Dietary Patterns and Long-Term Outcomes in Patients with NAFLD: A Prospective Analysis of 128,695 UK Biobank Participants
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Assessment of Dietary Quality
2.3. Assessment of Dietary Patterns
2.4. Ascertainment of Outcomes
2.5. Covariates
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Association of Diet Quality with Incident ESLD and Mortality
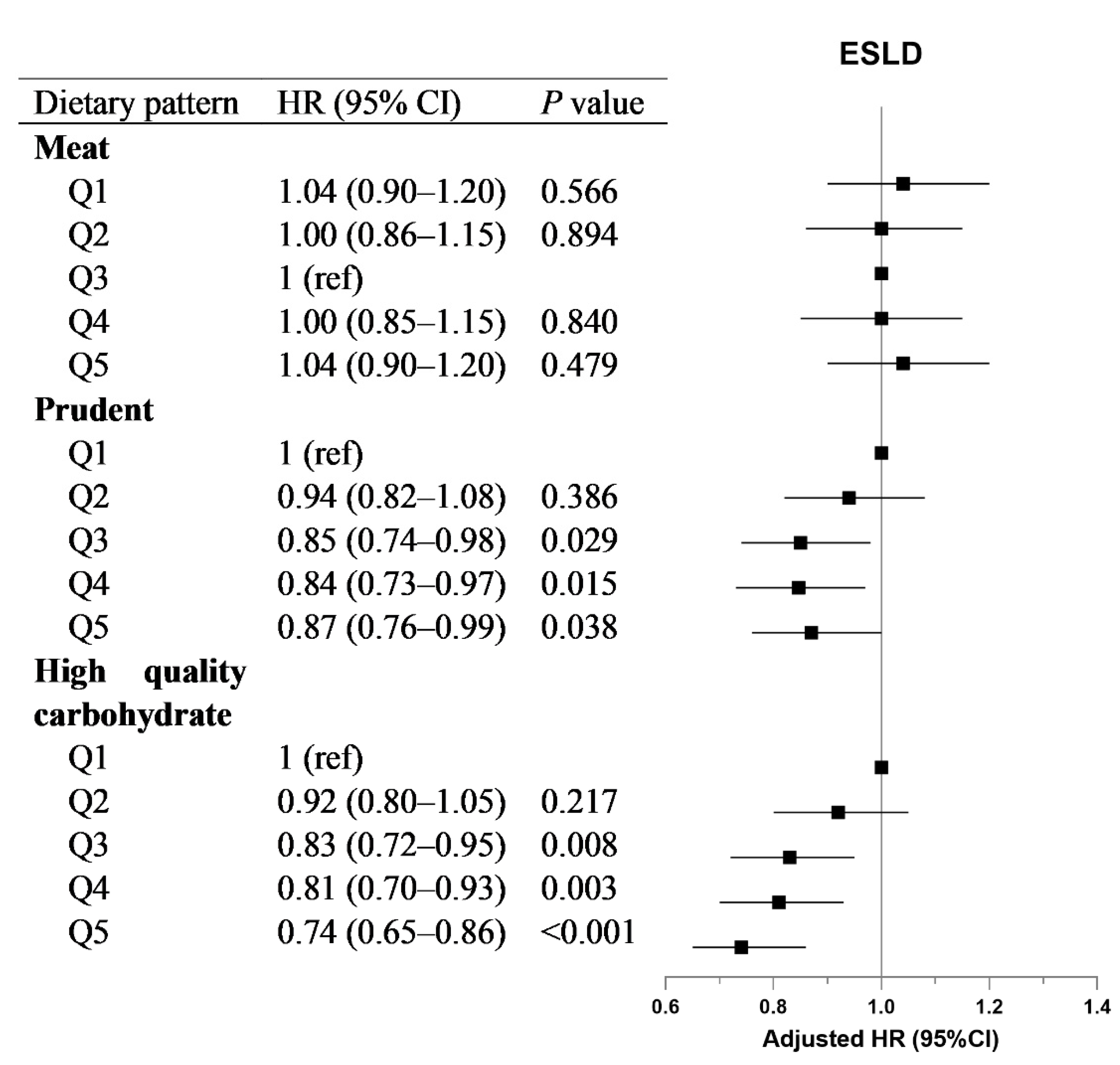
3.3. Association of Dietary Patterns with Incident ESLD and Mortality
3.4. Subgroup Analyses and Sensitivity Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazarus, J.V.; Mark, H.E.; Villota-Rivas, M.; Palayew, A.; Carrieri, P.; Colombo, M.; Ekstedt, M.; Esmat, G.; George, J.; Marchesini, G.; et al. The global NAFLD policy review and preparedness index: Are countries ready to address this silent public health challenge? J. Hepatol. 2022, 76, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Azhari, H.; Charette, J.H.; E Underwood, F.; A King, J.; Afshar, E.E.; Swain, M.G.; E Congly, S.; Kaplan, G.G.; Shaheen, A.-A. The prevalence and incidence of NAFLD worldwide: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2022, 7, 851–861. [Google Scholar] [CrossRef]
- Stefan, N.; Cusi, K. A global view of the interplay between non-alcoholic fatty liver disease and diabetes. Lancet Diabetes Endocrinol. 2022, 10, 284–296. [Google Scholar] [CrossRef]
- Li, J.; Ha, A.; Rui, F.; Zou, B.; Yang, H.; Xue, Q.; Hu, X.; Xu, Y.; Henry, L.; Barakat, M.; et al. Meta-analysis: Global prevalence, trend and forecasting of non-alcoholic fatty liver disease in children and adolescents, 2000–2021. Aliment. Pharmacol. Ther. 2022, 56, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.A.; Kendall, B.J.; Dalais, C.; Macdonald, G.A.; Thrift, A.P. Hepatocellular and extrahepatic cancers in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Eur. J. Cancer 2022, 173, 250–262. [Google Scholar] [CrossRef] [PubMed]
- Loomba, R.; Wong, R.; Fraysse, J.; Shreay, S.; Li, S.; Harrison, S.; Gordon, S.C. Nonalcoholic fatty liver disease progression rates to cirrhosis and progression of cirrhosis to decompensation and mortality: A real world analysis of Medicare data. Aliment. Pharmacol. Ther. 2020, 51, 1149–1159. [Google Scholar] [CrossRef]
- Lazarus, J.V.; Mark, H.E.; Anstee, Q.M.; Arab, J.P.; Batterham, R.L.; Castera, L.; Cortez-Pinto, H.; Crespo, J.; Cusi, K.; Dirac, M.A.; et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 60–78. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Alexander, M.; Loomis, A.K.; van der Lei, J.; Duarte-Salles, T.; Prieto-Alhambra, D.; Ansell, D.; Pasqua, A.; Lapi, F.; Rijnbeek, P.; Mosseveld, M.; et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: Real-world study of 18 million patients in four European cohorts. BMC Med. 2019, 17, 95. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef]
- Hashemian, M.; Merat, S.; Poustchi, H.; Jafari, E.; Radmard, A.-R.; Kamangar, F.; Freedman, N.; Hekmatdoost, A.; Sheikh, M.; Boffetta, P.; et al. Red Meat Consumption and Risk of Nonalcoholic Fatty Liver Disease in a Population with Low Meat Consumption: The Golestan Cohort Study. Am. J. Gastroenterol. 2021, 116, 1667–1675. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, J.; Zhang, Q.; Liu, L.; Lu, M.; Meng, G.; Yao, Z.; Wu, H.; Xia, Y.; Bao, X.; et al. Association between habitual yogurt consumption and newly diagnosed non-alcoholic fatty liver disease. Eur. J. Clin. Nutr. 2019, 74, 491–499. [Google Scholar] [CrossRef]
- Eslami, O.; Shidfar, F.; Maleki, Z.; Jazayeri, S.; Hosseini, A.F.; Agah, S.; Ardiyani, F. Effect of Soy Milk on Metabolic Status of Patients with Nonalcoholic Fatty Liver Disease: A Randomized Clinical Trial. J. Am. Coll. Nutr. 2018, 38, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Cespedes, E.M.; Hu, F.B. Dietary patterns: From nutritional epidemiologic analysis to national guidelines. Am. J. Clin. Nutr. 2015, 101, 899–900. [Google Scholar] [CrossRef]
- Strate, L.L.; Keeley, B.R.; Cao, Y.; Wu, K.; Giovannucci, E.L.; Chan, A.T. Western Dietary Pattern Increases, and Prudent Dietary Pattern Decreases, Risk of Incident Diverticulitis in a Prospective Cohort Study. Gastroenterology 2017, 152, 1023–1030.e2. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, C.; Iqbal, K.; Knüppel, S.; Schwingshackl, L.; Boeing, H. Contribution to the understanding of how principal component analysis–derived dietary patterns emerge from habitual data on food consumption. Am. J. Clin. Nutr. 2018, 107, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Zadeh, S.H.; Mansoori, A.; Hosseinzadeh, M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 1470–1478. [Google Scholar] [CrossRef]
- Ma, J.; Hennein, R.; Liu, C.; Long, M.T.; Hoffmann, U.; Jacques, P.F.; Lichtenstein, A.H.; Hu, F.B.; Levy, D. Improved Diet Quality Associates with Reduction in Liver Fat, Particularly in Individuals with High Genetic Risk Scores for Nonalcoholic Fatty Liver Disease. Gastroenterology 2018, 155, 107–117. [Google Scholar] [CrossRef]
- Mozaffarian, D. Dietary and Policy Priorities for Cardiovascular Disease, Diabetes, and Obesity: A Comprehensive Review. Circulation 2016, 133, 187–225. [Google Scholar] [CrossRef]
- Vilar-Gomez, E.; Nephew, L.D.; Vuppalanchi, R.; Gawrieh, S.; Mladenovic, A.; Pike, F.; Samala, N.; Chalasani, N. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 2022, 75, 1491–1506. [Google Scholar] [CrossRef]
- Khalatbari-Soltani, S.; Imamura, F.; Brage, S.; De Lucia Rolfe, E.; Griffin, S.J.; Wareham, N.J.; Marques-Vidal, P.; Forouhi, N.G. The association between adherence to the Mediterranean diet and hepatic steatosis: Cross-sectional analysis of two independent studies, the UK Fenland Study and the Swiss CoLaus Study. BMC Med. 2019, 17, 19. [Google Scholar] [CrossRef]
- Adriano, L.S.; de Carvalho Sampaio, H.A.; Arruda, S.P.M.; de Melo Portela, C.L.; de Melo, M.L.P.; Carioca, A.A.F.; Soares, N.T. Healthy dietary pattern is inversely associated with non-alcoholic fatty liver disease in elderly. Br. J. Nutr. 2016, 115, 2189–2195. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Q.; Shu, L.; Wang, S.; Wang, J.-J.; Zhou, Y.; Xuan, Y.-J.; Wang, S.-F. Dietary Patterns Modulate the Risk of Non-Alcoholic Fatty Liver Disease in Chinese Adults. Nutrients 2015, 7, 4778–4791. [Google Scholar] [CrossRef] [PubMed]
- Oddy, W.H.; Herbison, C.E.; Jacoby, P.; Ambrosini, G.L.; O’Sullivan, T.A.; Ayonrinde, O.T.; Olynyk, J.K.; Black, L.J.; Beilin, L.J.; Mori, T.A.; et al. The Western Dietary Pattern Is Prospectively Associated With Nonalcoholic Fatty Liver Disease in Adolescence. Am. J. Gastroenterol. 2013, 108, 778–785. [Google Scholar] [CrossRef]
- Said, M.A.; Verweij, N.; Van Der Harst, P. Associations of Combined Genetic and Lifestyle Risks with Incident Cardiovascular Disease and Diabetes in the UK Biobank Study. JAMA Cardiol. 2018, 3, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, P.; Liu, X.; Li, Y.; Wan, X.; Wu, Y.; Wu, F.; Zhang, Y.; Jiao, J. Effect of Diet Quality and Genetic Predisposition on Hemoglobin A1c and Type 2 Diabetes Risk: Gene-Diet Interaction Analysis of 357,419 Individuals. Diabetes Care 2021, 44, 2470–2479. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Z.; Gao, Q.; Zhao, H.; Chen, S.; Huang, L.; Wang, W.; Wang, T. A review of statistical methods for dietary pattern analysis. Nutr. J. 2021, 20, 37. [Google Scholar] [CrossRef]
- Murakami, K.; Shinozaki, N.; Fujiwara, A.; Yuan, X.; Hashimoto, A.; Fujihashi, H.; Wang, H.-C.; Livingstone, M.B.E.; Sasaki, S. A Systematic Review of Principal Component Analysis–Derived Dietary Patterns in Japanese Adults: Are Major Dietary Patterns Reproducible within a Country? Adv. Nutr. 2019, 10, 237–249. [Google Scholar] [CrossRef]
- Orr, J.G.; Homer, T.; Ternent, L.; Newton, J.; McNeil, C.J.; Hudson, M.; Jones, D.E. Health related quality of life in people with advanced chronic liver disease. J. Hepatol. 2014, 61, 1158–1165. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Li, L.; Dai, J.; Natarajan, Y.; Yu, X.; Asch, S.M.; El-Serag, H.B. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Salomone, F.; Mlynarsky, L. The Mediterranean dietary pattern as the diet of choice for non-alcoholic fatty liver disease: Evidence and plausible mechanisms. Liver Int. Off. J. Int. Assoc. Study Liver 2017, 37, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Salomone, F.; Godos, J.; Zelber-Sagi, S. Natural antioxidants for non-alcoholic fatty liver disease: Molecular targets and clinical perspectives. Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, M.; Yahagi, N.; Matsuzaka, T.; Najima, Y.; Nakakuki, M.; Nagai, R.; Ishibashi, S.; Osuga, J.; Yamada, N.; Shimano, H. Polyunsaturated fatty acids ameliorate hepatic steatosis in obese mice by SREBP-1 suppression. Hepatology 2003, 38, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.R.; Clore, J.N.; Stevens, W. Dietary n-3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 rats. Hepatology 2004, 39, 608–616. [Google Scholar] [CrossRef]
- Naowaboot, J.; Piyabhan, P.; Munkong, N.; Parklak, W.; Pannangpetch, P. Ferulic acid improves lipid and glucose homeostasis in high-fat diet-induced obese mice. Clin. Exp. Pharmacol. Physiol. 2016, 43, 242–250. [Google Scholar] [CrossRef]
- Kathirvel, E.; Morgan, K.; Nandgiri, G.; Sandoval, B.C.; Caudill, M.A.; Bottiglieri, T.; French, S.W.; Morgan, T.R. Betaine improves nonalcoholic fatty liver and associated hepatic insulin resistance: A potential mechanism for hepatoprotection by betaine. Am. J. Physiol. Gastrointest Liver Physiol. 2010, 299, G1068–G1077. [Google Scholar] [CrossRef]
- Rose, D.J. Impact of whole grains on the gut microbiota: The next frontier for oats? Br. J. Nutr. 2014, 112 (Suppl. S2), S44–S49. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Godos, J.; Loguercio, C.; Salomone, F. Targeting gut-liver axis for the treatment of nonalcoholic steatohepatitis: Translational and clinical evidence. Transl. Res. 2016, 167, 116–124. [Google Scholar] [CrossRef]


| Variables | Q1 | Q2 | Q3 | Q4 | Q5 | p Value |
|---|---|---|---|---|---|---|
| Male (%) | 70.9 | 62.4 | 56.5 | 53.2 | 53.7 | <0.001 |
| Age (years) | 55.7 ± 8.3 | 56.9 ± 8.1 | 57.3 ± 7.9 | 57.9 ± 7.6 | 59.1 ± 7.1 | <0.001 |
| White ethnicity (%) | 93.4 | 91.8 | 92.1 | 93.7 | 94.5 | <0.001 |
| Townsend deprivation index | −0.4 ± 3.4 | −0.8 ± 3.3 | −1.1 ± 3.2 | −1.3 ± 3.1 | −1.2 ± 3.1 | <0.001 |
| College or university degree (%) | 17.53 | 23.13 | 26.33 | 30.08 | 29.79 | <0.001 |
| Household income (£) | <0.001 | |||||
| <18,000 | 26.5 | 25.0 | 22.8 | 22.5 | 24.8 | |
| 18,000 to 30,999 | 22.2 | 22.1 | 22.5 | 22.5 | 23.6 | |
| 31,000 to 51,999 | 20.3 | 20.5 | 20.7 | 21.1 | 20.5 | |
| 52,000 to 100,000 | 12.5 | 13.7 | 15.0 | 15.4 | 13.5 | |
| >100,000 | 2.2 | 2.6 | 3.1 | 3.6 | 2.8 | |
| Sedentary behavior (%) | 46.5 | 42.9 | 40.9 | 39.1 | 39.1 | <0.001 |
| Smoking status (%) | <0.001 | |||||
| Never | 48.3 | 52.9 | 54.7 | 55.5 | 53.0 | |
| Previous | 34.0 | 35.7 | 36.6 | 37.1 | 41.0 | |
| Current | 17.7 | 11.4 | 8.7 | 7.3 | 6.0 | |
| Alcohol | ||||||
| Never or in special occasions only | 29.8 | 29.5 | 28.5 | 26.9 | 28.2 | |
| 1 to 3 times/month | 15.9 | 15.2 | 15.5 | 15.4 | 15.0 | |
| 1 to 4 times/week | 47.7 | 48.6 | 49.4 | 50.6 | 50.3 | |
| Daily or almost daily | 6.6 | 6.8 | 6.6 | 7.0 | 6.6 | |
| Body mass index (kg/m2) | 31.6 ± 4.7 | 31.8 ± 4.6 | 31.9 ± 4.6 | 31.8 ± 4.6 | 31.9 ± 4.6 | <0.001 |
| Waist circumference (cm) | 103.5 ± 10.2 | 102.9 ± 10.2 | 102.5 ± 10.1 | 102.2 ± 10.1 | 102.3 ± 10.1 | <0.001 |
| Hypertension (%) | 68.2 | 69.4 | 69.4 | 70.0 | 72.9 | <0.001 |
| Diabetes (%) | 10.0 | 11.2 | 12.2 | 12.2 | 17.4 | <0.001 |
| Alanine aminotransferase (U/L) | 30.3 ± 17.0 | 29.5 ± 16.5 | 29.1 ± 17.4 | 28.9 ± 16.1 | 28.8 ± 16.6 | <0.001 |
| Gamma glutamyltransferase (U/L) | 52.3 ± 52.3 | 50.6 ± 48.8 | 49.3 ± 49.8 | 48.0 ± 45.9 | 47.8 ± 48.4 | <0.001 |
| Triglycerides (mmol/L) | 2.5 ± 1.2 | 2.4 ± 1.2 | 2.4 ± 1.2 | 2.3 ± 1.1 | 2.3 ± 1.1 | <0.001 |
| Total cholesterol (mmol/L) | 5.7 ± 1.2 | 5.7 ± 1.2 | 5.7 ± 1.2 | 5.7 ± 1.2 | 5.6 ± 1.3 | <0.001 |
| Diet Score | ESLD | All-Cause Mortality | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | p Value | ptrend | HR (95% CI) | p Value | ptrend | |
| Model 1 | 0.001 | 0.004 | ||||
| Q1 | 1 (ref) | 1 (ref) | ||||
| Q2 | 0.95 (0.83–1.09) | 0.497 | 0.92 (0.87–0.97) | 0.003 | ||
| Q3 | 0.86 (0.75–0.99) | 0.035 | 0.88 (0.84–0.94) | <0.001 | ||
| Q4 | 0.85 (0.74–0.98) | 0.027 | 0.84 (0.80–0.89) | <0.001 | ||
| Q5 | 0.84 (0.73–0.96) | 0.010 | 0.82 (0.77–0.87) | <0.001 | ||
| Model 2 | <0.001 | <0.001 | ||||
| Q1 | 1 (ref) | 1 (ref) | ||||
| Q2 | 0.95 (0.83–1.08) | 0.412 | 0.94 (0.89–0.99) | 0.021 | ||
| Q3 | 0.83 (0.72–0.95) | 0.008 | 0.91 (0.86–0.96) | 0.001 | ||
| Q4 | 0.84 (0.73–0.96) | 0.013 | 0.85 (0.80–0.90) | <0.001 | ||
| Q5 | 0.76 (0.66–0.87) | <0.001 | 0.84 (0.79–0.88) | <0.001 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Huang, H.; Xie, J.; Xu, C. Dietary Patterns and Long-Term Outcomes in Patients with NAFLD: A Prospective Analysis of 128,695 UK Biobank Participants. Nutrients 2023, 15, 271. https://doi.org/10.3390/nu15020271
Liu Z, Huang H, Xie J, Xu C. Dietary Patterns and Long-Term Outcomes in Patients with NAFLD: A Prospective Analysis of 128,695 UK Biobank Participants. Nutrients. 2023; 15(2):271. https://doi.org/10.3390/nu15020271
Chicago/Turabian StyleLiu, Zhening, Hangkai Huang, Jiarong Xie, and Chengfu Xu. 2023. "Dietary Patterns and Long-Term Outcomes in Patients with NAFLD: A Prospective Analysis of 128,695 UK Biobank Participants" Nutrients 15, no. 2: 271. https://doi.org/10.3390/nu15020271
APA StyleLiu, Z., Huang, H., Xie, J., & Xu, C. (2023). Dietary Patterns and Long-Term Outcomes in Patients with NAFLD: A Prospective Analysis of 128,695 UK Biobank Participants. Nutrients, 15(2), 271. https://doi.org/10.3390/nu15020271








