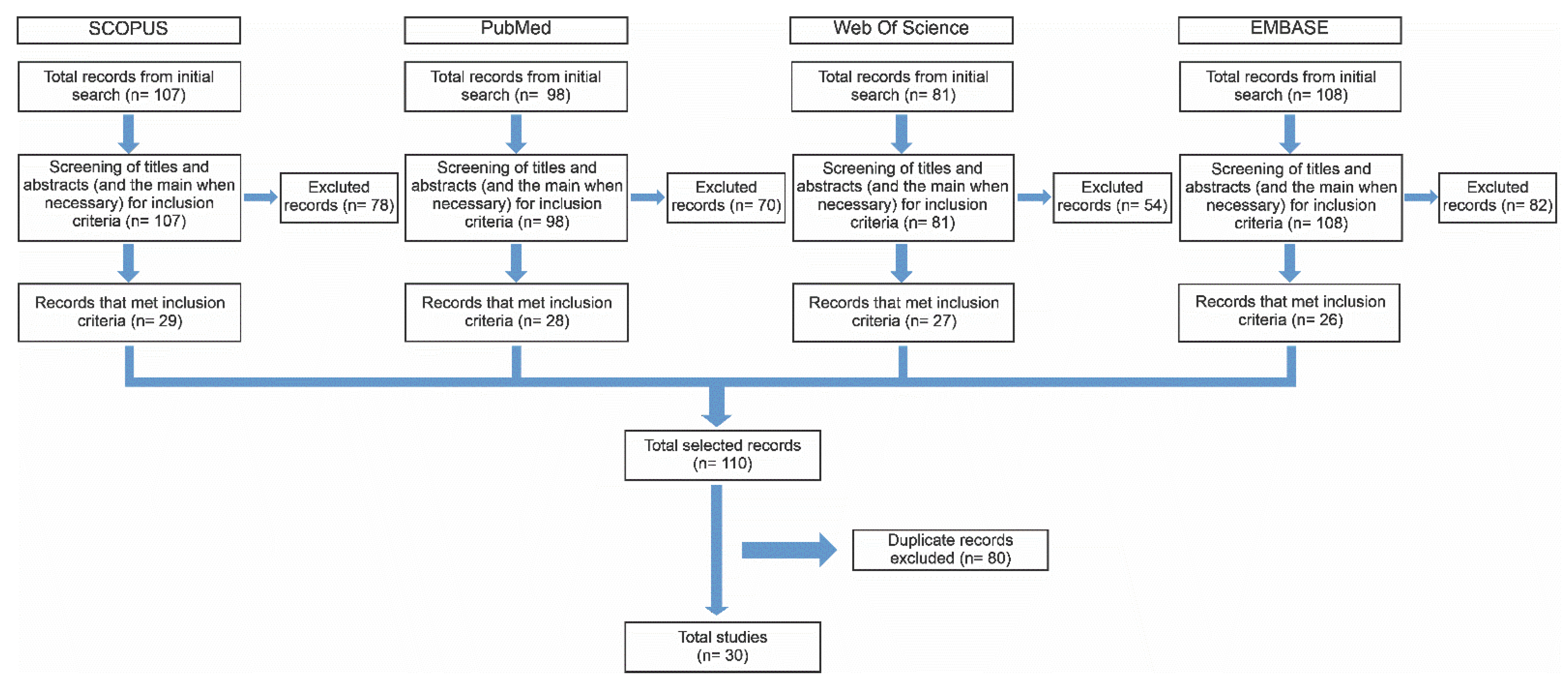
2. Materials and Methods
3. Results and Discussion
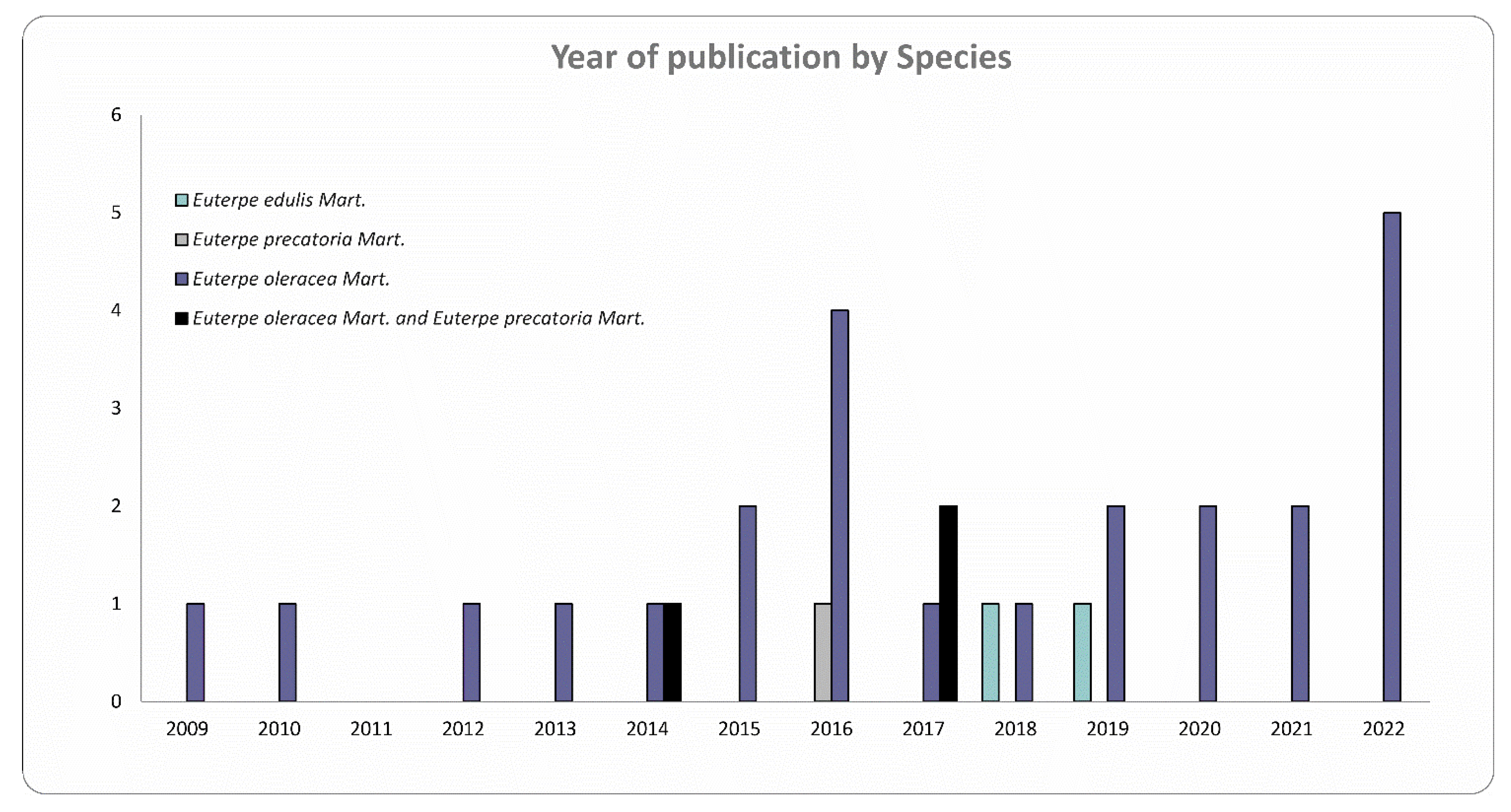
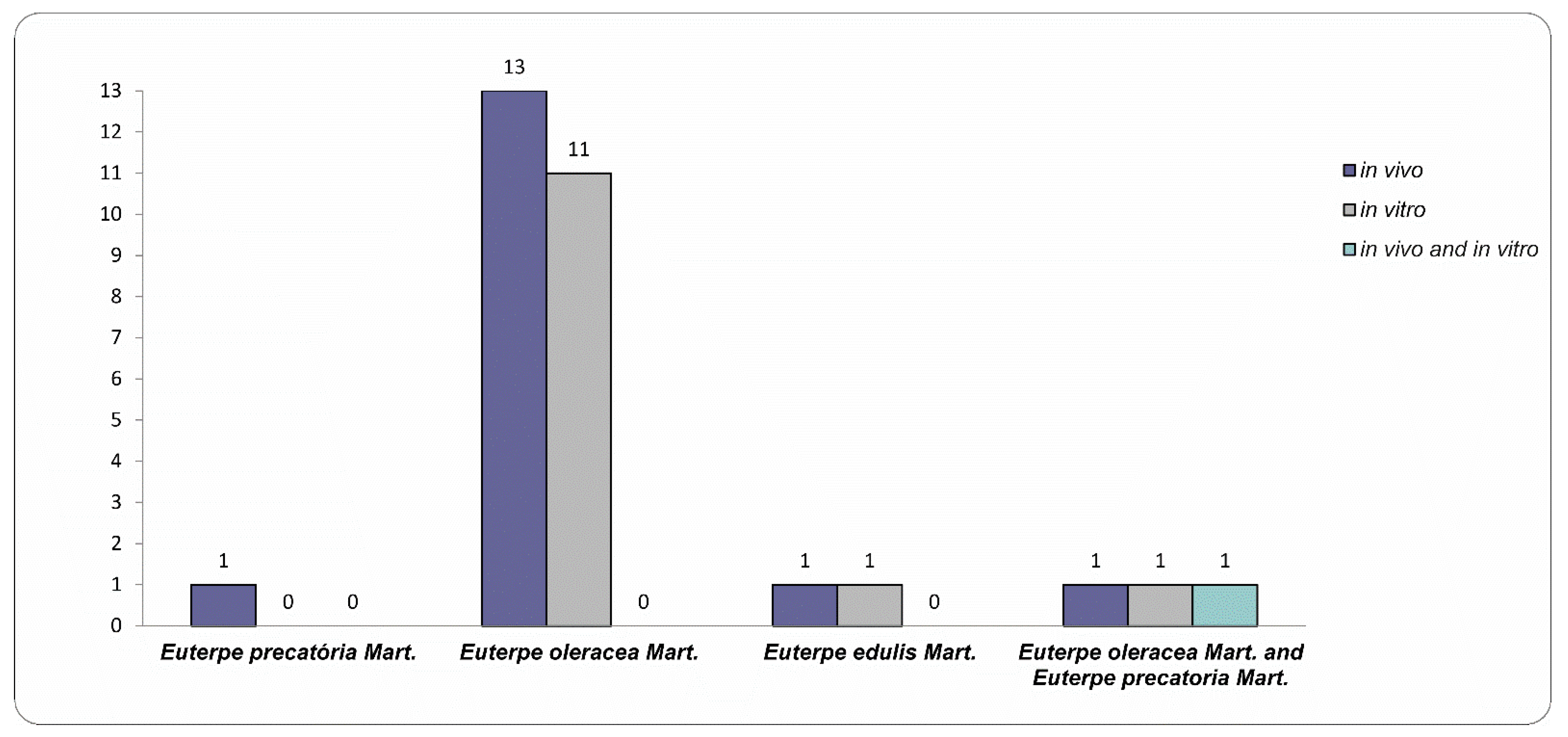
3.1. Features of Selected Studies
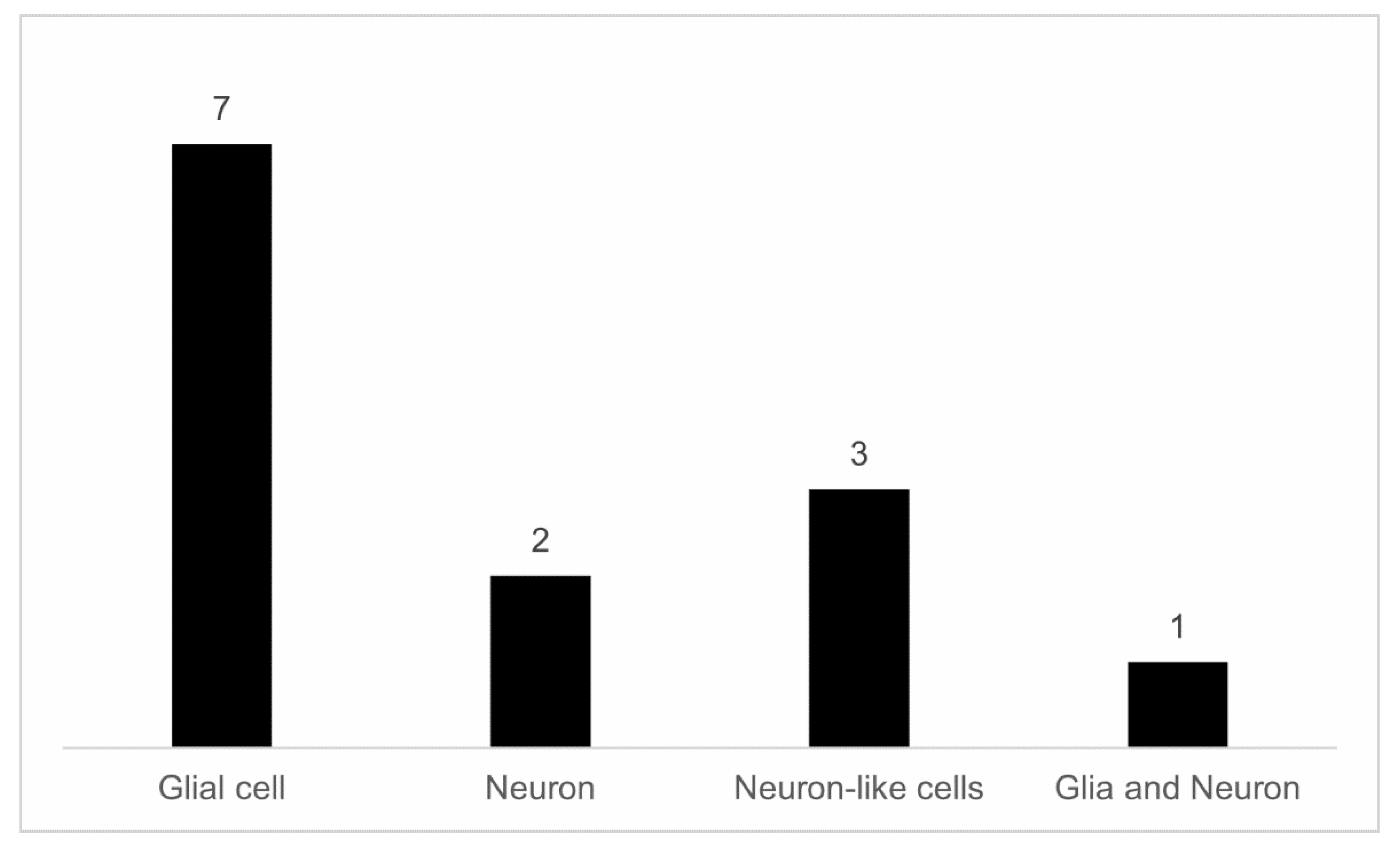
3.2. Experimental Models to Study Neuroprotection
3.3. Oxidative Stress and Neuroinflammation in the Brain
3.4. Signaling Pathways Targeted by Euterpe Species Associated with Neuroprotection
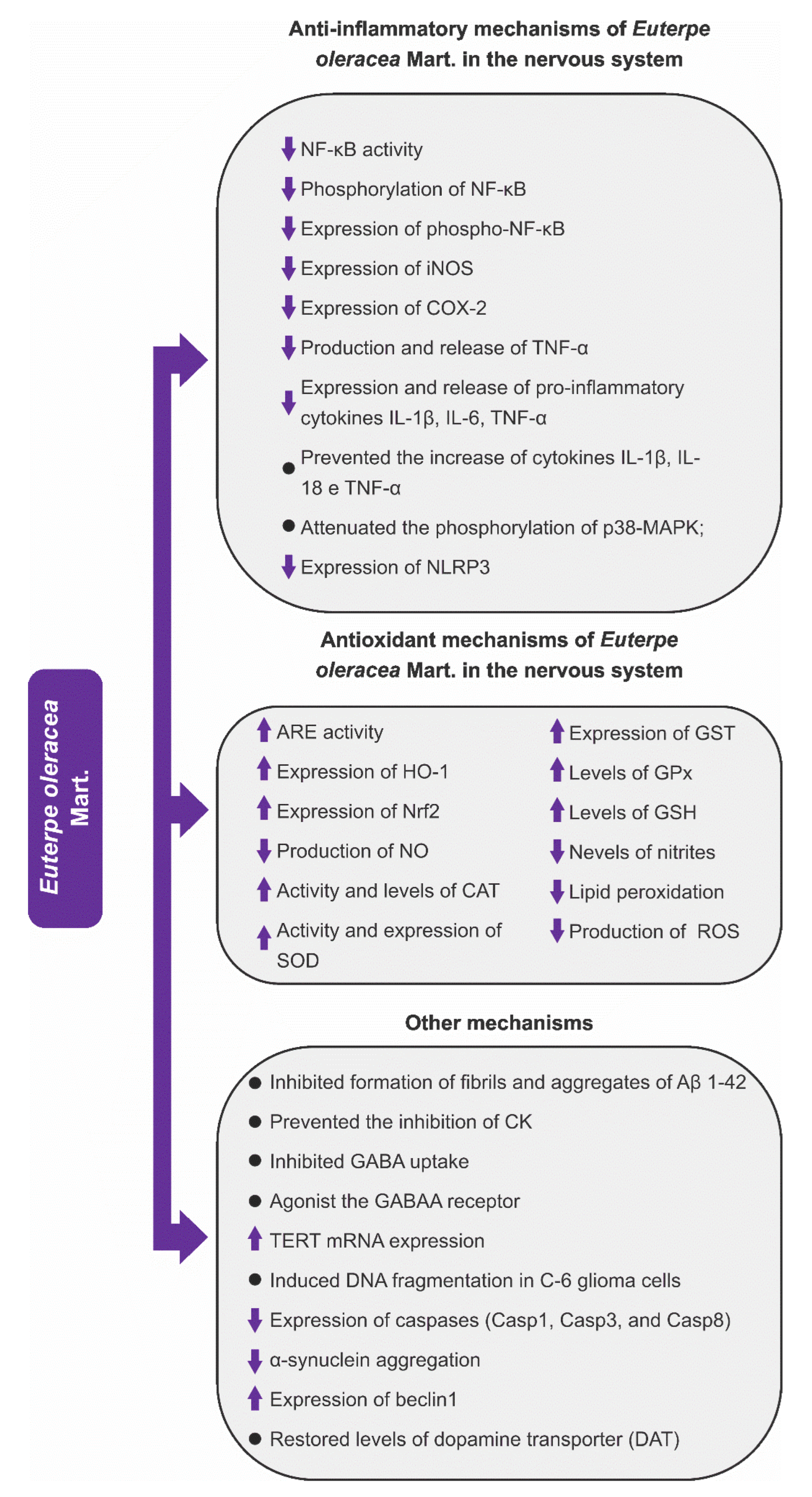
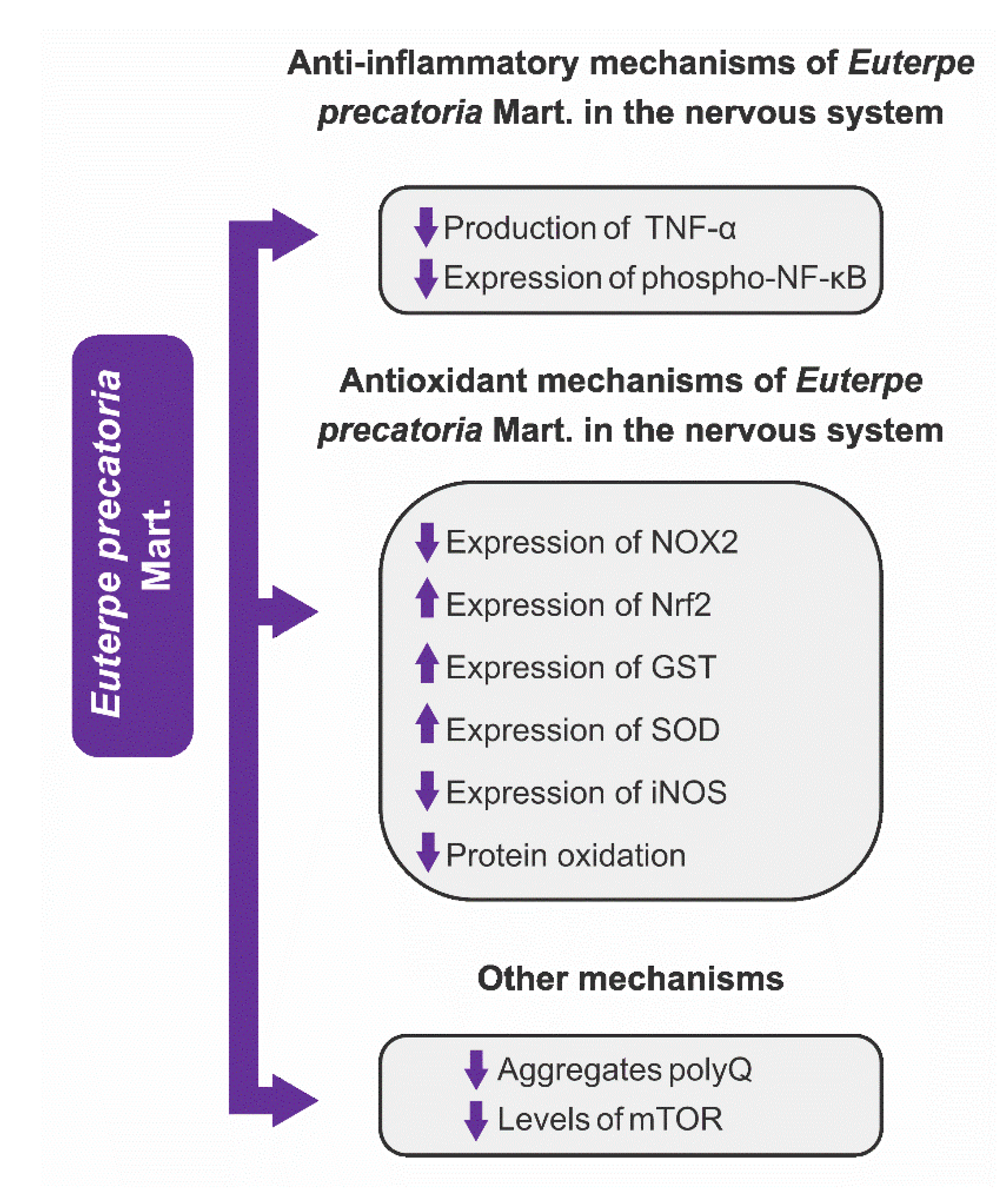
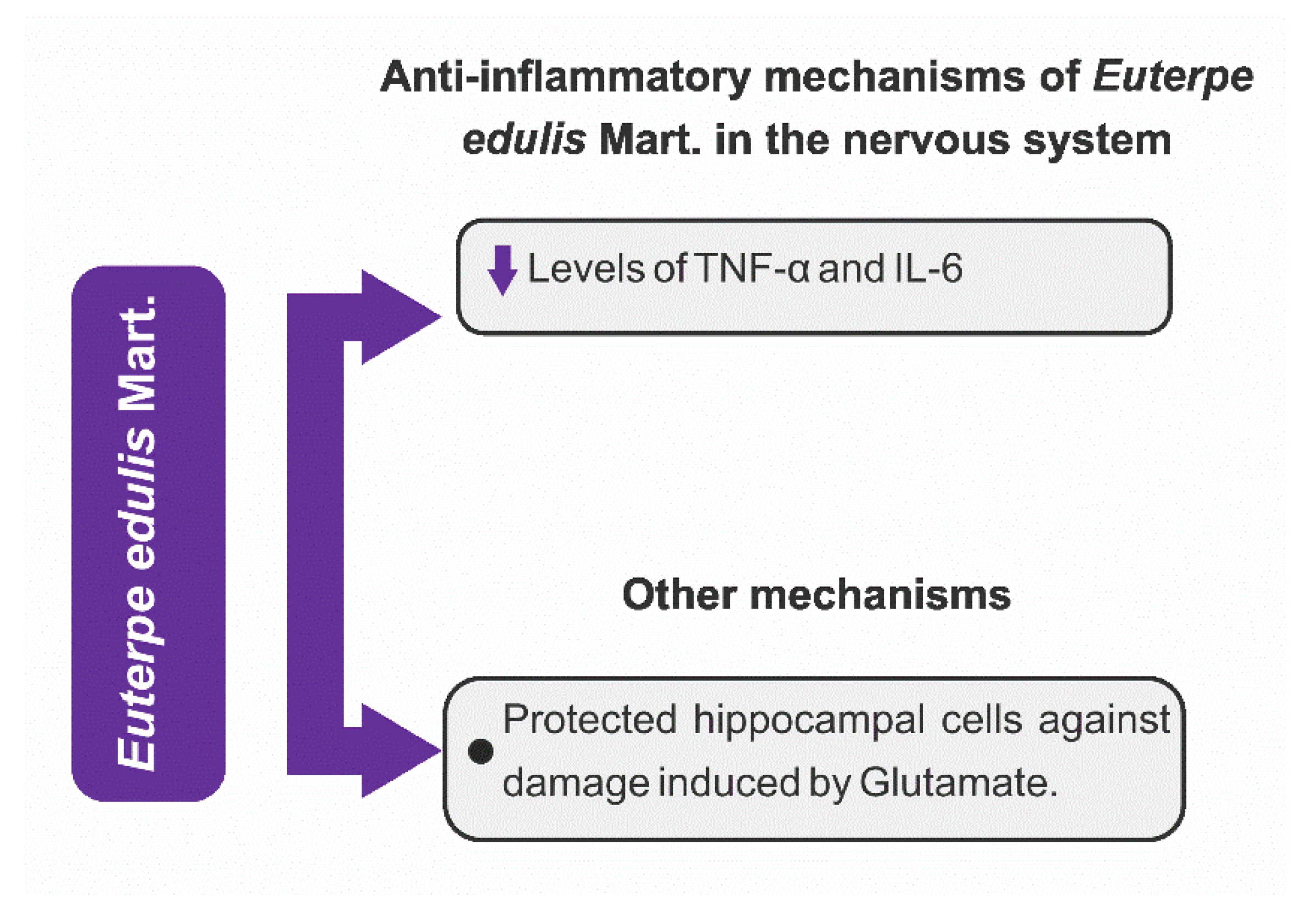
3.4.1. Anti-Inflammatory Mechanisms
3.4.2. Antioxidant Mechanisms
4. Future Directions
- Corroborating data from preclinical studies, the protective effects of açaí and JF on inflammation and oxidative stress were also observed in clinical studies. Studies in humans with the fruits of the three species of the genus Euterpe selected are limited; however, it is worth noting that the clinical studies already published evaluated only the effects of the fruits of EO and EE. So far, there are no studies evaluating the pharmacological effects of EP fruits in humans. Clinical studies with AEO and JF demonstrate that these species are able to reduce inflammatory markers (IL-6, INF-γ) and oxidative stress (8-isoprostane) and increase the activity of antioxidant enzymes (catalase, glutathione peroxidase) in the plasma and serum [9,12,81,152]. Although these effects were observed in clinical conditions unrelated to the CNS, they are important because they demonstrate the protective effects of these fruits in humans and consequently support the need for additional scientific studies, including clinical studies. Furthermore, the multiple mechanisms of action of the Euterpe species to exert neuroprotection also support the importance of future research to study the possible application in neurological disorders.
- It would be interesting if future scientific studies addressed, in their experimental designs, in addition to the pharmacodynamic aspects, the evaluation of pharmacokinetic properties (passage through BBB, absorption, distribution, metabolism, and excretion) of products derived (e.g., beverages) from EO, EE, and EP. An important pharmacokinetic property to be investigated in studies with fruits that have a vast phenolic composition is bioavailability since polyphenols have low bioavailability [153,154,155]. In this sense, it would be important to know the pharmacokinetic characteristics of products from EO, EE, and EP so that new discussions and eventual pharmaceutical solutions can be developed to overcome the problems with the bioavailability of polyphenols.
- Considering the data demonstrating that AEO and AEP are able to regulate microglial and astrocytic functions and that these cells perform homeostatic and immune functions in the CNS, it would be relevant to investigate not only the protective functions of AEO, AEP, and JF against brain injury or stimuli to mimic neuroinflammation but also to develop new research that can assess whether AEO, AEP, and JF are able to regulate brain functions under physiological conditions acting on microglia and astrocytes. That is, could EO, EP, or EE contribute to the maintenance of cerebral homeostasis? Could AEO, AEP, or JF modulate neurogenesis? These important questions, as well as others, are still unknown and can certainly contribute to the development of future therapies for brain health.
5. Conclusions
- The fruits of EO, EP, or EE species and EO seed extract protect the CNS using mechanisms that reduce/limit the neuroinflammatory process and oxidative stress, and because they are fruits with nutritional and functional appeal and are rich in phenolic compounds and anthocyanins, compounds that exert protective effects through mechanisms common to CNS pathologies, açaí (EO, EP) and juçara (EE) have the potential to impact conventional therapy or even prevent pathologies that affect the CNS.
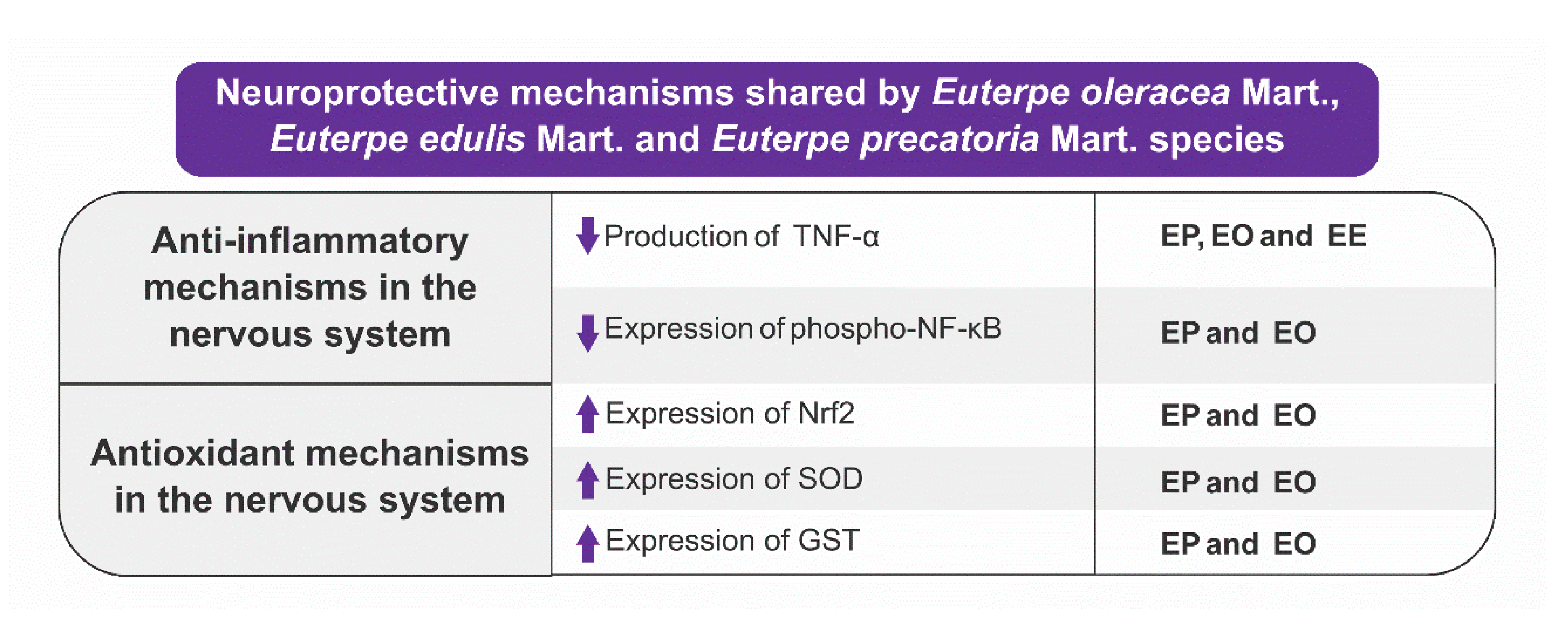
- EO, EE, and EP species have neuroprotective activity, but this effect is better consolidated in the literature for EO due to the greater amount of in vitro and in vivo studies.
- The neuroprotection exerted by EO, EE, and EP involves a series of molecular pathways: inhibition of GABA uptake, anti-aging effects, reduction of expression, production, release of inflammatory mediators, potentiation of antioxidant defenses via increased activity and expression of enzymes antioxidants, and reduced ROS production.
- The demand for new knowledge is necessary for the three Euterpe species, but based on the available literature evaluated in this article, it is essential that new neuropharmacological studies be directed to EP and EE species, as these two species are rich in phenolic compounds such as flavonoids and phenolic acids.
- In addition to new preclinical studies, there is also a need to carry out clinical studies aimed at evaluating the neuropharmacological activity of these three Euterpe species since, to date, there are no clinical studies aimed at evaluating the neuropharmacological activity of EO, EE, and EP. The protection already described in clinical studies with EO and EE (antioxidant and anti-inflammatory effects) is encouraging and may support new clinical studies targeting the CNS.
- It would be relevant if future preclinical and clinical studies were to verify the bioavailability of antioxidant molecules from açaí and juçara pulp or juice in the CNS, which would help determine the effectiveness of these beverages in reducing oxidative stress in the brain and neuroinflammation.
- Despite the low number of studies, one can suggest that açaí and juçara fruit have the potential to impact the therapy of diseases that affect the CNS because they induce neuroprotection through interaction with key pathways (e.g., neuroinflammation and oxidative stress) and alternatives (as autophagy) for the pathogenesis of diseases of the CNS.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melzer, T.M.; Manosso, L.M.; Yau, S.Y.; Gil-Mohapel, J.; Brocardo, P.S. In Pursuit of Healthy Aging: Effects of Nutrition on Brain Function. Int. J. Mol. Sci. 2021, 22, 5026. [Google Scholar] [CrossRef] [PubMed]
- Henriques, J.F.; Serra, D.; Dinis, T.C.P.; Almeida, L.M. The Anti-Neuroinflammatory Role of Anthocyanins and Their Metabolites for the Prevention and Treatment of Brain Disorders. Int. J. Mol. Sci. 2020, 21, 8653. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.N.; Bickford, P.C. Anthocyanins and Their Metabolites as Therapeutic Agents for Neurodegenerative Disease. Antioxidants 2019, 8, 333. [Google Scholar] [CrossRef] [PubMed]
- Vauzour, D. Dietary polyphenols as modulators of brain functions: Biological actions and molecular mechanisms underpinning their beneficial effects. Oxid. Med. Cell. Longev. 2012, 2012, 914273. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.K.; Alasalvar, C.; Shahidi, F. Superfruits: Phytochemicals, antioxidant efficacies, and health effects—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1580–1604. [Google Scholar] [CrossRef]
- Neri-Numa, I.A.; Soriano Sancho, R.A.; Pereira, A.P.A.; Pastore, G.M. Small Brazilian wild fruits: Nutrients, bioactive compounds, health-promotion properties and commercial interest. Food Res. Int. 2018, 103, 345–360. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Duncan, C.E.; Talcott, S.T. Phytochemical composition and thermal stability of two commercial açai species, Euterpe oleracea and Euterpe precatoria. Food Chem. 2009, 115, 1199–1205. [Google Scholar] [CrossRef]
- Bicudo, M.O.; Ribani, R.H.; Beta, T. Anthocyanins, phenolic acids and antioxidant properties of Jucara fruits (Euterpe edulis M.) along the on-tree ripening process. Plant Foods Hum. Nutr. 2014, 69, 142–147. [Google Scholar] [CrossRef]
- Aranha, L.N.; Silva, M.G.; Uehara, S.K.; Luiz, R.R.; Nogueira Neto, J.F.; Rosa, G.; Moraes de Oliveira, G.M. Effects of a hypoenergetic diet associated with acai (Euterpe oleracea Mart.) pulp consumption on antioxidant status, oxidative stress and inflammatory biomarkers in overweight, dyslipidemic individuals. Clin. Nutr. 2020, 39, 1464–1469. [Google Scholar] [CrossRef]
- Cardoso, A.L.; de Liz, S.; Rieger, D.K.; Farah, A.C.A.; Kunradi Vieira, F.G.; Altenburg de Assis, M.A.; Di Pietro, P.F. An Update on the Biological Activities of Euterpe edulis (Jucara). Planta Med. 2018, 84, 487–499. [Google Scholar] [CrossRef]
- Jensen, G.S.; Wu, X.; Patterson, K.M.; Barnes, J.; Carter, S.G.; Scherwitz, L.; Beaman, R.; Endres, J.R.; Schauss, A.G. In vitro and in vivo antioxidant and anti-inflammatory capacities of an antioxidant-rich fruit and berry juice blend. Results of a pilot and randomized, double-blinded, placebo-controlled, crossover study. J. Agric. Food Chem. 2008, 56, 8326–8333. [Google Scholar] [CrossRef]
- Kim, H.; Simbo, S.Y.; Fang, C.; McAlister, L.; Roque, A.; Banerjee, N.; Talcott, S.T.; Zhao, H.; Kreider, R.B.; Mertens-Talcott, S.U. Acai (Euterpe oleracea Mart.) beverage consumption improves biomarkers for inflammation but not glucose- or lipid-metabolism in individuals with metabolic syndrome in a randomized, double-blinded, placebo-controlled clinical trial. Food Funct. 2018, 9, 3097–3103. [Google Scholar] [CrossRef]
- Vianna, S.A. Euterpe in Flora do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB15713 (accessed on 21 February 2022).
- Vianna, S.A. Euterpe in Flora do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB22139 (accessed on 21 February 2022).
- Vianna, S.A. Euterpe in Flora do Brasil. Available online: https://floradobrasil.jbrj.gov.br/FB15712 (accessed on 21 February 2022).
- Brasil. Catálago de Produtos da Sociobiodiversidade do Brasil. Available online: https://www.gov.br/icmbio/pt-br/centrais-de-conteudo/publicacoes/publicacoes-diversas/catalago_de_produtos_da_sociobiodiversidade_do_brasil.pdf/view (accessed on 5 January 2022).
- Brasil. Alimentos Regionais Brasileiros. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/alimentos_regionais_brasileiros_2ed.pdf (accessed on 11 December 2021).
- Yamaguchi, K.K.; Pereira, L.F.; Lamarao, C.V.; Lima, E.S.; da Veiga-Junior, V.F. Amazon acai: Chemistry and biological activities: A review. Food Chem. 2015, 179, 137–151. [Google Scholar] [CrossRef]
- Oliveira, M.D.S.P.D.; Schwartz, G. Açaí—Euterpe oleracea. In Exotic Fruits Reference Guide; Rodrigues, S., Silva, E., de Brito, E., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–5. [Google Scholar]
- Schauss, A.G. The Effect of Acai (Euterpe spp.) Fruit Pulp on Brain Health and Performance. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 179–186. [Google Scholar]
- Boeira, L.S.; Freitas, P.H.B.; Uchôa, N.R.; Bezerra, J.A.; Cád, S.V.; Junior, S.D.; Albuquerque, P.M.; Mar, J.M.; Ramos, A.S.; Machado, M.B.; et al. Chemical and sensorial characterization of a novel alcoholic beverage produced with native acai (Euterpe precatoria) from different regions of the Amazonas state. Food Sci. Technol. 2020, 117, 108632. [Google Scholar] [CrossRef]
- Borges, G.D.S.C.; Gonzaga, L.V.; Jardini, F.A.; Filho, J.M.; Heller, M.; Micke, G.; Costa, A.C.O.; Fett, R. Protective effect of Euterpe edulis M. on Vero cell culture and antioxidant evaluation based on phenolic composition using HPLC−ESI-MS/MS. Food Res. Int. 2013, 51, 363–369. [Google Scholar] [CrossRef]
- Schulz, M.; da Silva Campelo Borges, G.; Gonzaga, L.V.; Oliveira Costa, A.C.; Fett, R. Jucara fruit (Euterpe edulis Mart.): Sustainable exploitation of a source of bioactive compounds. Food Res. Int. 2016, 89, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, A.C.D.; Fantini, A.C.; Schmitt-Filho, A.L.; Farley, J. Market for Amazonian Açaí (Euterpe oleraceae) Stimulates Pulp Production from Atlantic Forest Juçara Berries (Euterpe edulis). Agroecol. Sustain. Food Syst. 2015, 39, 762–781. [Google Scholar] [CrossRef]
- Schulz, M.; Borges, G.D.S.C.; Gonzaga, L.V.; Seraglio, S.K.T.; Olivo, I.S.; Azevedo, M.S.; Nehring, P.; de Gois, J.S.; de Almeida, T.S.; Vitali, L.; et al. Chemical composition, bioactive compounds and antioxidant capacity of juçara fruit (Euterpe edulis Martius) during ripening. Food Res. Int. 2015, 77, 125–131. [Google Scholar] [CrossRef]
- Schauss, A.G. Açai fruits: Potent antioxidant and anti-inflammatory superfruits with potential health benefits. In Dried Fruits: Phytochemicals and Health Effects; Alasalvar, C., Shahidi, F., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2013; p. 508. [Google Scholar]
- Vannuchi, N.; Jamar, G.; Pisani, L.; Braga, A.R.C.; de Rosso, V.V. Chemical composition, bioactive compounds extraction, and observed biological activities from jussara (Euterpe edulis): The exotic and endangered Brazilian superfruit. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3192–3224. [Google Scholar] [CrossRef]
- CONAB—Companhia Nacional De Abastecimento. Boletim da Sociobiodiversidade, Brasília, DF, v. 5, n. 5, Outubro 2021., 23. Available online: https://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/boletim-da-sociobiodiversidade/boletim-sociobio (accessed on 25 February 2022).
- Schauss, A.G.; Wu, X.; Prior, R.L.; Ou, B.; Patel, D.; Huang, D.; Kababick, J.P. Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe oleraceae mart. (acai). J. Agric. Food Chem. 2006, 54, 8598–8603. [Google Scholar] [CrossRef]
- Gordon, A.; Cruz, A.P.; Cabral, L.M.; de Freitas, S.C.; Taxi, C.M.; Donangelo, C.M.; de Andrade Mattietto, R.; Friedrich, M.; da Matta, V.M.; Marx, F. Chemical characterization and evaluation of antioxidant properties of acai fruits (Euterpe oleraceae Mart.) during ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef] [PubMed]
- D’Amico, R.; Impellizzeri, D.; Genovese, T.; Fusco, R.; Peritore, A.F.; Crupi, R.; Interdonato, L.; Franco, G.; Marino, Y.; Arangia, A.; et al. Acai Berry Mitigates Parkinson’s Disease Progression Showing Dopaminergic Neuroprotection via Nrf2-HO1 Pathways. Mol. Neurobiol. 2022, 59, 6519–6533. [Google Scholar] [CrossRef] [PubMed]
- Cardenas-Rodriguez, N.; Huerta-Gertrudis, B.; Rivera-Espinosa, L.; Montesinos-Correa, H.; Bandala, C.; Carmona-Aparicio, L.; Coballase-Urrutia, E. Role of oxidative stress in refractory epilepsy: Evidence in patients and experimental models. Int. J. Mol. Sci. 2013, 14, 1455–1476. [Google Scholar] [CrossRef] [PubMed]
- Popa-Wagner, A.; Mitran, S.; Sivanesan, S.; Chang, E.; Buga, A.M. ROS and brain diseases: The good, the bad, and the ugly. Oxid. Med. Cell. Longev. 2013, 2013, 963520. [Google Scholar] [CrossRef]
- Singh, E.; Devasahayam, G. Neurodegeneration by oxidative stress: A review on prospective use of small molecules for neuroprotection. Mol. Biol. Rep. 2020, 47, 3133–3140. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef]
- Rekatsina, M.; Paladini, A.; Piroli, A.; Zis, P.; Pergolizzi, J.V.; Varrassi, G. Pathophysiology and Therapeutic Perspectives of Oxidative Stress and Neurodegenerative Diseases: A Narrative Review. Adv. Ther. 2020, 37, 113–139. [Google Scholar] [CrossRef]
- Cobley, J.N.; Fiorello, M.L.; Bailey, D.M. 13 reasons why the brain is susceptible to oxidative stress. Redox Biol. 2018, 15, 490–503. [Google Scholar] [CrossRef]
- Lyman, M.; Lloyd, D.G.; Ji, X.; Vizcaychipi, M.P.; Ma, D. Neuroinflammation: The role and consequences. Neurosci. Res. 2014, 79, 1–12. [Google Scholar] [CrossRef]
- Kumar, V. Toll-like receptors in the pathogenesis of neuroinflammation. J. Neuroimmunol. 2019, 332, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Liu, L.; Zhang, S.; Nanda, A.; Li, G. Role of inflammation and its mediators in acute ischemic stroke. J. Cardiovasc. Transl. Res. 2013, 6, 834–851. [Google Scholar] [CrossRef] [PubMed]
- Solleiro-Villavicencio, H.; Rivas-Arancibia, S. Effect of Chronic Oxidative Stress on Neuroinflammatory Response Mediated by CD4(+)T Cells in Neurodegenerative Diseases. Front. Cell Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxid. Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef] [PubMed]
- Terrone, G.; Balosso, S.; Pauletti, A.; Ravizza, T.; Vezzani, A. Inflammation and reactive oxygen species as disease modifiers in epilepsy. Neuropharmacology 2020, 167, 107742. [Google Scholar] [CrossRef] [PubMed]
- Pajares, M.; Rojo, A.I.; Manda, G.; Boscá, L.; Cuadrado, A. Inflammation in Parkinson’s Disease: Mechanisms and Therapeutic Implications. Cells 2020, 9, 1687. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Soares, E.S.; Macchi, B.M.; Santos-Sacramento, L.; Takeda, P.Y.; Lopes-Araujo, A.; Paraense, R.S.O.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Luz, D.A.; et al. Towards Therapeutic Alternatives for Mercury Neurotoxicity in the Amazon: Unraveling the Pre-Clinical Effects of the Superfruit Acai (Euterpe oleracea, Mart.) as Juice for Human Consumption. Nutrients 2019, 11, 2585. [Google Scholar] [CrossRef]
- Farina, M.; Rocha, J.B.; Aschner, M. Mechanisms of methylmercury-induced neurotoxicity: Evidence from experimental studies. Life Sci. 2011, 89, 555–563. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Spada, P.D.; Dani, C.; Bortolini, G.V.; Funchal, C.; Henriques, J.A.; Salvador, M. Frozen fruit pulp of Euterpe oleraceae Mart. (Acai) prevents hydrogen peroxide-induced damage in the cerebral cortex, cerebellum, and hippocampus of rats. J. Med. Food 2009, 12, 1084–1088. [Google Scholar] [CrossRef]
- CONAB. Açaí-Análise Mensal-Março. 2019. Available online: https://www.conab.gov.br/info-agro/analises-do-mercado-agropecuario-e-extrativista/analises-do-mercado/historico-mensal-de-acai (accessed on 10 November 2021).
- Poulose, S.M.; Bielinski, D.F.; Carey, A.; Schauss, A.G.; Shukitt-Hale, B. Modulation of oxidative stress, inflammation, autophagy and expression of Nrf2 in hippocampus and frontal cortex of rats fed with acai-enriched diets. Nutr. Neurosci. 2017, 20, 305–315. [Google Scholar] [CrossRef]
- Heinrich, M.; Dhanji, T.; Casselman, I. Açai (Euterpe oleracea Mart.)—A phytochemical and pharmacological assessment of the species’ health claims. Phytochem. Lett. 2011, 4, 10–21. [Google Scholar] [CrossRef]
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.G.; Wu, X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Poulose, S.M.; Fisher, D.R.; Bielinski, D.F.; Gomes, S.M.; Rimando, A.M.; Schauss, A.G.; Shukitt-Hale, B. Restoration of stressor-induced calcium dysregulation and autophagy inhibition by polyphenol-rich acai (Euterpe spp.) fruit pulp extracts in rodent brain cells in vitro. Nutrition 2014, 30, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Souza-Monteiro, J.R.; Arrifano, G.P.F.; Queiroz, A.; Mello, B.S.F.; Custodio, C.S.; Macedo, D.S.; Hamoy, M.; Paraense, R.S.O.; Bittencourt, L.O.; Lima, R.R.; et al. Antidepressant and Antiaging Effects of Acai (Euterpe oleracea Mart.) in Mice. Oxid. Med. Cell. Longev. 2019, 2019, 3614960. [Google Scholar] [CrossRef]
- Souza-Monteiro, J.R.; Hamoy, M.; Santana-Coelho, D.; Arrifano, G.P.; Paraense, R.S.; Costa-Malaquias, A.; Mendonca, J.R.; da Silva, R.F.; Monteiro, W.S.; Rogez, H.; et al. Anticonvulsant properties of Euterpe oleracea in mice. Neurochem. Int. 2015, 90, 20–27. [Google Scholar] [CrossRef]
- da Silva, T.V.N.; Torres, M.F.; Sampaio, L.A.; Hamoy, M.; Monserrat, J.M.; Barbas, L.A.L. Dietary Euterpe oleracea Mart. attenuates seizures and damage to lipids in the brain of Colossoma macropomum. Fish Physiol. Biochem. 2021, 47, 1851–1864. [Google Scholar] [CrossRef]
- Muto, N.A.; Hamoy, M.; da Silva Ferreira, C.B.; Hamoy, A.O.; Lucas, D.C.R.; de Mello, V.J.; Rogez, H. Extract of Euterpe oleracea Martius Stone Presents Anticonvulsive Activity via the GABAA Receptor. Front. Cell Neurosci. 2022, 16, 872743. [Google Scholar] [CrossRef]
- de Bem, G.F.; Okinga, A.; Ognibene, D.T.; da Costa, C.A.; Santos, I.B.; Soares, R.A.; Silva, D.L.B.; da Rocha, A.P.M.; Isnardo Fernandes, J.; Fraga, M.C.; et al. Anxiolytic and antioxidant effects of Euterpe oleracea Mart. (acai) seed extract in adult rat offspring submitted to periodic maternal separation. Appl. Physiol. Nutr. Metab. 2020, 45, 1277–1286. [Google Scholar] [CrossRef]
- de Souza Machado, F.; Marinho, J.P.; Abujamra, A.L.; Dani, C.; Quincozes-Santos, A.; Funchal, C. Carbon Tetrachloride Increases the Pro-inflammatory Cytokines Levels in Different Brain Areas of Wistar Rats: The Protective Effect of Acai Frozen Pulp. Neurochem. Res. 2015, 40, 1976–1983. [Google Scholar] [CrossRef]
- de Souza Machado, F.; Kuo, J.; Wohlenberg, M.F.; da Rocha Frusciante, M.; Freitas, M.; Oliveira, A.S.; Andrade, R.B.; Wannmacher, C.M.; Dani, C.; Funchal, C. Subchronic treatment with acai frozen pulp prevents the brain oxidative damage in rats with acute liver failure. Metab. Brain Dis. 2016, 31, 1427–1434. [Google Scholar] [CrossRef]
- Nascimento, V.H.; Lima, C.D.; Paixao, J.T.; Freitas, J.J.; Kietzer, K.S. Antioxidant effects of acai seed ( Euterpe oleracea) in anorexia-cachexia syndrome induced by Walker-256 tumor. Acta Cir. Bras. 2016, 31, 597–601. [Google Scholar] [CrossRef]
- Yildirim, C.; Aydin, S.; Donertas, B.; Oner, S.; Kilic, F.S. Effects of Euterpe oleracea to Enhance Learning and Memory in a Conditioned Nicotinic and Muscarinic Receptor Response Paradigm by Modulation of Cholinergic Mechanisms in Rats. J. Med. Food 2020, 23, 388–394. [Google Scholar] [CrossRef]
- Oliveira, K.; Torres, M.L.M.; Kauffmann, N.; de Azevedo Ataide, B.J.; de Souza Franco Mendes, N.; Dos Anjos, L.M.; Dos Santos Borges, R.; Bahia, C.P.; Leao, L.K.R.; da Conceicao Fonseca Passos, A.; et al. Euterpe oleracea fruit (Acai)-enriched diet suppresses the development of experimental cerebral malaria induced by Plasmodium berghei (ANKA) infection. BMC Complement. Med. Ther. 2022, 22, 11. [Google Scholar] [CrossRef]
- Impellizzeri, D.; D’Amico, R.; Fusco, R.; Genovese, T.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Interdonato, L.; Di Paola, D.; Di Paola, R.; et al. Acai Berry Mitigates Vascular Dementia-Induced Neuropathological Alterations Modulating Nrf-2/Beclin1 Pathways. Cells 2022, 11, 2616. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, H.; Roxo, M.; Krstin, S.; Wang, X.; Wink, M. Anthocyanin-rich extract of Acai (Euterpe precatoria Mart.) mediates neuroprotective activities in Caenorhabditis elegans. J. Funct. Foods 2016, 26, 385–393. [Google Scholar] [CrossRef]
- Santamarina, A.B.; Jamar, G.; Mennitti, L.V.; de Rosso, V.V.; Cesar, H.C.; Oyama, L.M.; Pisani, L.P. The Use of Jucara (Euterpe edulis Mart.) Supplementation for Suppression of NF-kappaB Pathway in the Hypothalamus after High-Fat Diet in Wistar Rats. Molecules 2018, 23, 1814. [Google Scholar] [CrossRef]
- Machado, A.K.; Andreazza, A.C.; da Silva, T.M.; Boligon, A.A.; do Nascimento, V.; Scola, G.; Duong, A.; Cadona, F.C.; Ribeiro, E.E.; da Cruz, I.B. Neuroprotective Effects of Acai (Euterpe oleracea Mart.) against Rotenone In Vitro Exposure. Oxid. Med. Cell. Longev. 2016, 2016, 8940850. [Google Scholar] [CrossRef] [PubMed]
- Torma, P.D.; Brasil, A.V.; Carvalho, A.V.; Jablonski, A.; Rabelo, T.K.; Moreira, J.C.; Gelain, D.P.; Flores, S.H.; Augusti, P.R.; Rios, A.O. Hydroethanolic extracts from different genotypes of acai (Euterpe oleracea) presented antioxidant potential and protected human neuron-like cells (SH-SY5Y). Food Chem. 2017, 222, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Wong, D.Y.; Musgrave, I.F.; Harvey, B.S.; Smid, S.D. Acai (Euterpe oleraceae Mart.) berry extract exerts neuroprotective effects against beta-amyloid exposure in vitro. Neurosci. Lett. 2013, 556, 221–226. [Google Scholar] [CrossRef]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- da Silva Santos, V.; Bisen-Hersh, E.; Yu, Y.; Cabral, I.S.; Nardini, V.; Culbreth, M.; Teixeira da Rocha, J.B.; Barbosa, F., Jr.; Aschner, M. Anthocyanin-rich acai (Euterpe oleracea Mart.) extract attenuates manganese-induced oxidative stress in rat primary astrocyte cultures. J. Toxicol. Environ. Health A 2014, 77, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Ajit, D.; Simonyi, A.; Li, R.; Chen, Z.; Hannink, M.; Fritsche, K.L.; Mossine, V.V.; Smith, R.E.; Dobbs, T.K.; Luo, R.; et al. Phytochemicals and botanical extracts regulate NF-kappaB and Nrf2/ARE reporter activities in DI TNC1 astrocytes. Neurochem. Int. 2016, 97, 49–56. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Lichtenstein, M.P.; Souza-Monteiro, J.R.; Farina, M.; Rogez, H.; Carvalho, J.C.T.; Sunol, C.; Crespo-Lopez, M.E. Clarified Acai (Euterpe oleracea) Juice as an Anticonvulsant Agent: In Vitro Mechanistic Study of GABAergic Targets. Oxid. Med. Cell. Longev. 2018, 2018, 2678089. [Google Scholar] [CrossRef]
- de Souza, D.V.; Pappis, L.; Bandeira, T.T.; Sangoi, G.G.; Fontana, T.; Rissi, V.B.; Sagrillo, M.R.; Duarte, M.M.; Duarte, T.; Bodenstein, D.F.; et al. Acai (Euterpe oleracea Mart.) presents anti-neuroinflammatory capacity in LPS-activated microglia cells. Nutr. Neurosci. 2022, 25, 1188–1199. [Google Scholar] [CrossRef] [PubMed]
- Cadona, F.C.; de Souza, D.V.; Fontana, T.; Bodenstein, D.F.; Ramos, A.P.; Sagrillo, M.R.; Salvador, M.; Mota, K.; Davidson, C.B.; Ribeiro, E.E.; et al. Acai (Euterpe oleracea Mart.) as a Potential Anti-neuroinflammatory Agent: NLRP3 Priming and Activating Signal Pathway Modulation. Mol. Neurobiol. 2021, 58, 4460–4476. [Google Scholar] [CrossRef]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich acai (Euterpe oleracea Mart.) fruit pulp fractions attenuate inflammatory stress signaling in mouse brain BV-2 microglial cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef]
- Schulz, M.; Gonzaga, L.V.; de Souza, V.; Farina, M.; Vitali, L.; Micke, G.A.; Costa, A.C.O.; Fett, R. Neuroprotective effect of jucara (Euterpe edulis Martius) fruits extracts against glutamate-induced oxytosis in HT22 hippocampal cells. Food Res. Int. 2019, 120, 114–123. [Google Scholar] [CrossRef]
- Carey, A.N.; Miller, M.G.; Fisher, D.R.; Bielinski, D.F.; Gilman, C.K.; Poulose, S.M.; Shukitt-Hale, B. Dietary supplementation with the polyphenol-rich acai pulps (Euterpe oleracea Mart. and Euterpe precatoria Mart.) improves cognition in aged rats and attenuates inflammatory signaling in BV-2 microglial cells. Nutr. Neurosci. 2017, 20, 238–245. [Google Scholar] [CrossRef]
- Goh, J.-Y.; Weaver, R.J.; Dixon, L.; Platt, N.J.; Roberts, R.A. Development and use of in vitro alternatives to animal testing by the pharmaceutical industry 1980–2013. Toxicol. Res. 2015, 1297–1307. [Google Scholar] [CrossRef]
- de Liz, S.; Cardoso, A.L.; Copetti, C.L.K.; Hinnig, P.F.; Vieira, F.G.K.; da Silva, E.L.; Schulz, M.; Fett, R.; Micke, G.A.; Di Pietro, P.F. Acai (Euterpe oleracea Mart.) and jucara (Euterpe edulis Mart.) juices improved HDL-c levels and antioxidant defense of healthy adults in a 4-week randomized cross-over study. Clin. Nutr. 2020, 39, 3629–3636. [Google Scholar] [CrossRef]
- Pala, D.; Barbosa, P.O.; Silva, C.T.; de Souza, M.O.; Freitas, F.R.; Volp, A.C.P.; Maranhao, R.C.; Freitas, R.N. Acai (Euterpe oleracea Mart.) dietary intake affects plasma lipids, apolipoproteins, cholesteryl ester transfer to high-density lipoprotein and redox metabolism: A prospective study in women. Clin. Nutr. 2018, 37, 618–623. [Google Scholar] [CrossRef]
- Vigneron, M.; Deparis, X.; Deharo, E.; Bourdy, G. Antimalarial remedies in French Guiana: A knowledge attitudes and practices study. J. Ethnopharmacol. 2005, 98, 351–360. [Google Scholar] [CrossRef]
- Kffuri, C.W.; Lopes, M.A.; Ming, L.C.; Odonne, G.; Kinupp, V.F. Antimalarial plants used by indigenous people of the Upper Rio Negro in Amazonas, Brazil. J. Ethnopharmacol. 2016, 178, 188–198. [Google Scholar] [CrossRef]
- Gois, M.A.F.; Lucas, F.C.A.; Costa, J.C.M.; Moura, P.H.B.D.; Lobato, G.D.J.M. Etnobotânica de espécies vegetais medicinais no tratamento de transtornos do sistema gastrointestinal. Rev. Bras. Plant. Med. 2016, 18, 547–557. [Google Scholar] [CrossRef]
- Martins, A.G.; Rosário, D.L.d.; Barros, M.N.d.; Jardim, M.A.G. Etnobotanical research of medicinal, alimentary and toxic plants in Combu Island, County of Belém, Pará, Brazil. Rev. Bras. Farm. 2005, 86, 21–30. [Google Scholar]
- Pereira, M.D.G.D.S.; Coelho-Ferreira, M. Uso e diversidade de plantas medicinais em uma comunidade quilombola na Amazônia Oriental, Abaetetuba, Pará. Biota Amaz. 2017, 7, 57–68. [Google Scholar]
- Jardim, M.A.G.; Medeiros, T.D.S. Plantas oleaginosas do Estado do Pará: Composição florística e usos medicinais. Rev. Bras. Farmácia 2006, 87, 124–127. [Google Scholar]
- Carneiro, F.M.; Silva, M.J.P.d.; Borges, L.L.; Albernaz, L.C.; Costa, J.D.P. Trends of studies for medicinal plants in Brazil. Rev. Sapiência Soc. Saberes E Práticas Educ. 2014, 3, 44–75. [Google Scholar]
- da Silva Cristino Cordeiro, V.; de Bem, G.F.; da Costa, C.A.; Santos, I.B.; de Carvalho, L.; Ognibene, D.T.; da Rocha, A.P.M.; de Carvalho, J.J.; de Moura, R.S.; Resende, A.C. Euterpe oleracea Mart. seed extract protects against renal injury in diabetic and spontaneously hypertensive rats: Role of inflammation and oxidative stress. Eur. J. Nutr. 2018, 57, 817–832. [Google Scholar] [CrossRef]
- de Andrade Soares, R.; de Oliveira, B.C.; de Bem, G.F.; de Menezes, M.P.; Romao, M.H.; Santos, I.B.; da Costa, C.A.; de Carvalho, L.; Nascimento, A.L.R.; de Carvalho, J.J.; et al. Acai (Euterpe oleracea Mart.) seed extract improves aerobic exercise performance in rats. Food Res. Int. 2020, 136, 109549. [Google Scholar] [CrossRef] [PubMed]
- Martins, G.R.; Guedes, D.; Marques de Paula, U.L.; de Oliveira, M.; Lutterbach, M.T.S.; Reznik, L.Y.; Servulo, E.F.C.; Alviano, C.S.; Ribeiro da Silva, A.J.; Alviano, D.S. Acai (Euterpe oleracea Mart.) Seed Extracts from Different Varieties: A Source of Proanthocyanidins and Eco-Friendly Corrosion Inhibition Activity. Molecules 2021, 26, 3433. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham Ul, H.; Patel, S.; Pan, X.; Naz, S.; Sanches Silva, A.; Saeed, F.; Rasul Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef]
- Wang, X.; Cao, Y.; Chen, S.; Lin, J.; Bian, J.; Huang, D. Anti-Inflammation Activity of Flavones and Their Structure-Activity Relationship. J. Agric. Food Chem. 2021, 69, 7285–7302. [Google Scholar] [CrossRef]
- Matheus, M.E.; de Oliveira Fernandes, S.B.; Silveira, C.S.; Rodrigues, V.P.; de Sousa Menezes, F.; Fernandes, P.D. Inhibitory effects of Euterpe oleracea Mart. on nitric oxide production and iNOS expression. J. Ethnopharmacol. 2006, 107, 291–296. [Google Scholar] [CrossRef]
- Jha, M.K.; Jo, M.; Kim, J.H.; Suk, K. Microglia-Astrocyte Crosstalk: An Intimate Molecular Conversation. Neuroscientist 2019, 25, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Augusto-Oliveira, M.; Arrifano, G.P.; Lopes-Araujo, A.; Santos-Sacramento, L.; Takeda, P.Y.; Anthony, D.C.; Malva, J.O.; Crespo-Lopez, M.E. What Do Microglia Really Do in Healthy Adult Brain? Cells 2019, 8, 1293. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef]
- Augusto-Oliveira, M.; Arrifano, G.P.; Takeda, P.Y.; Lopes-Araujo, A.; Santos-Sacramento, L.; Anthony, D.C.; Verkhratsky, A.; Crespo-Lopez, M.E. Astroglia-specific contributions to the regulation of synapses, cognition and behaviour. Neurosci. Biobehav. Rev. 2020, 118, 331–357. [Google Scholar] [CrossRef]
- Crespo-Lopez, M.E.; Herculano, A.M.; Corvelo, T.C.; Do Nascimento, J.L. Mercury and neurotoxicity. Rev. Neurol. 2005, 40, 441–447. [Google Scholar] [PubMed]
- Berzas Nevado, J.J.; Rodriguez Martin-Doimeadios, R.C.; Guzman Bernardo, F.J.; Jimenez Moreno, M.; Herculano, A.M.; do Nascimento, J.L.; Crespo-Lopez, M.E. Mercury in the Tapajos River basin, Brazilian Amazon: A review. Environ. Int. 2010, 36, 593–608. [Google Scholar] [CrossRef]
- Arrifano, G.P.F.; Martin-Doimeadios, R.C.R.; Jimenez-Moreno, M.; Ramirez-Mateos, V.; da Silva, N.F.S.; Souza-Monteiro, J.R.; Augusto-Oliveira, M.; Paraense, R.S.O.; Macchi, B.M.; do Nascimento, J.L.M.; et al. Large-scale projects in the amazon and human exposure to mercury: The case-study of the Tucurui Dam. Ecotoxicol. Environ. Saf. 2018, 147, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Martin-Doimeadios, R.C.; Berzas Nevado, J.J.; Guzman Bernardo, F.J.; Jimenez Moreno, M.; Arrifano, G.P.; Herculano, A.M.; do Nascimento, J.L.; Crespo-Lopez, M.E. Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ. Sci. Pollut. Res. Int. 2014, 21, 7466–7479. [Google Scholar] [CrossRef]
- Kang, J.; Li, Z.; Wu, T.; Jensen, G.S.; Schauss, A.G.; Wu, X. Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). Food Chem. 2010, 122, 610–617. [Google Scholar] [CrossRef]
- de Almeida Magalhaes, T.S.S.; de Oliveira Macedo, P.C.; Converti, A.; Neves de Lima, A.A. The Use of Euterpe oleracea Mart. As a New Perspective for Disease Treatment and Prevention. Biomolecules 2020, 10, 813. [Google Scholar] [CrossRef]
- Pape, K.; Tamouza, R.; Leboyer, M.; Zipp, F. Immunoneuropsychiatry—Novel perspectives on brain disorders. Nat. Rev. Neurol. 2019, 15, 317–328. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Rai, S.; Swarnkar, S.; Tota, S.; Nath, C.; Tyagi, N. Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer’s Disease: Understanding the Therapeutics Strategies. Mol. Neurobiol. 2016, 53, 648–661. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Hannan, M.A.; Dash, R.; Sohag, A.A.M.; Haque, M.N.; Moon, I.S. Neuroprotection Against Oxidative Stress: Phytochemicals Targeting TrkB Signaling and the Nrf2-ARE Antioxidant System. Front. Mol. Neurosci. 2020, 13, 116. [Google Scholar] [CrossRef]
- Geronzi, U.; Lotti, F.; Grosso, S. Oxidative stress in epilepsy. Expert Rev. Neurother. 2018, 18, 427–434. [Google Scholar] [CrossRef]
- Teleanu, D.M.; Niculescu, A.G.; Lungu, I.l.; Radu, C.I.; Vladacenco, O.; Roza, E.; Costachescu, B.; Grumezescu, A.M.; Teleanu, R.I. An Overview of Oxidative Stress, Neuroinflammation, and Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 5938. [Google Scholar] [CrossRef]
- Morella, I.M.; Brambilla, R.; More, L. Emerging roles of brain metabolism in cognitive impairment and neuropsychiatric disorders. Neurosci. Biobehav. Rev. 2022, 142, 104892. [Google Scholar] [CrossRef]
- Faria-Pereira, A.; Morais, V.A. Synapses: The Brain’s Energy-Demanding Sites. Int. J. Mol. Sci. 2022, 23, 3627. [Google Scholar] [CrossRef]
- Du, F.; Zhu, X.H.; Zhang, Y.; Friedman, M.; Zhang, N.; Ugurbil, K.; Chen, W. Tightly coupled brain activity and cerebral ATP metabolic rate. Proc. Natl. Acad. Sci. USA 2008, 105, 6409–6414. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Neuroprotective Function of High Glycolytic Activity in Astrocytes: Common Roles in Stroke and Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 6568. [Google Scholar] [CrossRef]
- Takahashi, S. Metabolic Contribution and Cerebral Blood Flow Regulation by Astrocytes in the Neurovascular Unit. Cells 2022, 11, 813. [Google Scholar] [CrossRef] [PubMed]
- Claassen, J.A.H.R.; Dick, H.J.; Thijssen, R.B.P.; Faraci, F.M. Regulation of cerebral blood flow in humans: Physiology and clinical implications of autoregulation. Physiol. Rev. 2021, 101, 1487–1559. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Vezzani, A.; Friedman, A.; Dingledine, R.J. The role of inflammation in epileptogenesis. Neuropharmacology 2013, 69, 16–24. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chang, M. Roles of PRR-Mediated Signaling Pathways in the Regulation of Oxidative Stress and Inflammatory Diseases. Int. J. Mol. Sci. 2021, 22, 7688. [Google Scholar] [CrossRef]
- Vezzani, A.; Balosso, S.; Ravizza, T. Neuroinflammatory pathways as treatment targets and biomarkers in epilepsy. Nat. Rev. Neurol. 2019, 15, 459–472. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Bandopadhyay, R.; Singh, P.K.; Mishra, P.S.; Sharma, N.; Khurana, N. Neuroinflammation in neurological disorders: Pharmacotherapeutic targets from bench to bedside. Metab. Brain Dis. 2021, 36, 1591–1626. [Google Scholar] [CrossRef]
- Girish, C.; Raj, V.; Arya, J.; Balakrishnan, S. Evidence for the involvement of the monoaminergic system, but not the opioid system in the antidepressant-like activity of ellagic acid in mice. Eur. J. Pharmacol. 2012, 682, 118–125. [Google Scholar] [CrossRef]
- Zeni, A.L.; Zomkowski, A.D.; Maraschin, M.; Rodrigues, A.L.; Tasca, C.I. Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: Evidence for the involvement of the serotonergic system. Eur. J. Pharmacol. 2012, 679, 68–74. [Google Scholar] [CrossRef]
- Nakazawa, T.; Yasuda, T.; Ueda, J.; Ohsawa, K. Antidepressant-like effects of apigenin and 2,4,5-trimethoxycinnamic acid from Perilla frutescens in the forced swimming test. Biol. Pharm. Bull. 2003, 26, 474–480. [Google Scholar] [CrossRef]
- Yi, L.T.; Li, J.M.; Li, Y.C.; Pan, Y.; Xu, Q.; Kong, L.D. Antidepressant-like behavioral and neurochemical effects of the citrus-associated chemical apigenin. Life Sci. 2008, 82, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Machado, D.G.; Bettio, L.E.; Cunha, M.P.; Santos, A.R.; Pizzolatti, M.G.; Brighente, I.M.; Rodrigues, A.L. Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: Evidence for the involvement of the serotonergic and noradrenergic systems. Eur. J. Pharmacol. 2008, 587, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, R.; Chen, C.; Du, X.; Ruan, L.; Sun, J.; Li, J.; Zhang, L.; O’Donnell, J.M.; Pan, J.; et al. Antidepressant-like effect of trans-resveratrol in chronic stress model: Behavioral and neurochemical evidences. J. Psychiatr. Res. 2013, 47, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, D.; Chhillar, R. Antidepressant-like activity of ellagic acid in unstressed and acute immobilization-induced stressed mice. Pharmacol. Rep. 2012, 64, 796–807. [Google Scholar] [CrossRef]
- Dhapola, R.; Hota, S.S.; Sarma, P.; Bhattacharyya, A.; Medhi, B.; Reddy, D.H. Recent advances in molecular pathways and therapeutic implications targeting neuroinflammation for Alzheimer’s disease. Inflammopharmacology 2021, 29, 1669–1681. [Google Scholar] [CrossRef] [PubMed]
- Shabab, T.; Khanabdali, R.; Moghadamtousi, S.Z.; Kadir, H.A.; Mohan, G. Neuroinflammation pathways: A general review. Int. J. Neurosci. 2017, 127, 624–633. [Google Scholar] [CrossRef]
- Schain, M.; Kreisl, W.C. Neuroinflammation in Neurodegenerative Disorders—A Review. Curr. Neurol. Neurosci. Rep. 2017, 17, 25. [Google Scholar] [CrossRef]
- Vishwakarma, S.; Singh, S.; Singh, T.G. Pharmacological modulation of cytokines correlating neuroinflammatory cascades in epileptogenesis. Mol. Biol. Rep. 2022, 49, 1437–1452. [Google Scholar] [CrossRef]
- Soltani Khaboushan, A.; Yazdanpanah, N.; Rezaei, N. Neuroinflammation and Proinflammatory Cytokines in Epileptogenesis. Mol. Neurobiol. 2022, 59, 1724–1743. [Google Scholar] [CrossRef]
- Loscher, W.; Potschka, H.; Sisodiya, S.M.; Vezzani, A. Drug Resistance in Epilepsy: Clinical Impact, Potential Mechanisms, and New Innovative Treatment Options. Pharmacol. Rev. 2020, 72, 606–638. [Google Scholar] [CrossRef]
- Brandes, M.S.; Gray, N.E. NRF2 as a Therapeutic Target in Neurodegenerative Diseases. ASN Neuro 2020, 12, 1759091419899782. [Google Scholar] [CrossRef]
- Liddell, J.R. Are Astrocytes the Predominant Cell Type for Activation of Nrf2 in Aging and Neurodegeneration? Antioxidants 2017, 6, 65. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef]
- Zgorzynska, E.; Dziedzic, B.; Walczewska, A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2021, 22, 9592. [Google Scholar] [CrossRef]
- Sandberg, M.; Patil, J.; D’Angelo, B.; Weber, S.G.; Mallard, C. NRF2-regulation in brain health and disease: Implication of cerebral inflammation. Neuropharmacology 2014, 79, 298–306. [Google Scholar] [CrossRef]
- Osama, A.; Zhang, J.; Yao, J.; Yao, X.; Fang, J. Nrf2: A dark horse in Alzheimer’s disease treatment. Ageing Res. Rev. 2020, 64, 101206. [Google Scholar] [CrossRef]
- Clements, C.M.; McNally, R.S.; Conti, B.J.; Mak, T.W.; Ting, J.P. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. USA 2006, 103, 15091–15096. [Google Scholar] [CrossRef]
- Buendia, I.; Michalska, P.; Navarro, E.; Gameiro, I.; Egea, J.; Leon, R. Nrf2-ARE pathway: An emerging target against oxidative stress and neuroinflammation in neurodegenerative diseases. Pharmacol. Ther. 2016, 157, 84–104. [Google Scholar] [CrossRef]
- Cuadrado, A. Brain-Protective Mechanisms of Transcription Factor NRF2: Toward a Common Strategy for Neurodegenerative Diseases. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 255–277. [Google Scholar] [CrossRef]
- Morris, G.; Walker, A.J.; Walder, K.; Berk, M.; Marx, W.; Carvalho, A.F.; Maes, M.; Puri, B.K. Increasing Nrf2 Activity as a Treatment Approach in Neuropsychiatry. Mol. Neurobiol. 2021, 58, 2158–2182. [Google Scholar] [CrossRef]
- Brunschwig, C.; Leba, L.J.; Saout, M.; Martial, K.; Bereau, D.; Robinson, J.C. Chemical Composition and Antioxidant Activity of Euterpe oleracea Roots and Leaflets. Int. J. Mol. Sci. 2016, 18, 61. [Google Scholar] [CrossRef]
- Cardoso, A.L.; Pietro, P.F.D.; Vieira, F.G.K.; Boaventura, B.C.B.; Liz, S.d.; Borges, G.d.S.C.; Fett, R.; Andrade, D.F.d.; Silva, E.L.d. Acute consumption of juçara juice (Euterpe edulis) and antioxidant activity in healthy individuals. J. Funct. Foods 2015, 17, 152–162. [Google Scholar] [CrossRef]
- Manolescu, B.N.; Oprea, E.; Mititelu, M.; Ruta, L.L.; Farcasanu, I.C. Dietary Anthocyanins and Stroke: A Review of Pharmacokinetic and Pharmacodynamic Studies. Nutrients 2019, 11, 1479. [Google Scholar] [CrossRef]
- Leclerc, M.; Dudonne, S.; Calon, F. Can Natural Products Exert Neuroprotection without Crossing the Blood-Brain Barrier? Int. J. Mol. Sci. 2021, 22, 3356. [Google Scholar] [CrossRef]
- Di Lorenzo, C.; Colombo, F.; Biella, S.; Stockley, C.; Restani, P. Polyphenols and Human Health: The Role of Bioavailability. Nutrients 2021, 13, 273. [Google Scholar] [CrossRef]
| In Vivo Assays | |||
|---|---|---|---|
| Species | Experimental Model/Part of the Plants Used | Outcomes | References |
| Euterpe oleracea Mart. | Model of MeHg intoxication in mice—fruit |
| [46] |
| Depressive-like Behavior induced by LPS in mice—fruit |
| [55] | |
| Seizure induced by PTZ in mice—fruit |
| [56] | |
| Pentylenetetrazole (PTZ)-induced seizures in fish—fruit |
| [57] | |
| Seizure induced by PTZ in Rat—Stone |
| [58] | |
| Anxiety induced by periodic maternal separation (PMS) in rats—seed |
| [59] | |
| Hepatic encephalopathy in rats—fruit |
| [60] | |
| Hepatic encephalopathy in rats—fruit |
| [61] | |
| Anorexia-cachexia syndrome induced by Walker-256 tumor in rats—seed |
| [62] | |
| Evaluation of the effects of EO on learning and memory in rats |
| [63] | |
| Infection by Plasmodium berghei ANKA strain—fruit |
| [64] | |
| Experimental model of Parkinson’s disease (PD) MPTP-Induced in mice—Fruit |
| [31] | |
| Model of Vascular dementia (VaD) in mice—Fruit |
| [65] | |
| Euterpe oleracea Mart. and Euterpe Precatoria Mart. | Açaí-enriched diet—fruit |
| [51] |
| Euterpe precatoria Mart. | Caenorhabditis elegans—fruit |
| [66] |
| Euterpe edulis Mart. | High-Fat Diet—fruit |
| [67] |
| In Vitro Assays | |||
| Species | Experimental Model and Part of Plant | Outcomes | References |
| Euterpe oleracea Mart. | Tissues treated with hydrogen peroxide (H2O2)—fruit |
| [49] |
| Neuronal-like cells SHSY5Y—fruit |
| [68] | |
| human neuroblastoma cell line SH-SY5Y—fruit |
| [69] | |
| Primary hippocampal neurons and HT22 mouse hippocampal cells—fruit |
| [54] | |
| Rat phaeochromocytoma cells (PC12 cell)—fruit |
| [70] | |
| C-6 rat brain carcinoma cells—fruit |
| [71] | |
| Primary Cultures of Rat Astrocytes—fruit |
| [72] | |
| Immortalized rat astrocytes (DI TNC1)—fruit |
| [73] | |
| Primary cultures of cortical neurons and astrocytes—fruit |
| [74] | |
| BV-2 microglia cell line—fruit |
| [75] | |
| Microglia EOC 13.31 cell line—fruit |
| [76] | |
| BV-2 microglia cell line—fruit |
| [77] | |
| Euterpe edulis Mart. | Mouse hippocampal HT22 cells—fruit |
| [78] |
| In Vitro and In Vivo Assays | |||
| Species | Experimental Model | Outcomes | References |
| Euterpe oleracea Mart. and Euterpe precatoria Mart. | Dietary supplementation with EO and EP—in vivo—fruit BV-2 cells were treated with blood serum from both EO- and EP-fed rats—in vitro—fruit |
| [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Da Silva, I.O.; Crespo-Lopez, M.E.; Augusto-Oliveira, M.; Arrifano, G.d.P.; Ramos-Nunes, N.R.; Gomes, E.B.; da Silva, F.R.P.; de Sousa, A.A.; Leal, A.L.A.B.; Damasceno, H.C.; et al. What We Know about Euterpe Genus and Neuroprotection: A Scoping Review. Nutrients 2023, 15, 3189. https://doi.org/10.3390/nu15143189
Da Silva IO, Crespo-Lopez ME, Augusto-Oliveira M, Arrifano GdP, Ramos-Nunes NR, Gomes EB, da Silva FRP, de Sousa AA, Leal ALAB, Damasceno HC, et al. What We Know about Euterpe Genus and Neuroprotection: A Scoping Review. Nutrients. 2023; 15(14):3189. https://doi.org/10.3390/nu15143189
Chicago/Turabian StyleDa Silva, Ilano Oliveira, Maria Elena Crespo-Lopez, Marcus Augusto-Oliveira, Gabriela de Paula Arrifano, Natália Raphaela Ramos-Nunes, Elielton Barreto Gomes, Felipe Rodolfo Pereira da Silva, Aline Andrade de Sousa, Alessandro Luiz Araújo Bentes Leal, Helane Conceição Damasceno, and et al. 2023. "What We Know about Euterpe Genus and Neuroprotection: A Scoping Review" Nutrients 15, no. 14: 3189. https://doi.org/10.3390/nu15143189
APA StyleDa Silva, I. O., Crespo-Lopez, M. E., Augusto-Oliveira, M., Arrifano, G. d. P., Ramos-Nunes, N. R., Gomes, E. B., da Silva, F. R. P., de Sousa, A. A., Leal, A. L. A. B., Damasceno, H. C., de Oliveira, A. C. A., & Souza-Monteiro, J. R. (2023). What We Know about Euterpe Genus and Neuroprotection: A Scoping Review. Nutrients, 15(14), 3189. https://doi.org/10.3390/nu15143189








