Altered Expression of Antimicrobial Peptides in the Upper Gastrointestinal Tract of Patients with Diabetes Mellitus
Abstract
1. Introduction
2. Patients and Methods
- Group 1: Patients with inadequately controlled manifest diabetes mellitus (pathological fasting blood glucose levels > 126 mg% and HbA1c > 7.5%) or pathological OGTT (whole blood glucose capillary ≥ 200 mg%).
- Group 2: Patients with insulin resistance (HOMA index > 2.5 and euglycaemic metabolic state, HbA1c value < 6.5%).
- Group 3: Metabolic healthy subjects as controls (fasting blood glucose levels < 100 mg%, HbA1c < 5.8%, HOMA index ≤ 1).
3. Results
3.1. Patient Cohort
3.2. RNA Isolation and Gel Electrophoresis
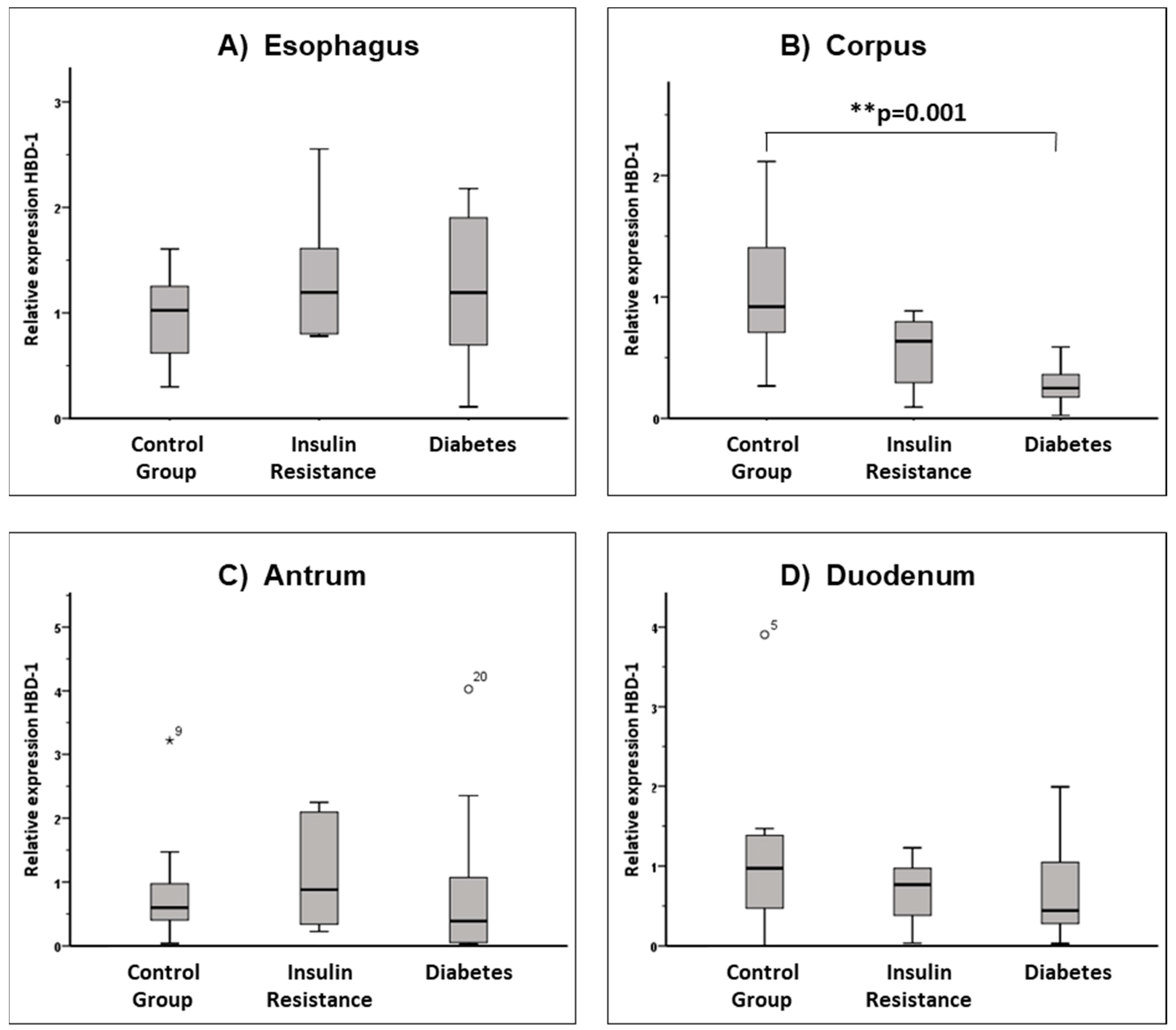
3.3. AMP Expression in the Upper Gastrointestinal Tract
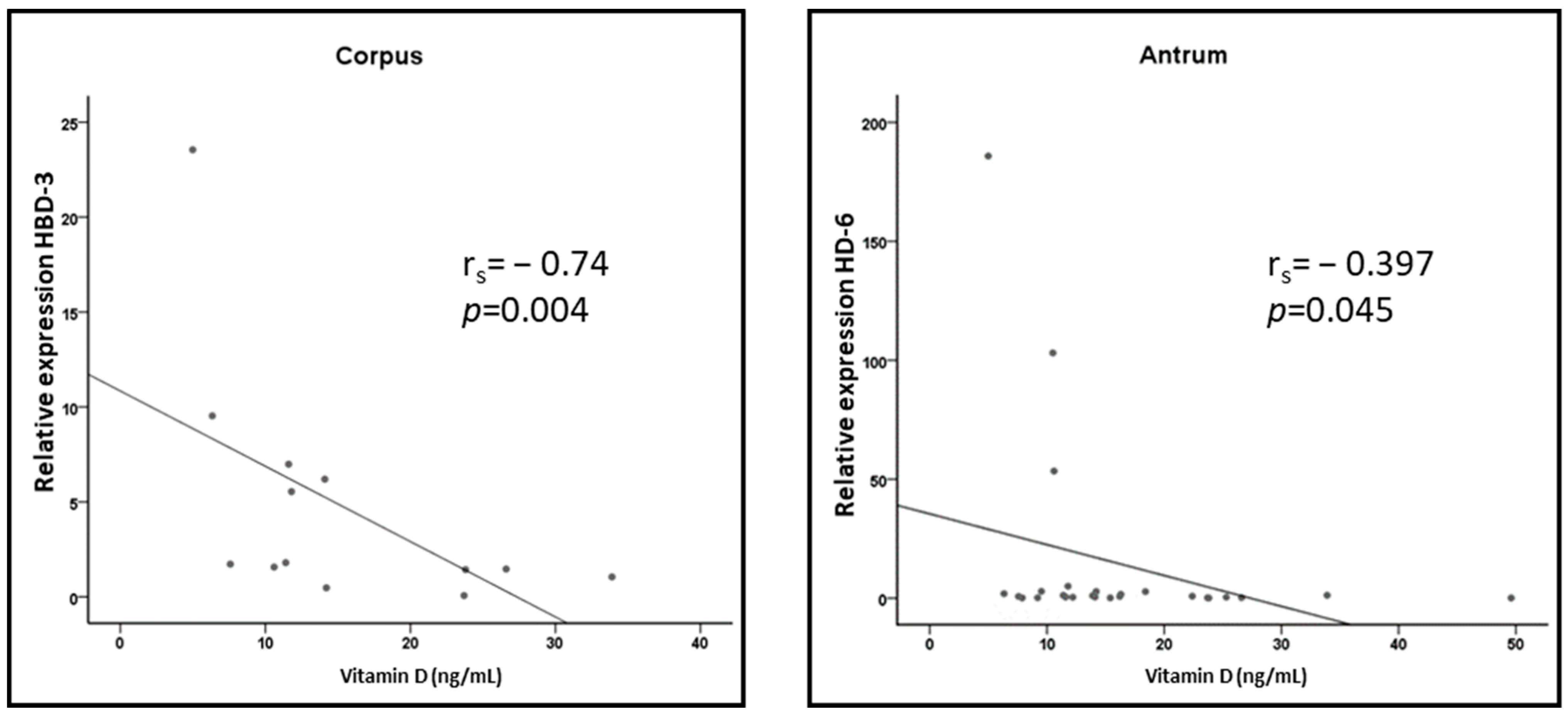
3.4. Correlation of Vitamin D Levels with AMP Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, K.; Raffatellu, M.G.I. Pros: Antimicrobial Defense in the Gastrointestinal Tract. Semin. Cell Dev. Biol. 2019, 88, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Hosaka, Y.; Koslowski, M.; Nuding, S.; Wang, G.; Schlee, M.; Schäfer, C.; Saigenji, K.; Stange, E.F.; Wehkamp, J. Antimicrobial host defense in the upper gastrointestinal tract. Eur. J. Gastroenterol. Hepatol. 2008, 20, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Fruitwala, S.; El-Naccache, D.W.; Chang, T.L. Multifaceted immune functions of human defensins and underlying mechanisms. Semin. Cell Dev. Biol. 2019, 88, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Solanki, S.S.; Singh, P.; Kashyap, P.; Sansi, M.S.; Ali, S.A. Promising role of defensins peptides as therapeutics to combat against viral infection. Microb. Pathog. 2021, 155, 104930. [Google Scholar] [CrossRef]
- Tonk, M.; Růžek, D.; Vilcinskas, A. Compelling Evidence for the Activity of Antiviral Peptides against SARS-CoV-2. Viruses 2021, 13, 912. [Google Scholar] [CrossRef]
- Pachón-Ibáñez, M.E.; Smani, Y.; Pachón, J.; Sánchez-Céspedes, J. Perspectives for clinical use of engineered human host defense antimicrobial peptides. FEMS Microbiol. Rev. 2017, 4, 323–342. [Google Scholar] [CrossRef]
- Boparai, J.K.; Sharma, P.K. Mini Review on Antimicrobial Peptides, Sources, Mechanism and Recent Applications. Protein Pept. Lett. 2020, 27, 4–16. [Google Scholar] [CrossRef]
- O’Neil, D.A.; Porter, E.M.; Elewaut, D.; Anderson, G.M.; Eckmann, L.; Ganz, T.; Kagnoff, M.F. Expression and regulation of the human β-defensins hBD-1 and hBD-2 in intestinal epithelium. J. Immunol. 1999, 163, 6718–6724. [Google Scholar] [CrossRef]
- Frye, M.; Bargon, J.; Lembcke, B.; Wagner, F.F.; Gropp, R. Differential expression of human α- and β-defensins mRNA in gastrointestinal epithelia. Eur. J. Clin. Investig. 2000, 30, 695–701. [Google Scholar] [CrossRef]
- Cunliffe, N.R.; Mahida, R.Y. Expression and regulation of antimicrobial peptides in the gastrointestinal tract. J. Leukoc. Biol. 2004, 75, 49–58. [Google Scholar] [CrossRef]
- Dommett, R.; Zilbauer, M.; George, J.T.; Bajaj-Elliott, M. Innate immune defence in the human gastrointestinal tract. Mol. Immunol. 2005, 42, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Kiehne, K.; Brunke, G.; Meyer, D.; Harder, J.; Herzig, K.-H. Oesophageal defensin expression during Candida infection and reflux disease. Scand. J. Gastroenterol. 2005, 40, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J.A. The Host Defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef]
- Imler, J.L.; Hoffmann, J.A. Toll receptors in innate immunity. Trends Cell Bio. 2001, 11, 304–311. [Google Scholar] [CrossRef]
- Takeda, K.; Akira, S. Roles of Toll-like receptors in innate immune responses. Genes Cells 2001, 6, 733–742. [Google Scholar] [CrossRef]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Ann. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Becker, T. Crossregulation of Insulin Signalling and Innate Immunity. Doctoral Dissertation, Rheinische Friedrich-Wilhelms-Universität, Bonn, Germany, 2009. [Google Scholar]
- Becker, T.; Loch, G.; Beyer, M.; Zinke, I.; Aschenbrenner, A.C.; Carrera, P.; Inhester, T.; Schultze, J.L.; Hoch, M. FOXO-dependent regulation of innate immune homeostasis. Nature 2010, 463, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Froy, O.; Hananel, A.; Chapnik, N.; Madar, Z. Differential effect of insulin treatment on decreased levels of beta-defensins and Toll-like receptors in diabetic rats. Mol. Immunol. 2007, 44, 796–802. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting Edge: 1,25-Dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol. Nutr. Food Res. 2011, 55, 96–108. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and abuse of HOMA modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using Real Time Quantitative PCR and the 2−ΔΔCT-method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.S.; Wang, P.-C.; Chen, J.-H.; Su, W.-C.; Tseng, T.-C.; Chen, H.-D.; Hsiao, T.-H.; Wang, C.-C.; Chao, Y.-C.; Lin, H.-H.; et al. Increasing insulin resistance is associated with increased severity and prevalence of gastroesophageal reflux disease. Aliment. Pharmacol. Ther. 2011, 34, 994–1004. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.H.; Lee, Y.-C.; Chiu, H.-M.; Chen, C.-C.; Liao, W.-C.; Tu, C.-H.; Yang, W.-S.; Wu, M.-S. Association of diabetes and HbA1c levels with gastrointestinal manifestations. Diabetes Care 2012, 35, 1053–1060. [Google Scholar] [CrossRef]
- Wehkamp, J.; Schmidt, K.; Herrlinger, K.R.; Baxmann, S.; Behling, S.; Wohlschläger, C.; Feller, A.C.; Stange, E.F.; Fellermann, K. Defensin pattern in chronic gastritis: HBD-2 is differentially expressed with respect to Helicobacter pylori status. J. Clin. Pathol. 2003, 56, 352–357. [Google Scholar] [CrossRef]
- Otte, J.M.; Neumann, H.M.; Brand, S.; Schrader, H.; Schmidt, W.E.; Schmitz, F. Expression of beta-defensin 4 is increased in human gastritis. Eur. J. Clin. Investig. 2009, 39, 126–138. [Google Scholar] [CrossRef]
- Kocsis, A.K.; Kiss, Z.; Tiszlavicz, L.; Tiszlavicz, Z.; Mandi, Y. Potential role of human β-defensin 1 in Helicobacter pylori-induced gastritis. Scand. J. Gastroenterol. 2009, 44, 289–295. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, I.; Lehrer, R.I. Widespread expression of beta-defensin hBD-1 in human secretory glands and epithelial cells. FEBS Lett. 1996, 396, 319–322. [Google Scholar] [CrossRef]
- Patel, S.R.; Smith, K.; Letley, D.P.; Cook, K.W.; Memon, A.A.; Ingram, R.J.M.; Staples, E.; Backerts, S.; Zaitoun, A.M.; Robinson, K.; et al. Helicobacter pylori downregulates expression of human β-defensin 1 in the gastric mucosa in a type IV secretion-dependent fashion. Cell. Microbiol. 2013, 15, 2080–2092. [Google Scholar] [CrossRef]
- Németh, B.C.; Várkonyi, T.; Somogyvári, F.; Lengyel, C.; Fehértemplomi, K.; Nyiraty, S.; Kempler, P.; Mándi, Y. Relevance of α-defensins (HNP1-3) and defensin β-1 in diabetes. World J. Gastroenterol. 2014, 20, 9128–9137. [Google Scholar] [CrossRef]
- Hiratsuka, T.; Nakazato, M.; Date, Y.; Mukae, H.; Matsukura, S. Nucleotide sequence and expression of rat β-Defensin-1: Its significance in diabetic rodent models. Nephron 2001, 88, 65–70. [Google Scholar] [CrossRef]
- Kawashima, R.; Shimizu, T.; To, M.; Saruta, J.; Jinbu, Y.; Kusama, M.; Tsukinoki, K. Effects of stress on mouse-β-defensin-3 expression in the upper digestive mucosa. Yonsei Med. J. 2014, 55, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Rachel, R.A.; Malik, A.N. Elevated levels of beta defensin-1 mRNA in diabetic kidneys of GK rats. Biochem. Biophys. Res. Commun. 2003, 310, 513–521. [Google Scholar]
- Barnea, M.; Madar, Z.; Froy, O. Glucose and insulin are needed for optimal defensin expression in human cell lines. Biochem. Biophys. Res. Commun. 2008, 367, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.N.; Al-Kafaji, G. Glucose regulation of beta-defensin-1 mRNA in human renal cells. Biochem. Biophys. Res. Commun. 2007, 353, 318–323. [Google Scholar] [CrossRef]
- Saraheimo, M.; Forsblom, C.; Pettersson-Fernholm, K.; Flyvbjerg, A.; Groop, P.-H.; Frystyk, J. Increased levels of α-defensin (-1, -2 and -3) in type 1 diabetic patients with nephropathy. Nephrol. Dial. Transplant. 2008, 23, 914–918. [Google Scholar] [CrossRef]
- Yilmaz, D.; Topcu, A.O.; Akcay, E.U.; Altındis, M.; Gursoy, U.K. Salivary human beta-defensins and cathelicidin levels in relation to periodontitis and type 2 diabetes mellitus. Acta Odontol. Scand. 2020, 78, 327–331. [Google Scholar] [CrossRef]
- Yilmaz, D.; Yilmaz, N.; Polat, R.; Nissilä, V.; Aydın, E.G.; Rautava, J.; Gürsoy, M.; Gürsoy, U.K. Salivary levels of hBDs in children and adolescents with type 1 diabetes mellitus and gingivitis. Clin. Oral Investig. 2022, 26, 4897–4904. [Google Scholar] [CrossRef]
- Mohanty, S.; Kamolvit, W.; Scheffschick, A.; Björklund, A.; Tovi, J.; Espinosa, A.; Brismar, K.; Nyström, T.; Schröder, J.M.; Östenson, C.-G.; et al. Diabetes downregulates the antimicrobial peptide psoriasin and increases E. coli burden in the urinary bladder. Nat. Commun. 2022, 20, 4983. [Google Scholar] [CrossRef]
- Lemaitre, B.; Reichhart, J.M.; Hoffmann, J.A. Drosophila host defense: Differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 1997, 94, 14614–14619. [Google Scholar] [CrossRef]
- Gross, D.N.; van den Heuvel, A.P.; Birnbaum, M.J. The role of FoxO in the regulation of metabolism. Oncogene 2008, 27, 2320–2336. [Google Scholar] [CrossRef] [PubMed]
- Arden, K.C. FOXO animal models reveal a variety of diverse roles for FOXO transcription factors. Oncogene 2008, 27, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- Teleman, A.A. Molecular mechanisms of metabolic regulation by insulin in Drosophila. Biochem. J. 2010, 425, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Seiler, F.; Hellberg, J.; Lepper, P.M.; Kamyschnikow, A.; Herr, C.; Bischoff, M.; Langer, F.; Schäfers, H.-J.; Lammert, F.; Menger, M.D.; et al. FOXO transcription factors regulate innate immune mechanisms in respiratory epithelial cells. J. Immunol. 2013, 190, 1603–1613. [Google Scholar] [CrossRef]
- White, J.H. Emerging Roles of Vitamin D-Induced Antimicrobial Peptides in Antiviral Innate Immunity. Nutrients 2022, 14, 284. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Park, M.S.; Kim, J.I.; Lee, I.; Park, S.; Bae, J.Y.; Park, M.S. Towards the Application of Human Defensins as Antivirals. Biomol. Ther. 2018, 26, 242–254. [Google Scholar] [CrossRef]
| Target Gene | Synonym | Assay Identification Number |
|---|---|---|
| GAPDH | GAPDH | Hs 02758991_g1 |
| HBD1 | DEFB 1 | Hs 00608345_m1 |
| HBD2 | DEFB 4 | Hs 00175474_m1 |
| HBD3 | DEFB 103 | Hs 00218678_m1 |
| HBD4 | DEFB 104 | Hs 00414476_m1 |
| HD5 | DEFA 5 | Hs 00360716_m1 |
| HD6 | DEFA 6 | Hs 00427001_m1 |
| LL-37 | CAMP | Hs 00189038_m1 |
| Control Group | Patients with Insulin Resistance | Patients with Diabetes | Total | |
|---|---|---|---|---|
| Number | 11 | 10 | 10 | 31 |
| Age (years) | 58 ± 16 | 68 ± 10 | 62 ± 14 | 63 ± 14 |
| Gender (male/female) | 3/8 | 3/7 | 4/6 | 10/21 |
| Weight (kg) | 65.64 ± 8.66 | 95.90 ± 15.77 ** | 85.90 ± 24.18 * | 81.94 ± 21.04 |
| Height (m) | 1.69 ± 0.09 | 1.65 ± 0.05 | 1.64 ± 0.14 | 1.66 ± 0.1 |
| BMI (kg/m2) | 23.03 ± 2.96 | 34.92 ± 5.03 ** | 31.69 ± 5.69 ** | 29.66 ± 6.85 |
| Pre-existing diabetes | 0 | 1 | 8 | 9 |
| Antidiabetic treatment (OAD/insulin/both) | 0/0/0 | 0/0/0 | 6/2/10 | 6/2/10 |
| Vitamin D supplementation | 0 | 1 | 0 | 1 |
| PPI therapy | 4 | 7 | 5 | 16 |
| NSAID therapy | 2 | 4 | 2 | 8 |
| Fasting glucose (mg%) | 91.73 ± 11.33 | 111.50 ± 13.88 | 126.40 ± 10.43 ** | 106.00 ± 17.98 |
| Capillary glucose (mg%) | 98.82 ± 7.82 | 118.67 ± 15.22 | 169.11 ± 81.66 ** | 126.79 ± 53.82 |
| HbA1c (%) | 5.60 ± 0.29 | 5.97 ± 0.44 | 8.82 ± 2.53 ** | 6.76 ± 2.03 |
| Insulin (ng/L) | 3.13 ± 1.53 | 20.51 ± 14.33 ** | 12.42 ± 9.62 | 11.56 ± 12.48 |
| HOMA index | 1.05 ± 0.75 | 5.88 ± 4.93 * | 3.77 ± 2.82 | 3.42 ± 3.93 |
| Vitamin D (ng/mL) | 16.9 ± 8.3 | 16.0 ± 7.8 | 17.2 ± 12.8 | 16.7 ± 9.5 |
| Normal Mucosa (EGD) | 6 | 2 | 1 | 9 |
| Esophagitis | 2 | 7 * | 7 * | 16 |
| Corpus gastritis | 1 | 1 | 4 | 6 |
| Antrum gastritis | 0 | 1 | 2 | 3 |
| Duodenitis | 2 | 0 | 0 | 2 |
| Helicobacter pylori positive | 0 | 0 | 4 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linn, O.; Menges, B.; Lammert, F.; Weber, S.N.; Krawczyk, M. Altered Expression of Antimicrobial Peptides in the Upper Gastrointestinal Tract of Patients with Diabetes Mellitus. Nutrients 2023, 15, 754. https://doi.org/10.3390/nu15030754
Linn O, Menges B, Lammert F, Weber SN, Krawczyk M. Altered Expression of Antimicrobial Peptides in the Upper Gastrointestinal Tract of Patients with Diabetes Mellitus. Nutrients. 2023; 15(3):754. https://doi.org/10.3390/nu15030754
Chicago/Turabian StyleLinn, Oliver, Bernhard Menges, Frank Lammert, Susanne N. Weber, and Marcin Krawczyk. 2023. "Altered Expression of Antimicrobial Peptides in the Upper Gastrointestinal Tract of Patients with Diabetes Mellitus" Nutrients 15, no. 3: 754. https://doi.org/10.3390/nu15030754
APA StyleLinn, O., Menges, B., Lammert, F., Weber, S. N., & Krawczyk, M. (2023). Altered Expression of Antimicrobial Peptides in the Upper Gastrointestinal Tract of Patients with Diabetes Mellitus. Nutrients, 15(3), 754. https://doi.org/10.3390/nu15030754






