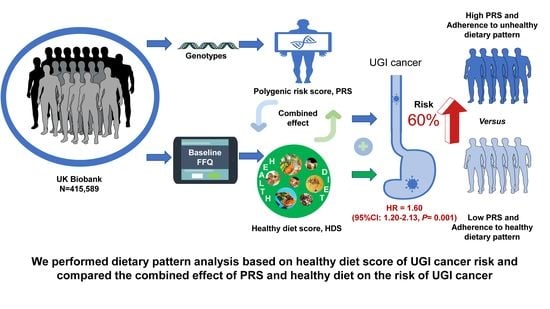
Healthy Diet, Polygenic Risk Score, and Upper Gastrointestinal Cancer Risk: A Prospective Study from UK Biobank
Abstract
1. Introduction
2. Materials and Methods
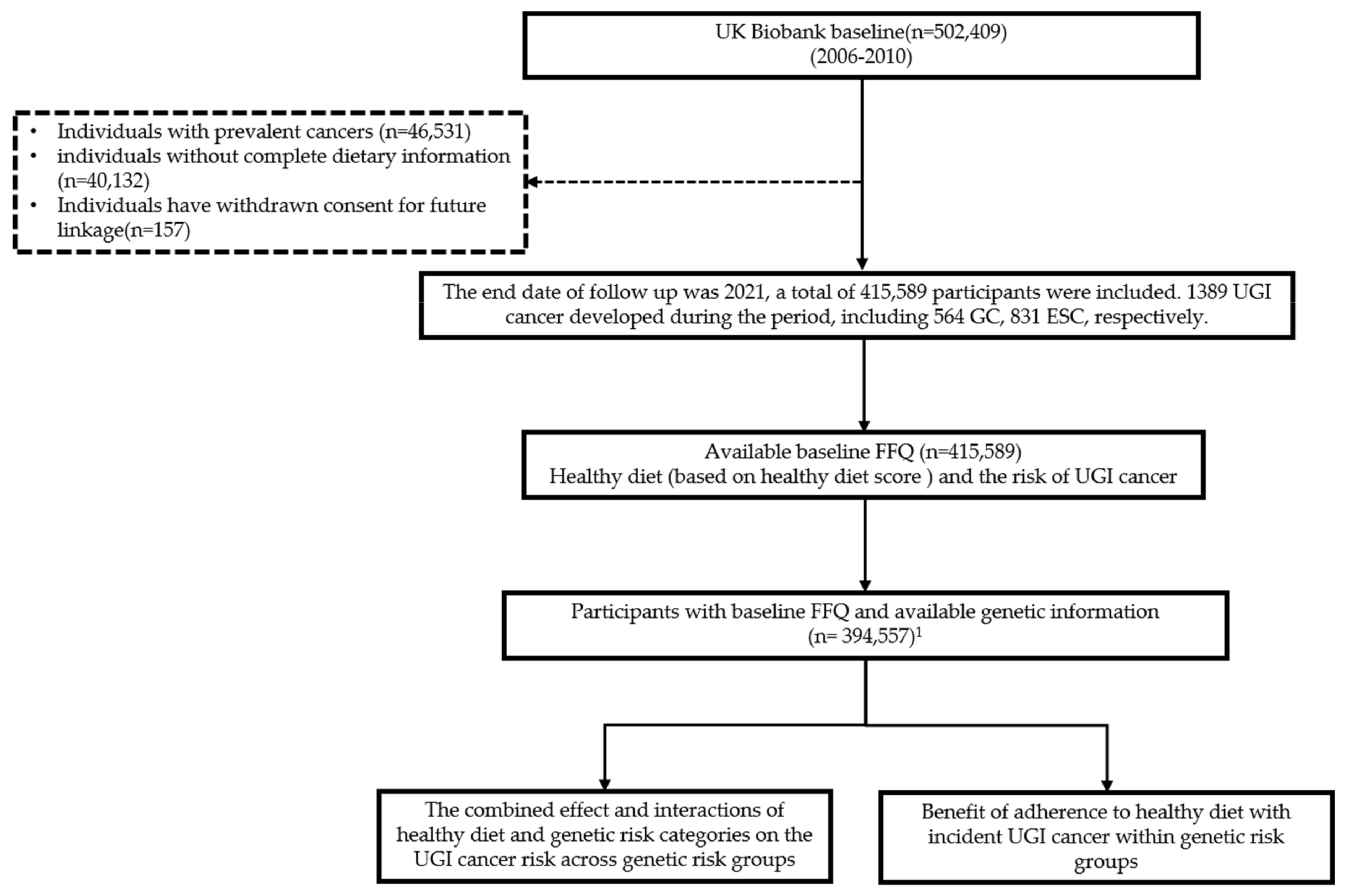
2.1. Study Design and Participants
2.2. Exposure Measurement
2.2.1. Dietary Intake Assessment
2.2.2. Healthy Diet Score Estimation
2.3. PRS Calculation and UGI-PRS Construction
2.4. Outcome Assessment
2.5. Statistical Analysis
3. Results
3.1. Participants and Characteristics
3.2. Healthy Diet and the Risk of UGI Cancer
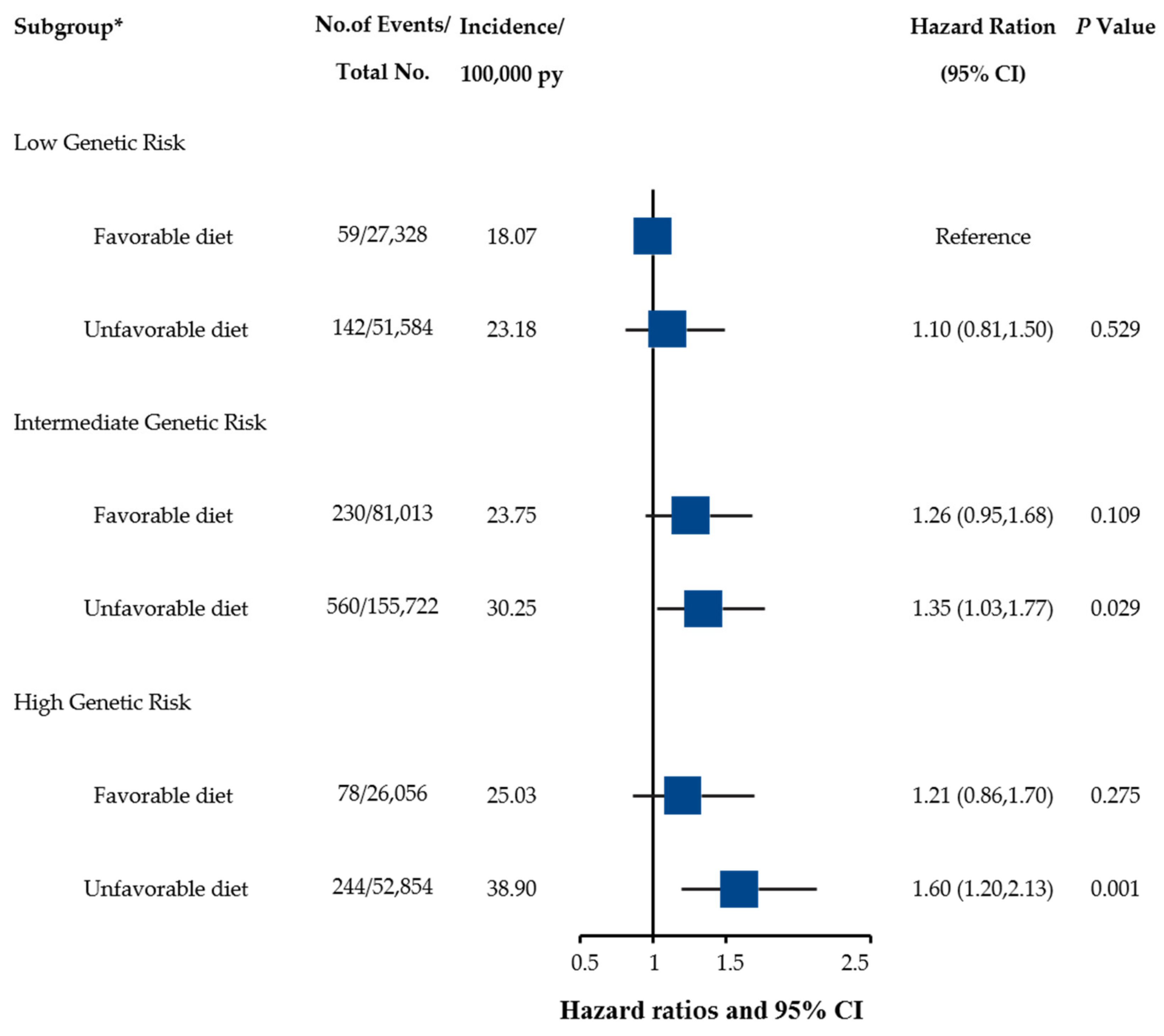
3.3. Combined Effect and Interactions of Healthy Diet and Genetic Risk on UGI Cancer Risk
3.4. Benefits of Adherence to a Healthy Diet with UGI Cancer Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Katki, H.A.; Graubard, B.I.; Kahle, L.L.; Chaturvedi, A.; Matthews, C.E.; Freedman, N.D.; Abnet, C.C. Population Attributable Risks of Subtypes of Esophageal and Gastric Cancers in the United States. Am. J. Gastroenterol. 2021, 116, 1844–1852. [Google Scholar] [CrossRef]
- Lu, L.; Mullins, C.S.; Schafmayer, C.; Zeißig, S.; Linnebacher, M. A global assessment of recent trends in gastrointestinal cancer and lifestyle-associated risk factors. Cancer Commun. 2021, 41, 1137–1151. [Google Scholar] [CrossRef] [PubMed]
- Avgerinos, K.I.; Spyrou, N.; Mantzoros, C.S.; Dalamaga, M. Obesity and cancer risk: Emerging biological mechanisms and perspectives. Metab. Clin. Exp. 2019, 92, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Behrens, G.; Jochem, C.; Keimling, M.; Ricci, C.; Schmid, D.; Leitzmann, M.F. The association between physical activity and gastroesophageal cancer: Systematic review and meta-analysis. Eur. J. Epidemiol. 2014, 29, 151–170. [Google Scholar] [CrossRef]
- Navarro Silvera, S.A.; Mayne, S.T.; Risch, H.A.; Gammon, M.D.; Vaughan, T.; Chow, W.H.; Dubin, J.A.; Dubrow, R.; Schoenberg, J.; Stanford, J.L.; et al. Principal component analysis of dietary and lifestyle patterns in relation to risk of subtypes of esophageal and gastric cancer. Ann. Epidemiol. 2011, 21, 543–550. [Google Scholar] [CrossRef]
- Gonzalez, C.A.; Riboli, E. Diet and cancer prevention: Contributions from the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Eur. J. Cancer 2010, 46, 2555–2562. [Google Scholar] [CrossRef]
- Abnet, C.C.; Corley, D.A.; Freedman, N.D.; Kamangar, F. Diet and upper gastrointestinal malignancies. Gastroenterology 2015, 148, 1234–1243.e4. [Google Scholar] [CrossRef]
- GBD 2019 Cancer Risk Factors Collaborators. The global burden of cancer attributable to risk factors, 2010–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2022, 400, 563–591. [Google Scholar] [CrossRef]
- World Cancer Research Fund; American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018. Available online: http://dietandcancerreport.org (accessed on 11 March 2019).
- Hu, F.B. Dietary pattern analysis: A new direction in nutritional epidemiology. Curr. Opin. Lipidol. 2002, 13, 3–9. [Google Scholar] [CrossRef]
- Sacks, F.M.; Obarzanek, E.; Windhauser, M.M.; Svetkey, L.P.; Vollmer, W.M.; McCullough, M.; Karanja, N.; Lin, P.H.; Steele, P.; Proschan, M.A.; et al. Rationale and design of the Dietary Approaches to Stop Hypertension trial (DASH). A multicenter controlled-feeding study of dietary patterns to lower blood pressure. Ann. Epidemiol. 1995, 5, 108–118. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. Toward a new philosophy of preventive nutrition: From a reductionist to a holistic paradigm to improve nutritional recommendations. Adv. Nutr. 2014, 5, 430–446. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. Perspective: Reductionist Nutrition Research Has Meaning Only within the Framework of Holistic and Ethical Thinking. Adv. Nutr. 2018, 9, 655–670. [Google Scholar] [CrossRef]
- Fardet, A.; Rock, E. Exclusive reductionism, chronic diseases and nutritional confusion: The degree of processing as a lever for improving public health. Crit. Rev. Food Sci. Nutr. 2022, 62, 2784–2799. [Google Scholar] [CrossRef]
- Steck, S.E.; Murphy, E.A. Dietary patterns and cancer risk. Nat. Rev. Cancer 2020, 20, 125–138. [Google Scholar] [CrossRef]
- Bertuccio, P.; Rosato, V.; Andreano, A.; Ferraroni, M.; Decarli, A.; Edefonti, V.; La Vecchia, C. Dietary patterns and gastric cancer risk: A systematic review and meta-analysis. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2013, 24, 1450–1458. [Google Scholar] [CrossRef]
- Kim, J.H.; Lee, J.; Choi, I.J.; Kim, Y.I.; Kim, J. Dietary patterns and gastric cancer risk in a Korean population: A case-control study. Eur. J. Nutr. 2021, 60, 389–397. [Google Scholar] [CrossRef]
- Mucci, L.A.; Hjelmborg, J.B.; Harris, J.R.; Czene, K.; Havelick, D.J.; Scheike, T.; Graff, R.E.; Holst, K.; Möller, S.; Unger, R.H.; et al. Familial Risk and Heritability of Cancer Among Twins in Nordic Countries. JAMA 2016, 315, 68–76. [Google Scholar] [CrossRef]
- Sun, W.Y.; Yang, H.; Wang, X.K.; Fan, J.H.; Qiao, Y.L.; Taylor, P.R. The Association Between Family History of Upper Gastrointestinal Cancer and the Risk of Death from Upper Gastrointestinal Cancer-based on Linxian Dysplasia Nutrition Intervention Trial (NIT) Cohort. Front. Oncol. 2022, 12, 897534. [Google Scholar] [CrossRef]
- Gharahkhani, P.; Fitzgerald, R.C.; Vaughan, T.L.; Palles, C.; Gockel, I.; Tomlinson, I.; Buas, M.F.; May, A.; Gerges, C.; Anders, M.; et al. Genome-wide association studies in oesophageal adenocarcinoma and Barrett’s oesophagus: A large-scale meta-analysis. Lancet Oncol. 2016, 17, 1363–1373. [Google Scholar] [CrossRef]
- Yan, C.; Zhu, M.; Ding, Y.; Yang, M.; Wang, M.; Li, G.; Ren, C.; Huang, T.; Yang, W.; He, B.; et al. Meta-analysis of genome-wide association studies and functional assays decipher susceptibility genes for gastric cancer in Chinese populations. Gut 2020, 69, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, A.T.; Cañadas Garre, M.; Thrift, A.P.; McMenamin, Ú.C.; Johnston, B.T.; Cardwell, C.R.; Anderson, L.A.; Spence, A.D.; Lagergren, J.; Xie, S.H.; et al. Information on Genetic Variants Does Not Increase Identification of Individuals at Risk of Esophageal Adenocarcinoma Compared to Clinical Risk Factors. Gastroenterology 2019, 156, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Lv, J.; Yang, M.; Wang, M.; Zhu, M.; Wang, T.; Yan, C.; Yu, C.; Ding, Y.; Li, G.; et al. Genetic risk, incident gastric cancer, and healthy lifestyle: A meta-analysis of genome-wide association studies and prospective cohort study. Lancet Oncol. 2020, 21, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Aschard, H.; Kang, J.H.; Lentjes, M.A.H.; Do, R.; Wiggs, J.L.; Khawaja, A.P.; Pasquale, L.R. Intraocular Pressure, Glaucoma, and Dietary Caffeine Consumption: A Gene-Diet Interaction Study from the UK Biobank. Ophthalmology 2021, 128, 866–876. [Google Scholar] [CrossRef]
- Zhang, H.; Zeng, Y.; Yang, H.; Hu, Y.; Hu, Y.; Chen, W.; Ying, Z.; Sun, Y.; Qu, Y.; Li, Q.; et al. Familial factors, diet, and risk of cardiovascular disease: A cohort analysis of the UK Biobank. Am. J. Clin. Nutr. 2021, 114, 1837–1846. [Google Scholar] [CrossRef]
- Collins, R. What makes UK Biobank special? Lancet 2012, 379, 1173–1174. [Google Scholar] [CrossRef]
- Palmer, L.J. UK Biobank: Bank on it. Lancet 2007, 369, 1980–1982. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef]
- Lourida, I.; Hannon, E.; Littlejohns, T.J.; Langa, K.M.; Hyppönen, E.; Kuzma, E.; Llewellyn, D.J. Association of Lifestyle and Genetic Risk With Incidence of Dementia. JAMA 2019, 322, 430–437. [Google Scholar] [CrossRef]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef]
- Choi, S.H.; Weng, L.C.; Roselli, C.; Lin, H.; Haggerty, C.M.; Shoemaker, M.B.; Barnard, J.; Arking, D.E.; Chasman, D.I.; Albert, C.M.; et al. Association Between Titin Loss-of-Function Variants and Early-Onset Atrial Fibrillation. JAMA 2018, 320, 2354–2364. [Google Scholar] [CrossRef]
- Helgason, H.; Rafnar, T.; Olafsdottir, H.S.; Jonasson, J.G.; Sigurdsson, A.; Stacey, S.N.; Jonasdottir, A.; Tryggvadottir, L.; Alexiusdottir, K.; Haraldsson, A.; et al. Loss-of-function variants in ATM confer risk of gastric cancer. Nat. Genet. 2015, 47, 906–910. [Google Scholar] [CrossRef]
- Dai, J.; Lv, J.; Zhu, M.; Wang, Y.; Qin, N.; Ma, H.; He, Y.Q.; Zhang, R.; Tan, W.; Fan, J.; et al. Identification of risk loci and a polygenic risk score for lung cancer: A large-scale prospective cohort study in Chinese populations. Lancet Respir. Med. 2019, 7, 881–891. [Google Scholar] [CrossRef]
- Office for National Statistics. Cancer Registration Statistics, England. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/datasets/cancerregistrationstatisticscancerregistrationstatisticsengland (accessed on 26 April 2019).
- Zhu, M.; Wang, T.; Huang, Y.; Zhao, X.; Ding, Y.; Zhu, M.; Ji, M.; Wang, C.; Dai, J.; Yin, R.; et al. Genetic Risk for Overall Cancer and the Benefit of Adherence to a Healthy Lifestyle. Cancer Res. 2021, 81, 4618–4627. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, C.; Yin, S.; Wang, T.; Zhu, M.; Liu, L.; Jin, G. Genetic risk, metabolic syndrome, and gastrointestinal cancer risk: A prospective cohort study. Cancer Med. 2022, 12, 597–605. [Google Scholar] [CrossRef]
- Knol, M.J.; VanderWeele, T.J. Recommendations for presenting analyses of effect modification and interaction. Int. J. Epidemiol. 2012, 41, 514–520. [Google Scholar] [CrossRef]
- Li, R.; Chambless, L. Test for additive interaction in proportional hazards models. Ann. Epidemiol. 2007, 17, 227–236. [Google Scholar] [CrossRef]
- Assmann, S.F.; Hosmer, D.W.; Lemeshow, S.; Mundt, K.A. Confidence intervals for measures of interaction. Epidemiology 1996, 7, 286–290. [Google Scholar] [CrossRef]
- Arthur, R.S.; Wang, T.; Xue, X.; Kamensky, V.; Rohan, T.E. Genetic Factors, Adherence to Healthy Lifestyle Behavior, and Risk of Invasive Breast Cancer Among Women in the UK Biobank. J. Natl. Cancer Inst. 2020, 112, 893–901. [Google Scholar] [CrossRef]
- Grosso, G.; Bella, F.; Godos, J.; Sciacca, S.; Del Rio, D.; Ray, S.; Galvano, F.; Giovannucci, E.L. Possible role of diet in cancer: Systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr. Rev. 2017, 75, 405–419. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Ward, M.H.; Graubard, B.I.; Heineman, E.F.; Markin, R.M.; Potischman, N.A.; Russell, R.M.; Weisenburger, D.D.; Tucker, K.L. Dietary patterns and adenocarcinoma of the esophagus and distal stomach. Am. J. Clin. Nutr. 2002, 75, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, X.; Lin, S.; Yuan, J.; Yu, I.T. Dietary patterns and oesophageal squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Cancer 2014, 110, 2785–2795. [Google Scholar] [CrossRef] [PubMed]
- Cancer Research UK. Cancer Statistics for the UK. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics-for-the-uk (accessed on 5 April 2019).
- Ioannidis, J.P. Why most discovered true associations are inflated. Epidemiology 2008, 19, 640–648. [Google Scholar] [CrossRef]
- Ioannidis, J.P. Why most published research findings are false. PLoS Med. 2005, 2, e124. [Google Scholar] [CrossRef]
- Dwan, K.; Gamble, C.; Williamson, P.R.; Kirkham, J.J. Systematic review of the empirical evidence of study publication bias and outcome reporting bias—An updated review. PLoS ONE 2013, 8, e66844. [Google Scholar] [CrossRef]
- Lin, M.; Lucas, H.C.; Shmueli, G. Research Commentary—Too Big to Fail: Large Samples and the p-Value Problem. Inf. Syst. Res. 2013, 24, 906–917. [Google Scholar] [CrossRef]
- Willett, W.C. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
| Participants | ||
|---|---|---|
| With Incident UGI | Without UGI | |
| Cancer (n = 1389) | Cancer (n = 414,200) | |
| Age at baseline, y | 61.21 ± 6.29 | 56.17 ± 8.09 |
| Female | 388 (27.93) | 222,118 (53.63) |
| Townsend deprivation index, means ± SD | −1.04 ± 3.23 | −1.40 ± 3.03 |
| BMI, means ± SD | 28.61 ± 5.19 | 27.39 ± 4.75 |
| HbA1c, mmol/mol, means ± SD | 38.12 ± 7.98 | 35.94 ± 6.47 |
| Physical activity, MET minutes/week | ||
| <600 | 259 (18.65) | 63,772 (15.4) |
| 600–3000 | 774 (55.72) | 244,531 (59.04) |
| >3000 | 356 (25.63) | 105,897 (25.57) |
| Ethnicity | ||
| White | 1346 (96.9) | 392,733 (94.82) |
| Nonwhite | 38 (2.74) | 20,197 (4.88) |
| Unknown | 5 (0.36) | 1270 (0.31) |
| Education | ||
| College or university degree | 352 (25.34) | 139,657 (33.72) |
| No degree | 1023 (73.65) | 271,190 (65.47) |
| Unknown | 14 (1.01) | 3353 (0.81) |
| Smoking status | ||
| Never | 506 (36.43) | 228,680 (55.21) |
| Former | 640 (46.08) | 141,909 (34.26) |
| Current | 236 (16.99) | 42,454 (10.25) |
| Unknown | 7 (0.5) | 1157 (0.28) |
| Alcohol intake frequency | ||
| Never/rare | 621 (44.71) | 184,431 (44.53) |
| Twice or less per week | 445 (32.04) | 153,836 (37.14) |
| At least three times per week | 322 (23.18) | 75,752 (18.29) |
| Unknown | 1 (0.07) | 181 (0.04) |
| Health status | ||
| Multimorbidity, n (%) | ||
| None | 216 (15.55) | 107,012 (25.84) |
| ≥1 | 1172 (84.38) | 306,847 (74.08) |
| Unknown | 1 (0.07) | 341 (0.08) |
| Family cancer history | ||
| yes | 832 (59.9) | 257,969 (62.28) |
| no | 380 (27.36) | 109,695 (26.48) |
| Unknown | 177 (12.74) | 46,536 (11.24) |
| Healthy Diet | Total No. (Cases) | Minimally Adjusted Model 1 | Fully Adjusted Model 2 | ||
|---|---|---|---|---|---|
| HR (95% CI) | p Value | HR (95% CI) | p Value | ||
| Healthy diet score 3 | |||||
| Low-quality diet (0–1) | 64,171 (304) | 1.00 (ref) | 1.00 (ref) | ||
| Intermediate-quality diet (2–4) | 297,417 (943) | 0.81 (0.71, 0.92) | 0.001 | 0.87 (0.77, 1.00) | 0.047 |
| High-quality diet (5–7) | 54,001 (142) | 0.66 (0.54, 0.81) | <0.001 | 0.76 (0.62, 0.93) | 0.009 |
| Per two-point score increase | 415,589 (1389) | 0.84 (0.78, 0.91) | <0.001 | 0.90 (0.83, 0.97) | 0.006 |
| p for trend | <0.001 | 0.007 | |||
| PRS * | ||
|---|---|---|
| Intermediate | High | |
| RERI (95% CI) | −0.01 (−0.47–0.31) | 0.28 (−0.23–0.67) |
| AP (95% CI) | −0.01 (−0.29–0.26) | 0.18 (−0.13–0.45) |
| RHR (95% CI) | 1.03 (0.73–1.45) | 0.84 (0.56–1.24) |
| Low Genetic Risk | Intermediate Genetic Risk | High Genetic Risk | ||||
|---|---|---|---|---|---|---|
| Dietary Pattern | Unfavorable | Favorable | Unfavorable | Favorable | Unfavorable | Favorable |
| No. of cases/Person-years | 142/61,2672 | 59/326,606 | 560/185,1465 | 230/968,645 | 244/627,221 | 78/311,605 |
| HR (95% CI) | Ref. | 0.85 (0.63–1.17) | Ref. | 0.94 (0.80–1.10) | Ref. | 0.78 (0.60–1.01) |
| p value | 0.323 | 0.417 | 0.057 | |||
| Absolute risk (%)-5 years (95% CI) | 0.10 (0.07–0.12) | 0.08 (0.05–0.10) | 0.13 (0.12–0.15) | 0.11 (0.09–0.12) | 0.16 (0.13–0.19) | 0.10 (0.07–0.13) |
| Absolute risk reduction (%)-5 years (95% CI) | Ref. | 0.02 (−0.06–0.49) | Ref. | 0.03 (0.01–0.05) | Ref. | 0.06 (0.02–0.09) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, T.; Zhu, M.; Jin, G. Healthy Diet, Polygenic Risk Score, and Upper Gastrointestinal Cancer Risk: A Prospective Study from UK Biobank. Nutrients 2023, 15, 1344. https://doi.org/10.3390/nu15061344
Liu W, Wang T, Zhu M, Jin G. Healthy Diet, Polygenic Risk Score, and Upper Gastrointestinal Cancer Risk: A Prospective Study from UK Biobank. Nutrients. 2023; 15(6):1344. https://doi.org/10.3390/nu15061344
Chicago/Turabian StyleLiu, Wenmin, Tianpei Wang, Meng Zhu, and Guangfu Jin. 2023. "Healthy Diet, Polygenic Risk Score, and Upper Gastrointestinal Cancer Risk: A Prospective Study from UK Biobank" Nutrients 15, no. 6: 1344. https://doi.org/10.3390/nu15061344
APA StyleLiu, W., Wang, T., Zhu, M., & Jin, G. (2023). Healthy Diet, Polygenic Risk Score, and Upper Gastrointestinal Cancer Risk: A Prospective Study from UK Biobank. Nutrients, 15(6), 1344. https://doi.org/10.3390/nu15061344