Dietary Effects on Pain Symptoms in Patients with Fibromyalgia Syndrome: Systematic Review and Future Directions
Abstract
1. Introduction
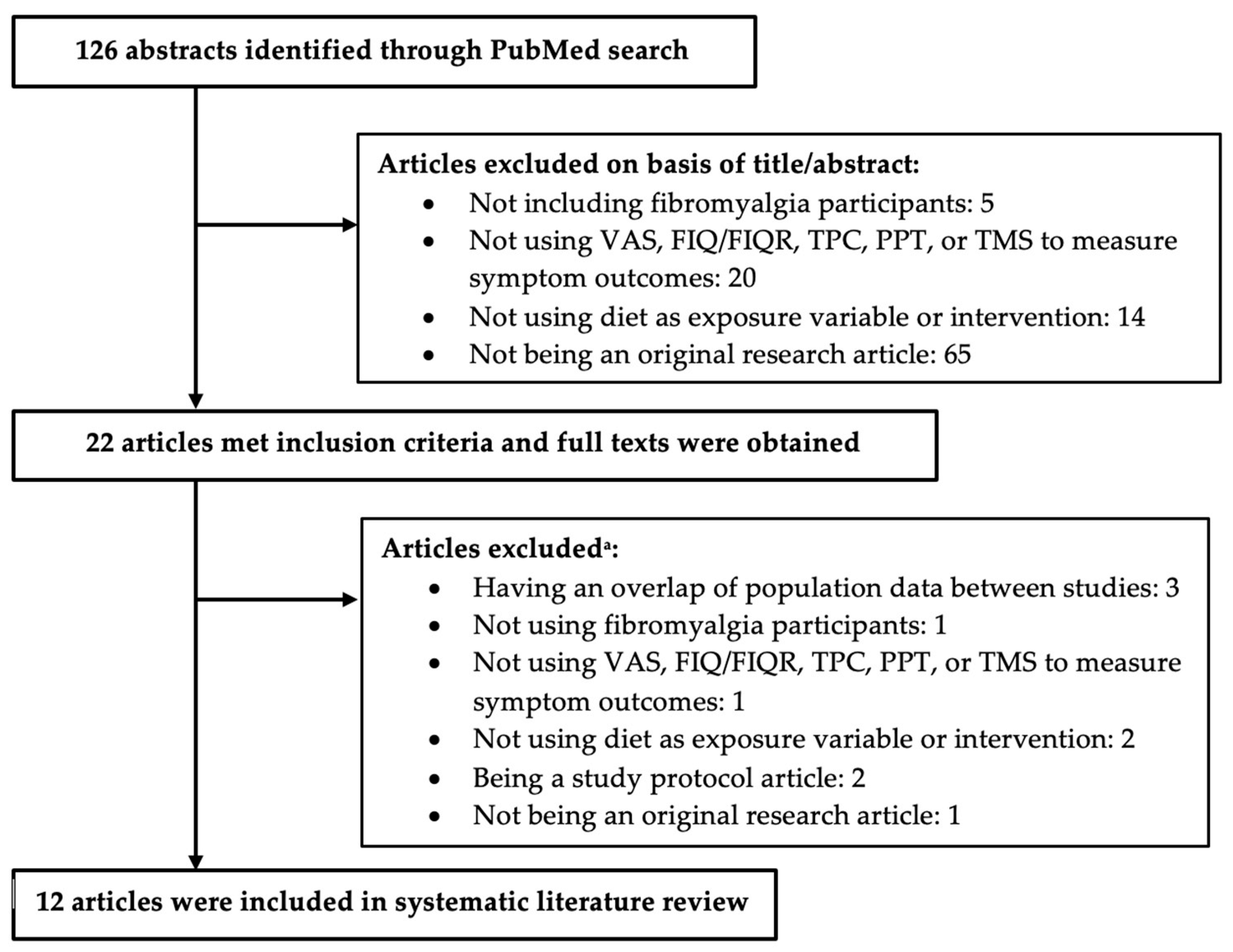
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.D.; Albenberg, L.; Lee, D.; Kratz, M.; Gottlieb, K.; Reinisch, W. The Importance and Challenges of Dietary Intervention Trials for Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2017, 23, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Clem, J.; Barthel, B. A Look at Plant-Based Diets. Missouri Med. 2021, 118, 233–238. [Google Scholar] [PubMed]
- Haefeli, M.; Elfering, A. Pain assessment. Eur. Spine J. 2006, 15 (Suppl. 1), S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, F.; Smythe, H.A.; Yunus, M.B.; Bennett, R.M.; Bombardier, C.; Goldenberg, D.L.; Tugwell, P.; Campbell, S.M.; Abeles, M.; Clark, P.; et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990, 33, 160–172. [Google Scholar] [CrossRef] [PubMed]
- National Heart Lung and Blood Institute. Study Quality Assessment Tools. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 5 July 2022).
- Kaartinen, K.; Lammi, K.; Hypen, M.; Nenonen, M.; Hanninen, O.; Rauma, A.L. Vegan diet alleviates fibromyalgia symptoms. Scand. J. Rheumatol. 2000, 29, 308–313. [Google Scholar] [CrossRef]
- Marum, A.P.; Moreira, C.; Tomas-Carus, P.; Saraiva, F.; Guerreiro, C.S. A low fermentable oligo-di-mono-saccharides and polyols (FODMAP) diet is a balanced therapy for fibromyalgia with nutritional and symptomatic benefits. Nutr. Hosp. 2017, 34, 667–674. [Google Scholar] [CrossRef]
- Rodrigo, L.; Blanco, I.; Bobes, J.; de Serres, F.J. Effect of one year of a gluten-free diet on the clinical evolution of irritable bowel syndrome plus fibromyalgia in patients with associated lymphocytic enteritis: A case-control study. Arthritis Res. Ther. 2014, 16, 421. [Google Scholar] [CrossRef] [PubMed]
- Schrepf, A.; Harte, S.E.; Miller, N.; Fowler, C.; Nay, C.; Williams, D.A.; Clauw, D.J.; Rothberg, A. Improvement in the Spatial Distribution of Pain, Somatic Symptoms, and Depression After a Weight Loss Intervention. J. Pain 2017, 18, 1542–1550. [Google Scholar] [CrossRef] [PubMed]
- Isasi, C.; Colmenero, I.; Casco, F.; Tejerina, E.; Fernandez, N.; Serrano-Vela, J.I.; Castro, M.J.; Villa, L.F. Fibromyalgia and non-celiac gluten sensitivity: A description with remission of fibromyalgia. Rheumatol. Int. 2014, 34, 1607–1612. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, L.B.; Mikkelsen, K.; Haugen, M.; Pripp, A.H.; Fields, J.Z.; Forre, O.T. Treatment of fibromyalgia at the Maharishi Ayurveda Health Centre in Norway II--a 24-month follow-up pilot study. Clin. Rheumatol. 2012, 31, 821–827. [Google Scholar] [CrossRef]
- Rasmussen, L.B.; Mikkelsen, K.; Haugen, M.; Pripp, A.H.; Forre, O.T. Treatment of fibromyalgia at the Maharishi Ayurveda Health Centre in Norway. A six-month follow-up study. Clin. Exp. Rheumatol. 2009, 27, S46–S50. [Google Scholar]
- Bennett, R.M. A raw vegetarian diet for patients with fibromyalgia. Curr. Rheumatol. Rep. 2002, 4, 284. [Google Scholar] [CrossRef] [PubMed]
- Correa-Rodriguez, M.; Casas-Barragan, A.; Gonzalez-Jimenez, E.; Schmidt-RioValle, J.; Molina, F.; Aguilar-Ferrandiz, M.E. Dietary Inflammatory Index Scores Are Associated with Pressure Pain Hypersensitivity in Women with Fibromyalgia. Pain Med. 2020, 21, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, L.; Blanco, I.; Bobes, J.; de Serres, F.J. Clinical impact of a gluten-free diet on health-related quality of life in seven fibromyalgia syndrome patients with associated celiac disease. BMC Gastroenterol. 2013, 13, 157. [Google Scholar] [CrossRef]
- Lamb, J.J.; Konda, V.R.; Quig, D.W.; Desai, A.; Minich, D.M.; Bouillon, L.; Chang, J.L.; Hsi, A.; Lerman, R.H.; Kornberg, J.; et al. A program consisting of a phytonutrient-rich medical food and an elimination diet ameliorated fibromyalgia symptoms and promoted toxic-element detoxification in a pilot trial. Altern. Ther. Health Med. 2011, 17, 36–44. [Google Scholar] [PubMed]
- Marum, A.P.; Moreira, C.; Saraiva, F.; Tomas-Carus, P.; Sousa-Guerreiro, C. A low fermentable oligo-di-mono saccharides and polyols (FODMAP) diet reduced pain and improved daily life in fibromyalgia patients. Scand. J. Pain 2016, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Vellisca, M.Y.; Latorre, J.I. Monosodium glutamate and aspartame in perceived pain in fibromyalgia. Rheumatol. Int. 2014, 34, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Azad, K.A.; Alam, M.N.; Haq, S.A.; Nahar, S.; Chowdhury, M.A.; Ali, S.M.; Ullah, A.K. Vegetarian diet in the treatment of fibromyalgia. Bangladesh Med. Res. Counc. Bull. 2000, 26, 41–47. [Google Scholar]
- Donaldson, M.S.; Speight, N.; Loomis, S. Fibromyalgia syndrome improved using a mostly raw vegetarian diet: An observational study. BMC Complement Altern. Med. 2001, 1, 7. [Google Scholar] [CrossRef]
- Holton, K.F.; Taren, D.L.; Thomson, C.A.; Bennett, R.M.; Jones, K.D. The effect of dietary glutamate on fibromyalgia and irritable bowel symptoms. Clin. Exp. Rheumatol. 2012, 30, 10–17. [Google Scholar] [PubMed]
- Slim, M.; Calandre, E.P.; Garcia-Leiva, J.M.; Rico-Villademoros, F.; Molina-Barea, R.; Rodriguez-Lopez, C.M.; Morillas-Arques, P. The Effects of a Gluten-free Diet Versus a Hypocaloric Diet Among Patients with Fibromyalgia Experiencing Gluten Sensitivity-like Symptoms: A Pilot, Open-Label Randomized Clinical Trial. J. Clin. Gastroenterol. 2017, 51, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Pagliai, G.; Colombini, B.; Dinu, M.; Whittaker, A.; Masoni, A.; Danza, G.; Amedei, A.; Ballerini, G.; Benedettelli, S.; Sofi, F. Effectiveness of a Khorasan Wheat-Based Replacement on Pain Symptoms and Quality of Life in Patients with Fibromyalgia. Pain Med. 2020, 21, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Senna, M.K.; Sallam, R.A.; Ashour, H.S.; Elarman, M. Effect of weight reduction on the quality of life in obese patients with fibromyalgia syndrome: A randomized controlled trial. Clin. Rheumatol. 2012, 31, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Hanninen, O.; Kaartinen, K.; Rauma, A.L.; Nenonen, M.; Torronen, R.; Hakkinen, A.S.; Adlercreutz, H.; Laakso, J. Antioxidants in vegan diet and rheumatic disorders. Toxicology 2000, 155, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, B.F.; Okyay, R.A. The relationship between body mass index and pain, disease activity, depression and anxiety in women with fibromyalgia. PeerJ 2018, 6, e4917. [Google Scholar] [CrossRef] [PubMed]
- Spencer, E.A.; Appleby, P.N.; Davey, G.K.; Key, T.J. Diet and body mass index in 38000 EPIC-Oxford meat-eaters, fish-eaters, vegetarians and vegans. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 728–734. [Google Scholar] [CrossRef]
- Barone, M.; Della Valle, N.; Rosania, R.; Facciorusso, A.; Trotta, A.; Cantatore, F.P.; Falco, S.; Pignatiello, S.; Viggiani, M.T.; Amoruso, A.; et al. A comparison of the nutritional status between adult celiac patients on a long-term, strictly gluten-free diet and healthy subjects. Eur. J. Clin. Nutr. 2016, 70, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Field, R.; Field, T.; Pourkazemi, F.; Rooney, K. Low-carbohydrate and ketogenic diets: A scoping review of neurological and inflammatory outcomes in human studies and their relevance to chronic pain. Nutr. Res. Rev. 2022. [Google Scholar] [CrossRef]
| First Author, Publication Year | Study Design | Year(s) | Country | Number of Participants | Sex a | Age b | Race or Ethnicity | Diagnosis c |
|---|---|---|---|---|---|---|---|---|
| Azad 2000 [21] | Intervention Control Trial | No data | Bangladesh | 78 | Female 78% Male 22% | 30.9 (12–60) | No data | FMS |
| Correa-Rodríguez 2020 [16] | Observational, Cross-sectional | 2018 | Spain | 95 | Female 100% | 55.76 | No data | FMS |
| Donaldson 2001 [22] | Intervention Pre and Post Trial | No data | United States | 20 | Female 93% Male 7% | (45–54) | No data | FMS |
| Hänninen 2000 [27] | Intervention Pre and Post Control Trial | No data | Finland | 33 | No data | 51 | No data | FMS |
| Holton 2012 [23] | Intervention Pre and Post Trial | No data | United States | 37 | Female 92% Male 8% | 51.6 | No data | FMS and IBS |
| Lamb 2011 [18] | Intervention Cross-over Trial | 2008 | United States | 8 | Female 100% | 55.6 (48–74) | White | FMS |
| Marum 2016 [19] | Intervention Pre and Post Trial | 2015 | Portugal | 38 | Female 100% | 51 | No data | FMS and GID |
| Pagliai 2020 [25] | Intervention Cross-over Trial | No data | Italy | 20 | Female 95% Male 5% | 48.95 | No data | FMS |
| Rodrigo 2013 [17] | Intervention Pre and Post Trial | 2007–2012 | Spain | 7 | Female 100% | 49 (34–68) | White | FMS, IBS and CD |
| Senna 2012 [26] | Intervention Control Trial | 2011 | Egypt | 83 | Female 90% Male 10% | 45.6 | No data | FMS |
| Slim 2017 [24] | Intervention Parallel-group Trial | 2012–2014 | Spain | 55 | Female 97% Male 3% | HCD: 53 (32–65) GFD: 52 (36–66) | No data | FMS and GS |
| Vellisca 2014 [20] | Intervention Control Trial | No data | Spain | 72 | Female 100% | 40.98 (24–65) | No data | FMS |
| First Author of Article | Dietary Variable or Intervention | Length of Intervention | FIQ/FIQR c | VAS d | |||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | End | ||||||||
| Azad | Vegetarian | 6 weeks | 5.7 ± 1.8 | 5.0 ± 1.8 * | |||||
| Control | 6 weeks | 6.2 ± 1.9 | 2.3 ± 1.3 * | ||||||
| Cross-sectional | Cross-sectional | ||||||||
| Correa-Rodríguez a | Anti-Inflammatory diet: DII score Quartile 1 | N/A | 70.5 ± 13.3 | 7.20 ± 1.64 | |||||
| Quartile 2 | 79.9 ± 10.5 | 7.91 ± 1.57 | |||||||
| Quartile 3 | 69.2 ± 20.1 | 7.48 ± 2.10 | |||||||
| Quartile 4 | 71.9 ± 15.4 | 7.40 ± 1.14 | |||||||
| Baseline | 2 months | End | Baseline | 2 months | End | ||||
| Donaldson | Raw Vegetarian | 7 months | 51.4 ± 14.2 | 33.6 ± 15.6 | 27.6 ± 19.0 * | ||||
| Baseline | Middle | End | |||||||
| Hänninen b | Living Food | 3 months | 6.0 | 3.0 | 3.2 * | ||||
| Control | 3 months | 5.8 | 4.8 | 6.5 | |||||
| Baseline | End | Baseline | End | ||||||
| Holton | Dietary Additive Excitotoxin Elimination | 4 weeks | 58.6 | 36.4 * | 13.1 | 7.7 * | |||
| Baseline | End | ||||||||
| Lamb | Control | 4 weeks | 46.3 ± 3.4 | 43.6 ± 5.1 | |||||
| Modified Elimination | 4 weeks | 36.6 ± 8.2 | |||||||
| Baseline | End | Baseline | End | ||||||
| Marum | Low FODMAP | 4 weeks | 61.6 | 47.9 * | 6.6 | 4.9 * | |||
| Baseline | End | Baseline | End | ||||||
| Rodrigo | Gluten-Free | 1 year | 74.3 ± 2.9 | 36.6 ± 4.0 * | 8.0 ± 0.5 | 3.9 ± 1.0 * | |||
| Baseline | End | ||||||||
| Senna | Energy-restricted | 6 months | 54.6 ± 13.1 | 47 ± 5.1 * | |||||
| Control | 6 months | 53.2 ± 11.55 | 51.6 ± 9.4 | ||||||
| Baseline | End | ||||||||
| Slim | Gluten-Free | 24 weeks | 69.5 ± 16.3 | 60.3 ± 19.6 | |||||
| Hypocaloric (Control) | 24 weeks | 70.4 ± 16.1 | 61.7 ± 22.2 | ||||||
| Baseline | End | ||||||||
| Pagliai | Khorasan Wheat Replacement | 8 weeks | 54.3 | 42.06 * | |||||
| Control | 8 weeks | 54.06 | 53.87 | ||||||
| Baseline | 1 month | 2 months | End | ||||||
| Vellisca | Control | 3 months | 5.63 ± 0.86 | 5.41 ± 0.73 | 5.05 ± 0.82 | 5.31 ± 0.88 | |||
| Dietary Additive Excitotoxin Elimination | 3 months | 5.58 ± 0.91 | 5.05 ± 0.82 | 4.88 ± 0.97 | 5.15 ± 0.95 | ||||
| First Author of Article | Dietary Variable or Intervention | Length of Intervention | TPC | TMS | ||
|---|---|---|---|---|---|---|
| Baseline | End | Baseline | End | |||
| Azad | Vegetarian | 6 weeks | 15.7 ± 2.4 | 14.7 ± 3.6 * | - | - |
| Control | 6 weeks | 16.1 ± 2.3 | 6.4 ± 3.0 * | - | - | |
| Holton | Dietary Additive Excitotoxin Elimination | 4 weeks | 16.5 | 14.0 * | 35.2 | 25.7 * |
| Rodrigo | Gluten-Free | 1 year | 16.3 ± 2.4 | 8.0 ± 1.6 * | - | - |
| Senna | Energy-restricted | 6 months | 4.9 ± 0.8 | - | ||
| Control | 6 months | 5.7 ± 1 | ||||
| First Author of Article | Dietary Variable or Intervention | Length of Intervention | PPT | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Occiput | Trapezius | Zygapophyseal Joint | Supraspinatus | Second Rib | Epicondyle | Gluteus | Greater Trochanter | Knee | ||||||||||||
| Cross-Sectional | ||||||||||||||||||||
| Correa-Rodríguez a | Anti-Inflammatory: DII score Quartile 1 Quartile 2 Quartile 3 Quartile 4 | N/A | 1.18 ± 0.79 | 1.37 ± 0.84 | 1.50 ± 1.12 | 1.61 ± 1.14 | 1.09 ± 0.49 | 1.24 ± 0.74 | 2.43 ± 1.63 | 2.39 ± 0.95 | 2.17 ± 1.00 | |||||||||
| 0.94 ± 0.75 | 0.97 ± 0.65 | 0.97 ± 0.72 | 1.31 ± 0.89 | 0.87 ± 0.52 | 0.95 ± 0.59 | 2.12 ± 1.70 | 2.36 ± 1.47 | 1.91 ± 1.43 | ||||||||||||
| 0.72 ± 0.44 | 0.83 ± 0.41 | 0.90 ± 0.53 | 1.08 ± 0.52 | 0.80 ± 0.37 | 0.85 ± 0.43 | 1.65 ± 1.32 | 1.78 ± 0.87 | 1.05 ± 1.05 | ||||||||||||
| 0.57 ± 0.37 * | 0.73 ± 0.45 * | 0.80 ± 0.53 * | 0.99 ± 0.51 | 0.67 ± 0.25 * | 0.78 ± 0.35 | 1.44 ± 0.78 | 1.65 ± 0.72 * | 1.20 ± 0.65 * | ||||||||||||
| BL | End | BL | End | BL | End | BL | End | BL | End | BL | End | BL | End | BL | End | BL | End | |||
| Lamb | Control | 4 weeks | 0.89 | 1.35 | 1.62 | 2.11 | 1.11 | 1.64 | 1.85 | 2.19 | 1.11 | 1.03 | 0.94 | 1.13 | 2.38 | 2.73 | 1.78 | 2.26 | 1.41 | 1.29 |
| Modified Elimination | 4 weeks | 1.48 | 2.33 | 2.05 | 2.50 | 0.93 | 1.54 | 2.81 | 2.41 | 1.87 | ||||||||||
| End | End | End | End | End | End | End | End | End | ||||||||||||
| Senna | Energy-restricted | 6 months | 4.5 ± 2.9 | 6 ± 2.3 * | 4.7 ± 1.9 | 4.6 ± 2.1 * | 5.8 ± 1.5 | 4.9 ± 1.8 | 4.3 ± 1.8 * | 4.9 ± 2.1 * | 4.2 ± 1.8 ** | |||||||||
| Control | 5.1 ± 2.7 | 7.3 ± 2.4 | 4.5 ± 1.7 | 5.9 ± 2.7 | 6 ± 1.7 | 4.3 ± 2.2 | 5.7 ± 2.2 | 6.2 ± 2.4 | 6.1 ± 2 | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddox, E.K.; Massoni, S.C.; Hoffart, C.M.; Takata, Y. Dietary Effects on Pain Symptoms in Patients with Fibromyalgia Syndrome: Systematic Review and Future Directions. Nutrients 2023, 15, 716. https://doi.org/10.3390/nu15030716
Maddox EK, Massoni SC, Hoffart CM, Takata Y. Dietary Effects on Pain Symptoms in Patients with Fibromyalgia Syndrome: Systematic Review and Future Directions. Nutrients. 2023; 15(3):716. https://doi.org/10.3390/nu15030716
Chicago/Turabian StyleMaddox, Emma K., Shawn C. Massoni, Cara M. Hoffart, and Yumie Takata. 2023. "Dietary Effects on Pain Symptoms in Patients with Fibromyalgia Syndrome: Systematic Review and Future Directions" Nutrients 15, no. 3: 716. https://doi.org/10.3390/nu15030716
APA StyleMaddox, E. K., Massoni, S. C., Hoffart, C. M., & Takata, Y. (2023). Dietary Effects on Pain Symptoms in Patients with Fibromyalgia Syndrome: Systematic Review and Future Directions. Nutrients, 15(3), 716. https://doi.org/10.3390/nu15030716






