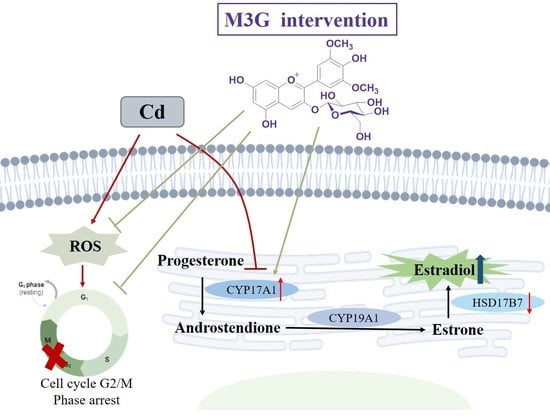
Malvidin-3-O-Glucoside Ameliorates Cadmium-Mediated Cell Dysfunction in the Estradiol Generation of Human Granulosa Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
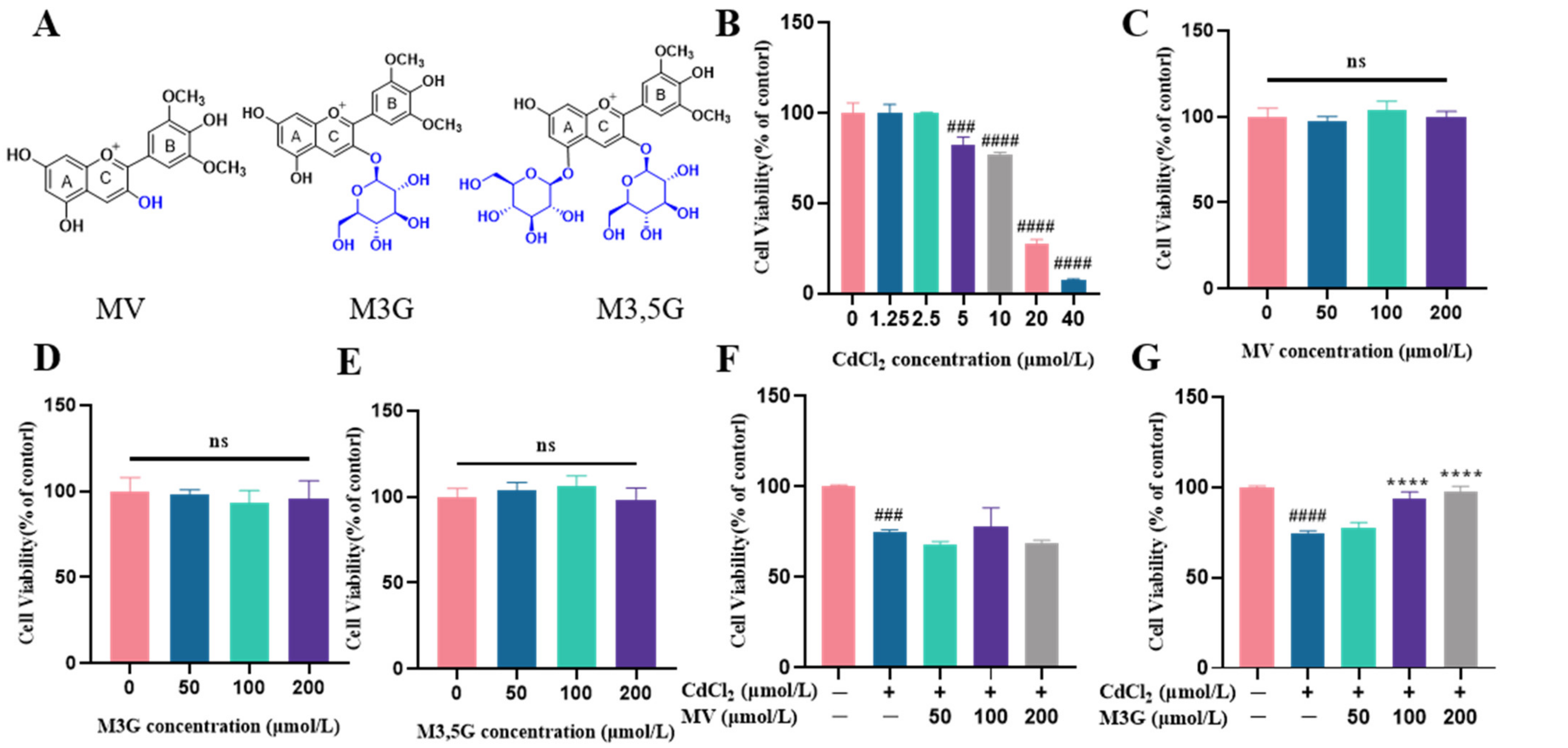
2.2. Cell Viability
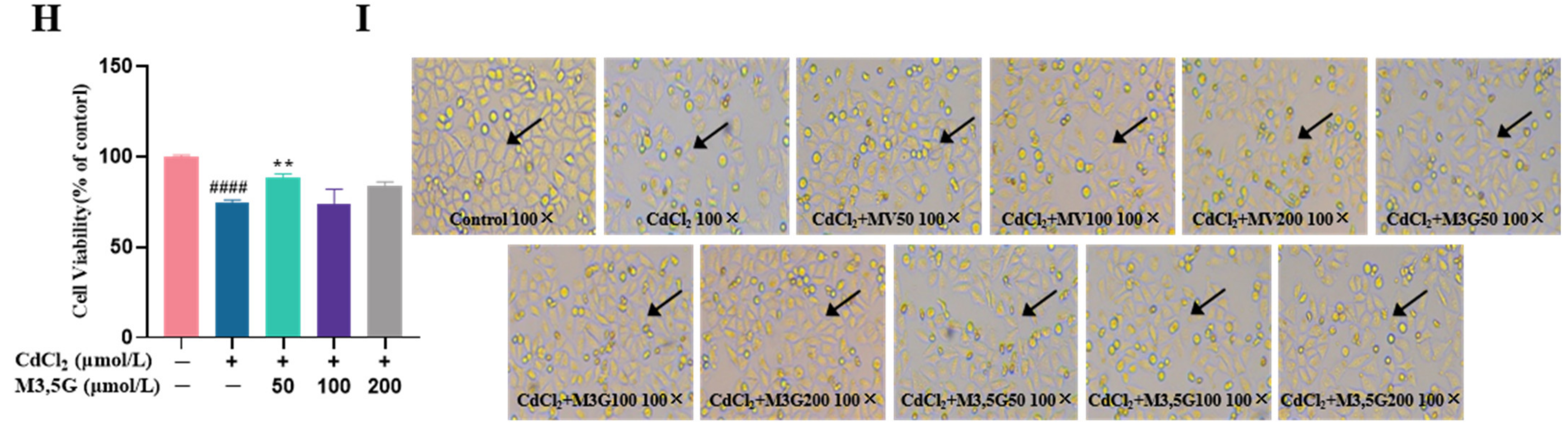
2.3. Cell Morphology Observation
2.4. Detection of E2
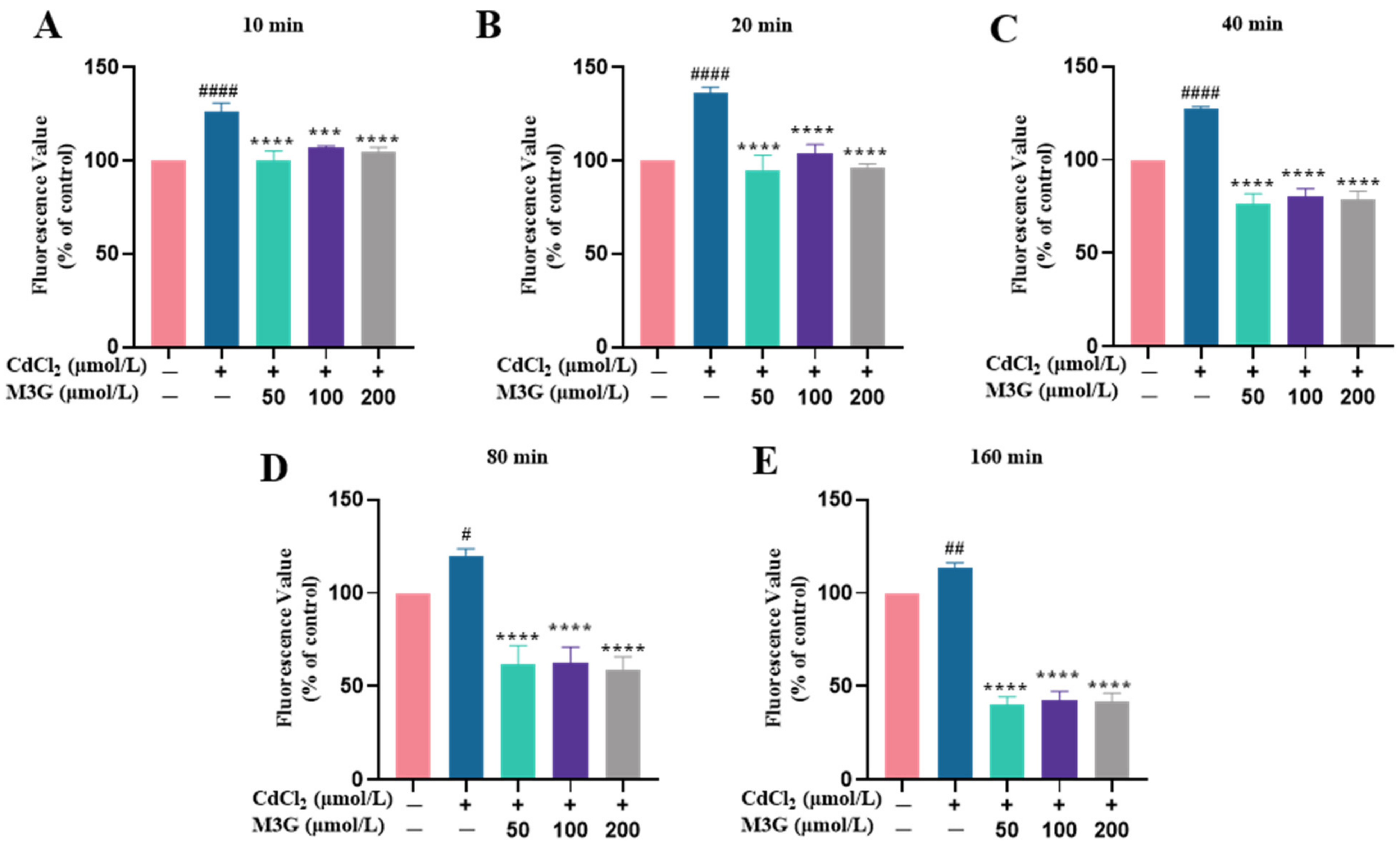
2.5. Determination of Reactive Oxygen Species
2.6. Cell Cycle Analysis
2.7. Transcriptomic Analysis
2.8. Quantitative Real-Time Polymerase Chain Reaction
2.9. Western Blot
2.10. Statistical Analysis
3. Results
3.1. Effects of M3G on the Viability and Morphological Changes of CdCl2-Exposed KGN Cells
3.2. Effects of M3G on E2 Levels of CdCl2-Exposed KGN Cells
3.3. Protective Effect of M3G against ROS Production Induced by CdCl2
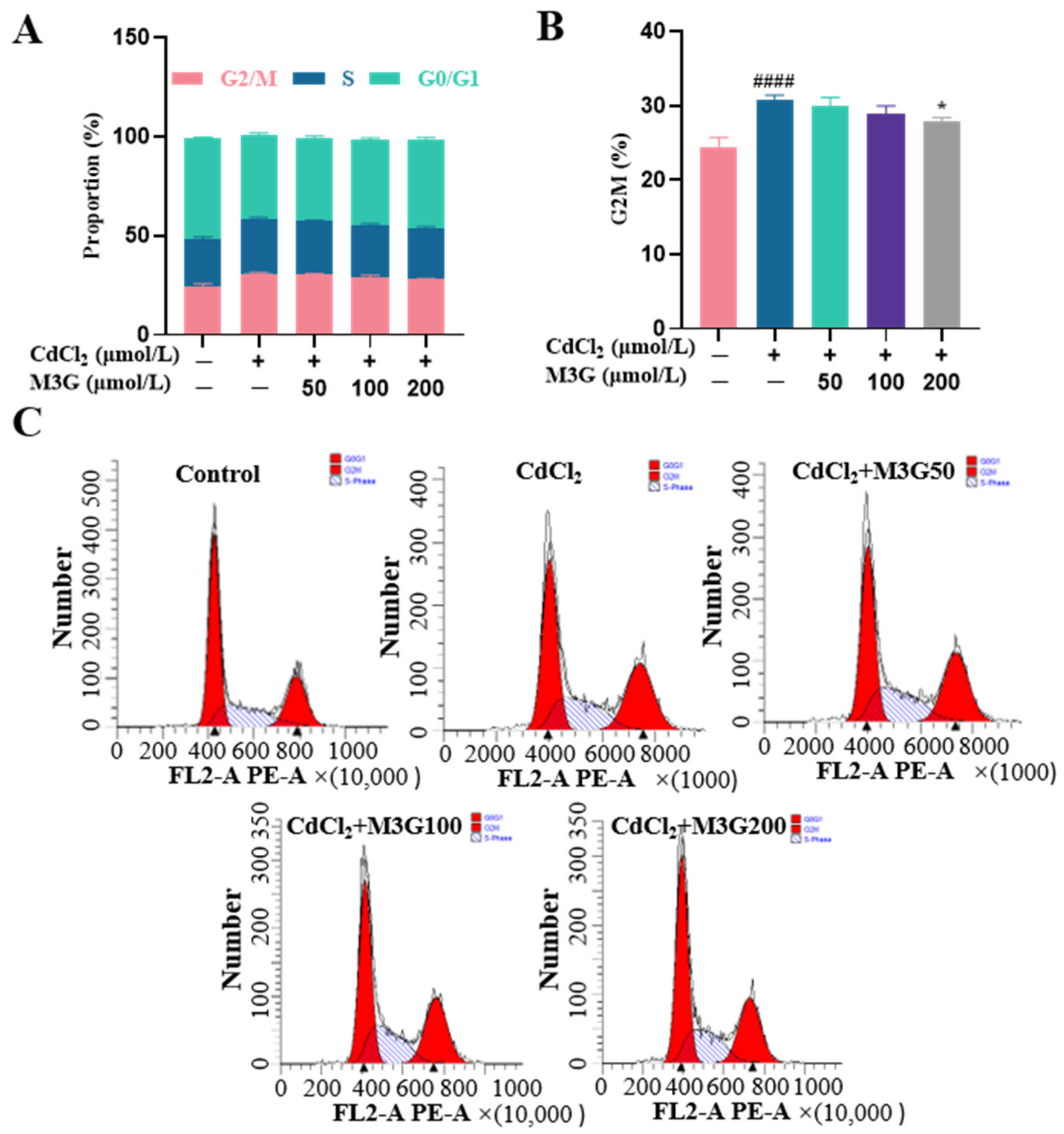
3.4. Protective Effect of M3G on Cell Cycle Arrest of CdCl2-Exposed KGN Cells
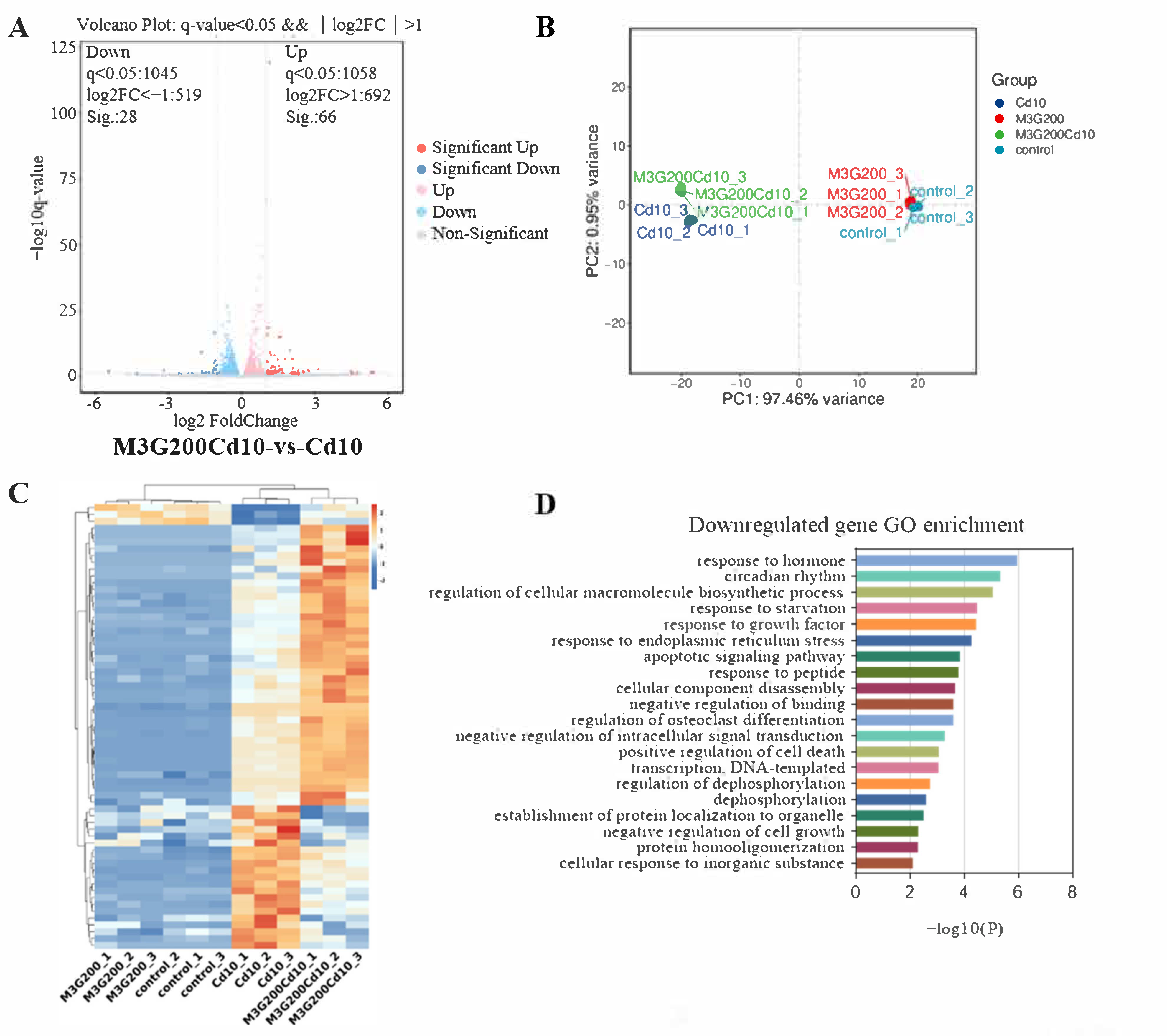
3.5. Effects of M3G on the Transcriptome of CdCl2-Exposed KGN Cells
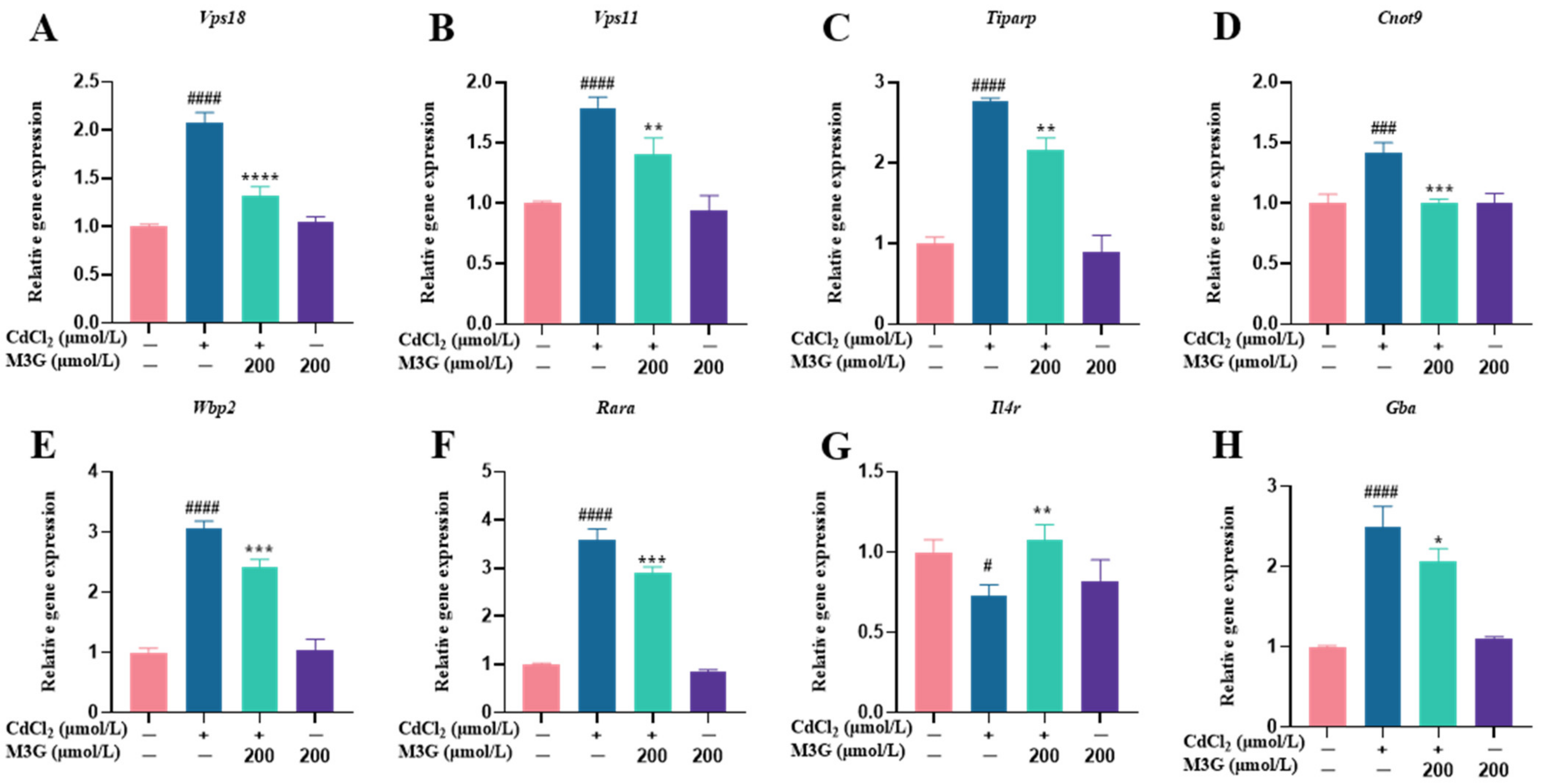
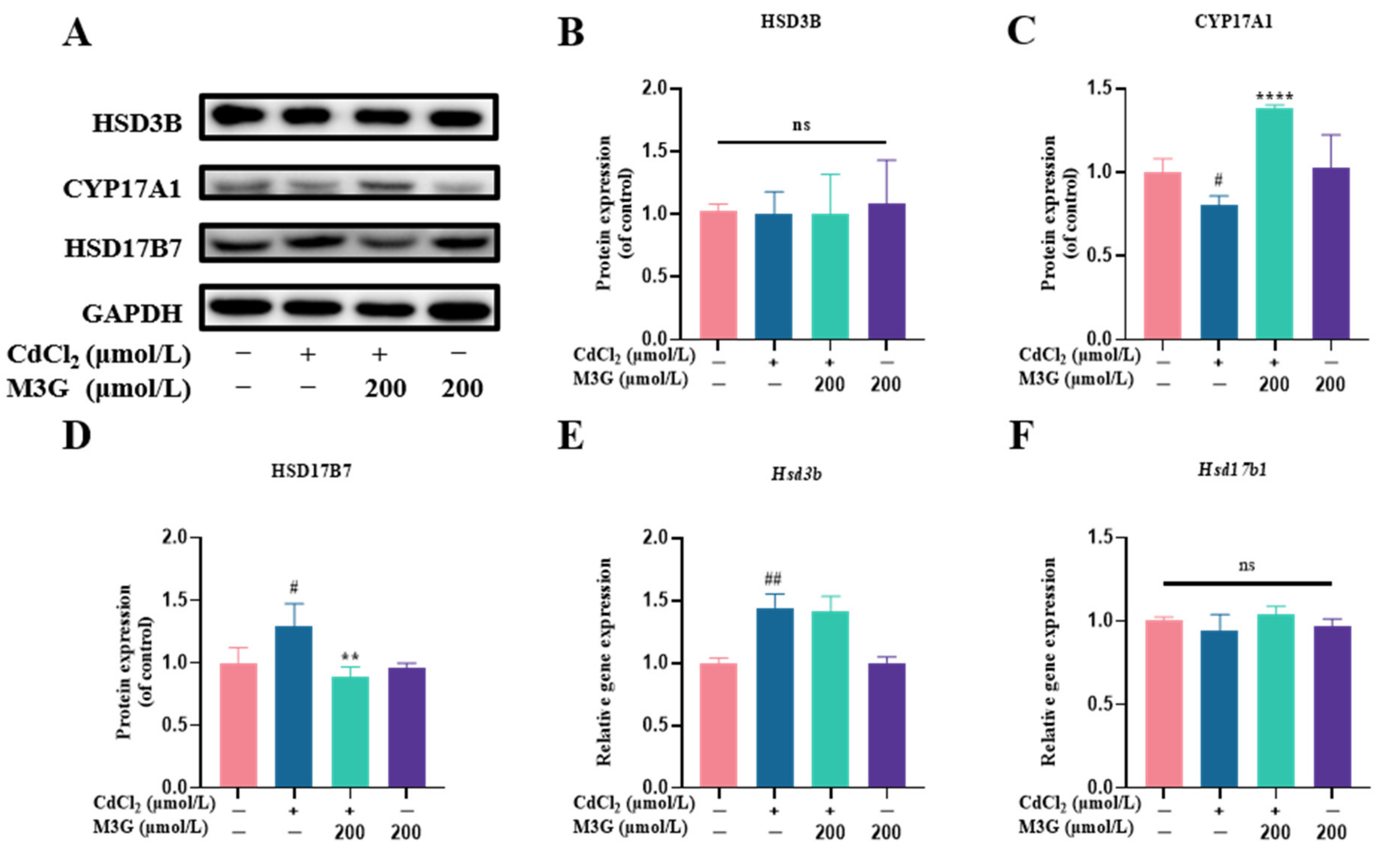
3.6. Effects of M3G on E2-Related Protein and Gene Expression of CdCl2-Exposed KGN Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Sharma, A. Cadmium toxicity: Effects on human reproduction and fertility. Rev. Environ. Health 2019, 34, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, H.R.; Dennis, S.; Fitzpatrick, S. Cadmium: Mitigation strategies to reduce dietary exposure. J. Food Sci. 2020, 85, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Mezynska, M.; Brzoska, M.M. Environmental exposure to cadmium-a risk for health of the general population in industrialized countries and preventive strategies. Environ. Sci. Pollut. Res. 2018, 25, 3211–3232. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yao, Z.; Yang, D.; Jiang, X.; Sun, J.; Tian, L.; Hu, J.; Wu, B.; Bai, W. Cyanidin-3-O-glucoside restores spermatogenic dysfunction in cadmium-exposed pubertal mice via histone ubiquitination and mitigating oxidative damage. J. Hazard. Mater. 2020, 387, 121706. [Google Scholar] [CrossRef]
- Bhardwaj, J.K.; Paliwal, A.; Saraf, P. Effects of heavy metals on reproduction owing to infertility. J. Biochem. Mol. Toxicol. 2021, 35, e22823. [Google Scholar] [CrossRef]
- Wang, X.; Tian, J. Health risks related to residential exposure to cadmium in Zhenhe County, China. Arch. Environ. Health 2004, 59, 324–330. [Google Scholar] [CrossRef]
- Lee, S.; Min, J.Y.; Min, K.B. Female infertility associated with blood lead and cadmium levels. Int. J. Environ. Res. Public Health 2020, 17, 1794. [Google Scholar] [CrossRef]
- Nasiadek, M.; Danilewicz, M.; Klimczak, M.; Stragierowicz, J.; Kilanowicz, A. Subchronic exposure to cadmium causes persistent changes in the reproductive system in female wistar rats. Oxid. Med. Cell. Longev. 2019, 2019, 6490820. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, X.; Wang, Y.; Fan, R.; Qiu, C.; Zhong, S.; Wei, L.; Luo, D. Effect of cadmium on cellular ultrastructure in mouse ovary. Ultrastruct. Pathol. 2015, 39, 324–328. [Google Scholar] [CrossRef]
- Yang, D.; Ran, Y.; Li, X.; Jiang, X.; Chen, J.; Sun, J.; Tian, L.; Teerds, K.; Bai, W. Cyanidin-3-O-glucoside ameliorates cadmium induced uterine epithelium proliferation in mice. J. Hazard. Mater. 2022, 425, 127571. [Google Scholar] [CrossRef]
- Xu, G.; Liu, S.; Huang, M.; Jiang, X.; Yang, M. Cadmium induces apoptosis of human granulosa cell line KGN via mitochondrial dysfunction-mediated pathways. Ecotoxicol. Environ. Saf. 2021, 220, 112341. [Google Scholar] [CrossRef]
- Jiang, X.; Li, X.; Zhu, C.; Sun, J.; Tian, L.; Chen, W.; Bai, W. The target cells of anthocyanins in metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2019, 59, 921–946. [Google Scholar] [CrossRef]
- Li, X.; Jiang, X.; Sun, J.; Zhu, C.; Li, X.; Tian, L.; Liu, L.; Bai, W. Cytoprotective effects of dietary flavonoids against cadmium-induced toxicity. Ann. N. Y. Acad. Sci. 2017, 1398, 5–19. [Google Scholar] [CrossRef]
- Gong, P.; Chen, F.X.; Wang, L.; Wang, J.; Jin, S.; Ma, Y.M. Protective effects of blueberries (Vaccinium corymbosum L.) extract against cadmium-induced hepatotoxicity in mice. Environ. Toxicol. Pharmacol. 2014, 37, 1015–1027. [Google Scholar] [CrossRef]
- Ahmed, J.K.; Salih, H.A.; Hadi, A.G. Anthocyanins in Red Beet Juice Act as Scavengers for Heavy Metals Ions. Int. J. Sci. Technol. 2013, 2, 269–273. [Google Scholar]
- Tu, J.; Chen, Y.; Li, Z.; Yang, H.; Chen, H.; Yu, Z. Long non-coding RNAs in ovarian granulosa cells. J. Ovarian Res. 2020, 13, 63. [Google Scholar] [CrossRef]
- Nishi, Y.; Yanase, T.; Mu, Y.-M.; Oba, K.; Ichino, I.; Saito, M.; Nomura, M.; Mukasa, C.; Okabe, T.; Goto, K.; et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology 2001, 142, 437–445. [Google Scholar] [CrossRef]
- Hussain, T.; Murtaza, G.; Metwally, E.; Kalhoro, D.H.; Kalhoro, M.S.; Rahu, B.A.; Sahito, R.G.A.; Yin, Y.; Yang, H.; Chughtai, M.I.; et al. The role of oxidative stress and antioxidant balance in pregnancy. Mediat. Inflamm. 2021, 2021, 9962860. [Google Scholar] [CrossRef]
- Thevenod, F.; Lee, W.K. Cadmium and cellular signaling cascades: Interactions between cell death and survival pathways. Arch. Toxicol. 2013, 87, 1743–1786. [Google Scholar] [CrossRef]
- Goncalves, A.C.; Nunes, A.R.; Falcao, A.; Alves, G.; Silva, L.R. Dietary effects of anthocyanins in human health: A comprehensive review. Pharmaceuticals 2021, 14, 690. [Google Scholar] [CrossRef]
- Yi, L.; Chen, C.Y.; Jin, X.; Mi, M.T.; Yu, B.; Chang, H.; Ling, W.H.; Zhang, T. Structural requirements of anthocyanins in relation to inhibition of endothelial injury induced by oxidized low-density lipoprotein and correlation with radical scavenging activity. FEBS Lett. 2010, 584, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Jing, N.; Song, J.; Liu, Z.; Wang, L.; Jiang, G. Glycosylation of anthocyanins enhances the apoptosis of colon cancer cells by handicapping energy metabolism. BMC Complement. Med. Ther. 2020, 20, 312. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Miao, S.; Zhou, W.; Elnesr, S.S.; Dong, X.; Zou, X. MAPK, AKT/FoxO3a and mTOR pathways are involved in cadmium regulating the cell cycle, proliferation and apoptosis of chicken follicular granulosa cells. Ecotoxicol. Environ. Saf. 2021, 214, 112091. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhou, J.; Liu, W.; Tao, W.; He, J.; Jin, W.; Guo, H.; Yang, N.; Li, Y. The anti-inflammatory potential of protein-bound anthocyanin compounds from purple sweet potato in LPS-induced RAW264.7 macrophages. Food Res. Int. 2020, 137, 109647. [Google Scholar] [CrossRef]
- Wen, L.; Jiang, X.; Sun, J.; Li, X.; Li, X.; Tian, L.; Li, Y.; Bai, W. Cyanidin-3-O-glucoside promotes the biosynthesis of progesterone through the protection of mitochondrial function in Pb-exposed rat leydig cells. Food Chem. Toxicol. 2018, 112, 427–434. [Google Scholar] [CrossRef]
- Chao, J.I.; Yang, J.L. Opposite Roles of ERK and p38 mitogen-activated protein kinases in cadmium-induced genotoxicity and mitotic arrest. Chem. Res. Toxicol. 2001, 14, 1193–1202. [Google Scholar] [CrossRef]
- Xie, J.; Shaikh, Z.A. Cadmium induces cell cycle arrest in rat kidney epithelial cells in G2/M phase. Toxicology 2006, 224, 56–65. [Google Scholar] [CrossRef]
- Yang, P.M.; Chiu, S.J.; Lin, K.A.; Lin, L.Y. Effect of cadmium on cell cycle progression in Chinese hamster ovary cells. Chem.-Biol. Interact. 2004, 149, 125–136. [Google Scholar] [CrossRef]
- Wang, K.; Ma, J.Y.; Li, M.Y.; Qin, Y.S.; Bao, X.C.; Wang, C.C.; Cui, D.L.; Xiang, P.; Ma, L.Q. Mechanisms of Cd and Cu induced toxicity in human gastric epithelial cells: Oxidative stress, cell cycle arrest and apoptosis. Sci. Total Environ. 2021, 756, 143951. [Google Scholar] [CrossRef]
- Shimada, K.; Reznik, E.; Stokes, M.E.; Krishnamoorthy, L.; Bos, P.H.; Song, Y.; Quartararo, C.E.; Pagano, N.C.; Carpizo, D.R.; deCarvalho, A.C.; et al. Copper-binding small molecule induces oxidative stress and cell-cycle arrest in glioblastoma-patient-derived cells. Cell Chem. Biol. 2018, 25, 585–594. [Google Scholar] [CrossRef]
- Elisia, I.; Kitts, D.D. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol. Cell. Biochem. 2008, 312, 139–145. [Google Scholar] [CrossRef]
- Hwang, J.W.; Kim, E.K.; Lee, S.J.; Kim, Y.S.; Moon, S.H.; Jeon, B.T.; Sung, S.H.; Kim, E.T.; Park, P.J. Antioxidant activity and protective effect of anthocyanin oligomers on H2O2-triggered G2/M arrest in retinal cells. J. Agric. Food Chem. 2012, 60, 4282–4288. [Google Scholar] [CrossRef]
- Baker, M.E.; Lathe, R. The promiscuous estrogen receptor: Evolution of physiological estrogens and response to phytochemicals and endocrine disruptors. J. Steroid Biochem. Mol. Biol. 2018, 184, 29–37. [Google Scholar] [CrossRef]
- Nasiadek, M.; Danilewicz, M.; Sitarek, K.; Swiatkowska, E.; Darago, A.; Stragierowicz, J.; Kilanowicz, A. The effect of repeated cadmium oral exposure on the level of sex hormones, estrous cyclicity, and endometrium morphometry in female rats. Environ. Sci. Pollut. Res. 2018, 25, 28025–28038. [Google Scholar] [CrossRef]
- Ma, Y.; Zheng, L.; Wang, Y.; Gao, Y.; Xu, Y. Arachidonic acid in follicular fluid of PCOS induces oxidative stress in a human ovarian granulosa tumor cell line (KGN) and upregulates GDF15 expression as a response. Front. Endocrinol. 2022, 13, 865748. [Google Scholar] [CrossRef]
- Belani, M.; Shah, P.; Banker, M.; Gupta, S. Dual effect of insulin resistance and cadmium on human granulosa cells—In vitro study. Toxicol. Appl. Pharmacol. 2016, 313, 119–130. [Google Scholar] [CrossRef]
- Wu, X.; Guo, X.; Wang, H.; Zhou, S.; Li, L.; Chen, X.; Wang, G.; Liu, J.; Ge, H.S.; Ge, R.S. A brief exposure to cadmium impairs Leydig cell regeneration in the adult rat testis. Sci. Rep. 2017, 7, 6337. [Google Scholar] [CrossRef] [PubMed]
- Kemilainen, H.; Adam, M.; Maki-Jouppila, J.; Damdimopoulou, P.; Damdimopoulos, A.E.; Kere, J.; Hovatta, O.; Laajala, T.D.; Aittokallio, T.; Adamski, J.; et al. The hydroxysteroid (17β) dehydrogenase family gene HSD17B12 is involved in the prostaglandin synthesis pathway, the ovarian function, and regulation of fertility. Endocrinology 2016, 157, 3719–3730. [Google Scholar] [CrossRef]
- Hilborn, E.; Stål, O.; Jansson, A. Estrogen and androgen-converting enzymes 17β-hydroxysteroid dehydrogenase and their involvement in cancer: With a special focus on 17β-hydroxysteroid dehydrogenase type 1, 2, and breast cancer. Oncotarget 2017, 8, 30552. [Google Scholar] [CrossRef]
- Aryan, L.; Younessi, D.; Zargari, M.; Banerjee, S.; Agopian, J.; Rahman, S.; Borna, R.; Ruffenach, G.; Umar, S.; Eghbali, M. The role of estrogen receptors in cardiovascular disease. Int. J. Mol. Sci. 2020, 21, 4313. [Google Scholar] [CrossRef]
- Filardo, E.; Quinn, J.; Pang, Y.; Graeber, C.; Shaw, S.; Dong, J.; Thomas, P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 2007, 148, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Doi, A.; Ishikawa, K.; Shibata, N.; Ito, E.; Fujimoto, J.; Yamamoto, M.; Shiga, H.; Mochizuki, H.; Kawamura, Y.; Goshima, N.; et al. Enhanced expression of retinoic acid receptor alpha (RARA) induces epithelial-to-mesenchymal transition and disruption of mammary acinar structures. Mol. Oncol. 2015, 9, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Buffa, L.; Saeed, A.M.; Nawaz, Z. Molecular mechanism of WW-domain binding protein-2 coactivation function in estrogen receptor signaling. IUBMB Life 2013, 65, 76–84. [Google Scholar] [CrossRef]




| Gene Name | Primer Sequence (5′→3′) | |
|---|---|---|
| Hsd3b | Forward | CACATGGCCCGCTCCATAC |
| Reverse | GTGCCGCCGTTTTTCAGATTC | |
| Hsd17b1 | Forward | ACGTGAATGTAGTAGGGACTGT |
| Reverse | GCGCAATAAACGTCATTGAAAGG | |
| Hsd17b7 | Forward | ATCTGGACATCATCTCGCAGT |
| Reverse | AAGAGCTGTAGGGTTCCTTGC | |
| Hsd17b12 | Forward | TGTCCCACTCTTGACCATCTAT |
| Reverse | CTTGCTCCTATACTCCTCATGGA | |
| Vps18 | Forward | ACACTGCTCCGCATTGACTT |
| Reverse | TTTTGCGTCATCCTTACGTCC | |
| Vps11 | Forward | CAATCCACTCTGCACTCGAAT |
| Reverse | CGGGTGATGTCTCCTTTGTTCA | |
| Cnot9 | Forward | CACTGGCACAAGTGGATAGAG |
| Reverse | GCTTCTTACTTAGCTCCAGCAAA | |
| Wbp2 | Forward | GCGGAGTGATCGTCAATAACAT |
| Reverse | GACCCGGTAAGGGGTAAGGT | |
| Tiparp | Forward | AATTTGACCAACTACGAAGGCTG |
| Reverse | CAGACTCGGGATACTCTCTCC | |
| Rara | Forward | GGGCAAATACACTACGAACAACA |
| Reverse | CTCCACAGTCTTAATGATGCACT | |
| Il4r | Forward | ACACCAATGTCTCCGACACTC |
| Reverse | TGTTGACTGCATAGGTGAGATGA | |
| Gba | Forward | GCAGGGCTAACCTAGTGCCT |
| Reverse | GCTTGGGACATTCCTCTCTGG | |
| Kat5 | Forward | AACAAACGTCTGGATGAATGGG |
| Reverse | AGGAAGTCCGTTCTTAGTGGG | |
| Uba5 | Forward | GTTGGTGGAGTAGGTAGTGTGA |
| Reverse | GTTCCTCAGAGTATGTTCTGCTG | |
| Gper1 | Forward | TCACGGGCCACATTGTCAAC |
| Reverse | GTCTCCCCGAGAAAGCTGTAG | |
| Stxbp1 | Forward | AAAGCTGTTGTCGGAGAGAAG |
| Reverse | CACAATCGTTATGCCCTCGG | |
| Gapdh | Forward | GTCGGAGTCAACGGATTTGG |
| Reverse | GGGTGGAATCAATTGGAACAT | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Li, X.; Liu, R.; Hu, J.; Li, Y.; Sun, J.; Bai, W. Malvidin-3-O-Glucoside Ameliorates Cadmium-Mediated Cell Dysfunction in the Estradiol Generation of Human Granulosa Cells. Nutrients 2023, 15, 753. https://doi.org/10.3390/nu15030753
Liang S, Li X, Liu R, Hu J, Li Y, Sun J, Bai W. Malvidin-3-O-Glucoside Ameliorates Cadmium-Mediated Cell Dysfunction in the Estradiol Generation of Human Granulosa Cells. Nutrients. 2023; 15(3):753. https://doi.org/10.3390/nu15030753
Chicago/Turabian StyleLiang, Shuer, Xusheng Li, Ruijing Liu, Jun Hu, Yue Li, Jianxia Sun, and Weibin Bai. 2023. "Malvidin-3-O-Glucoside Ameliorates Cadmium-Mediated Cell Dysfunction in the Estradiol Generation of Human Granulosa Cells" Nutrients 15, no. 3: 753. https://doi.org/10.3390/nu15030753
APA StyleLiang, S., Li, X., Liu, R., Hu, J., Li, Y., Sun, J., & Bai, W. (2023). Malvidin-3-O-Glucoside Ameliorates Cadmium-Mediated Cell Dysfunction in the Estradiol Generation of Human Granulosa Cells. Nutrients, 15(3), 753. https://doi.org/10.3390/nu15030753