Timing of Meals and Sleep in the Mediterranean Population: The Effect of Taste, Genetics, Environmental Determinants, and Interactions on Obesity Phenotypes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Demographic, Anthropometric, Biochemical, Clinical, and Lifestyle Variables
2.3. Taste Perception Tests
2.4. Temporal Eating Patterns
2.5. Sleep Patterns
2.6. DNA Isolation and DNA Genotyping
2.7. Statistical Analysis
3. Results
3.1. General Characteristics of the Population
3.2. Association between Phenotypic (Measured) Taste Perception, Candidate SNP, and Eating Patterns
3.3. Association between Phenotypic (Measured) and Genotypic Taste Perception and Sleep Patterns
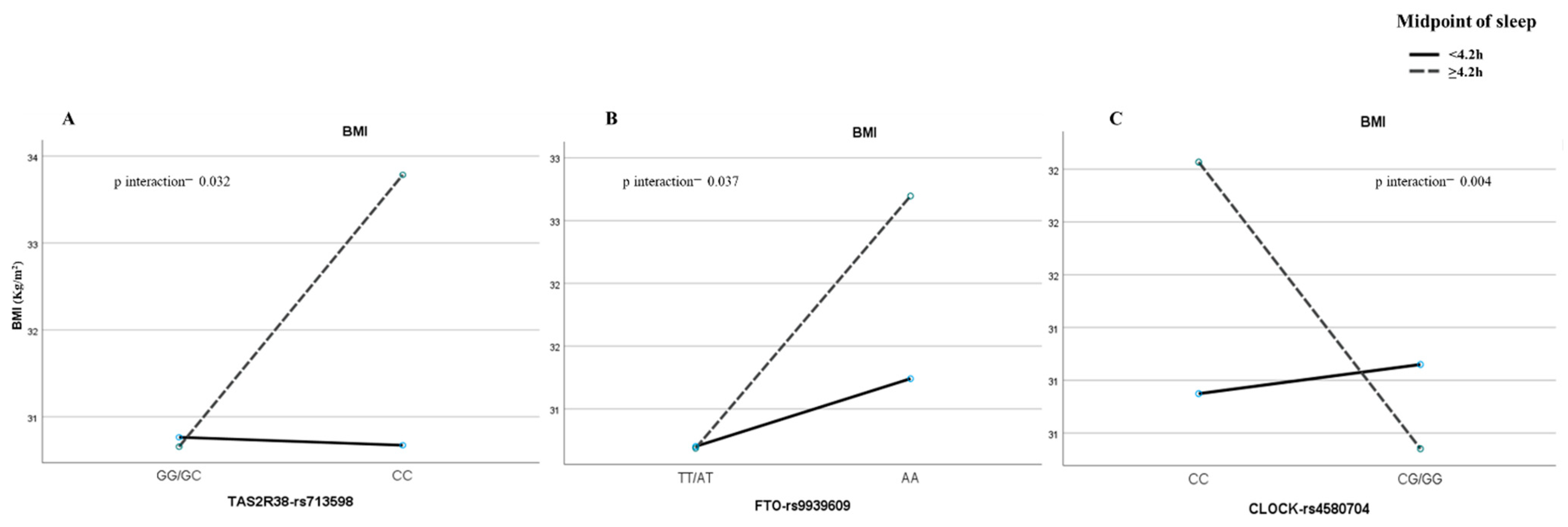
3.4. Association between Candidate Genes and Obesity-Related Phenotypes: Selected Gene–Pattern Interactions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Froy, O.; Garaulet, M. The Circadian Clock in White and Brown Adipose Tissue: Mechanistic, Endocrine, and Clinical Aspects. Endocr. Rev. 2018, 39, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Boege, H.L.; Bhatti, M.Z.; St-Onge, M.P. Circadian Rhythms and Meal Timing: Impact on Energy Balance and Body Weight. Curr. Opin. Biotechnol. 2021, 70, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Molina-Montes, E.; Rodríguez-Barranco, M.; Ching-López, A.; Artacho, R.; Huerta, J.M.; Amiano, P.; Lasheras, C.; Moreno-Iribas, C.; Jimenez-Zabala, A.; Chirlaque, M.D.; et al. Circadian Clock Gene Variants and Their Link with Chronotype, Chrononutrition, Sleeping Patterns and Obesity in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. Clin. Nutr. 2022, 41, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian Clocks and Insulin Resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- Rijo-Ferreira, F.; Takahashi, J.S. Genomics of Circadian Rhythms in Health and Disease. Genome Med. 2019, 11, 82. [Google Scholar] [CrossRef]
- Ko, C.H.; Takahashi, J.S. Molecular Components of the Mammalian Circadian Clock. Hum. Mol. Genet. 2006, 15, R271–R277. [Google Scholar] [CrossRef]
- Scott, E.M.; Carter, A.M.; Grant, P.J. Association between Polymorphisms in the Clock Gene, Obesity and the Metabolic Syndrome in Man. Int. J. Obes. 2008, 32, 658–662. [Google Scholar] [CrossRef]
- Sookoian, S.; Gemma, C.; Gianotti, T.F.; Burgueño, A.; Castaño, G.; Pirola, C.J. Genetic Variants of Clock Transcription Factor Are Associated with Individual Susceptibility to Obesity. Am. J. Clin. Nutr. 2008, 87, 1606–1615. [Google Scholar] [CrossRef]
- Uemura, H.; Katsuura-Kamano, S.; Yamaguchi, M.; Arisawa, K.; Hamajima, N.; Hishida, A.; Kawai, S.; Oze, I.; Shinchi, K.; Takashima, N.; et al. Variant of the Clock Circadian Regulator (CLOCK) Gene and Related Haplotypes Are Associated with the Prevalence of Type 2 Diabetes in the Japanese Population. J. Diabetes 2016, 8, 667–676. [Google Scholar] [CrossRef]
- Corella, D.; Asensio, E.M.; Coltell, O.; Sorlí, J.V.; Estruch, R.; Martínez-González, M.Á.; Salas-Salvadó, J.; Castañer, O.; Arós, F.; Lapetra, J.; et al. CLOCK Gene Variation Is Associated with Incidence of Type-2 Diabetes and Cardiovascular Diseases in Type-2 Diabetic Subjects: Dietary Modulation in the PREDIMED Randomized Trial. Cardiovasc. Diabetol. 2016, 15, 4. [Google Scholar] [CrossRef]
- Hublin, C.; Haasio, L.; Kaprio, J. Changes in Self-Reported Sleep Duration with Age—36-Year Longitudinal Study of Finnish Adults. BMC Public Health 2020, 20, 1373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wheaton, A.G.; Chapman, D.P.; Cunningham, T.J.; Lu, H.; Croft, J.B. Prevalence of Healthy Sleep Duration among Adults--United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhai, L.; Zhang, D. Sleep Duration and Obesity among Adults: A Meta-Analysis of Prospective Studies. Sleep Med. 2014, 15, 1456–1462. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Grandner, M.A.; Brown, D.; Conroy, M.B.; Jean-Louis, G.; Coons, M.; Bhatt, D.L. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e367–e386. [Google Scholar] [CrossRef] [PubMed]
- al Khatib, H.K.; Harding, S.V.; Darzi, J.; Pot, G.K. The Effects of Partial Sleep Deprivation on Energy Balance: A Systematic Review and Meta-Analysis. Eur. J. Clin. Nutr. 2017, 71, 614–624. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.P.; Pizinger, T.; Kovtun, K.; RoyChoudhury, A. Sleep and Meal Timing Influence Food Intake and Its Hormonal Regulation in Healthy Adults with Overweight/Obesity. Eur. J. Clin. Nutr. 2019, 72, 76–82. [Google Scholar] [CrossRef]
- Spaeth, A.M.; Goel, N.; Dinges, D.F. Caloric and Macronutrient Intake and Meal Timing Responses to Repeated Sleep Restriction Exposures Separated by Varying Intervening Recovery Nights in Healthy Adults. Nutrients 2020, 12, 2694. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.J.L.; Jacques, P.F.; Lamon-Fava, S.; Ordovás, J.M. Short Sleep Duration and Dietary Intake: Epidemiologic Evidence, Mechanisms, and Health Implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef]
- St-Onge, M.P.; Ard, J.; Baskin, M.L.; Chiuve, S.E.; Johnson, H.M.; Kris-Etherton, P.; Varady, K. Meal Timing and Frequency: Implications for Cardiovascular Disease Prevention: A Scientific Statement From the American Heart Association. Circulation 2017, 135, e96–e121. [Google Scholar] [CrossRef]
- Chow, L.S.; Manoogian, E.N.C.; Alvear, A.; Fleischer, J.G.; Thor, H.; Dietsche, K.; Wang, Q.; Hodges, J.S.; Esch, N.; Malaeb, S.; et al. Time-Restricted Eating Effects on Body Composition and Metabolic Measures in Humans Who Are Overweight: A Feasibility Study. Obesity 2020, 28, 860–869. [Google Scholar] [CrossRef]
- Gu, C.; Brereton, N.; Schweitzer, A.; Cotter, M.; Duan, D.; Børsheim, E.; Wolfe, R.R.; Pham, L.V.; Polotsky, V.Y.; Jun, J.C. Metabolic Effects of Late Dinner in Healthy Volunteers-A Randomized Crossover Clinical Trial. J. Clin. Endocrinol. Metab. 2020, 105, 2789–2802. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Sears, D.D.; St-Onge, M.P.; Zuraikat, F.M.; Gallo, L.C.; Talavera, G.A.; Castaneda, S.F.; Lai, Y.; Mi, J.; Aggarwal, B. Habitual Nightly Fasting Duration, Eating Timing, and Eating Frequency Are Associated with Cardiometabolic Risk in Women. Nutrients 2020, 12, 3043. [Google Scholar] [CrossRef] [PubMed]
- Zerón-Rugerio, M.F.; Hernáez, Á.; Porras-Loaiza, A.P.; Cambras, T.; Izquierdo-Pulido, M. Eating Jet Lag: A Marker of the Variability in Meal Timing and Its Association with Body Mass Index. Nutrients 2019, 11, 2980. [Google Scholar] [CrossRef] [PubMed]
- Dashti, H.S.; Gómez-Abellán, P.; Qian, J.; Esteban, A.; Morales, E.; Scheer, F.A.J.L.; Garaulet, M. Late Eating Is Associated with Cardiometabolic Risk Traits, Obesogenic Behaviors, and Impaired Weight Loss. Am. J. Clin. Nutr. 2021, 113, 154–161. [Google Scholar] [CrossRef]
- Vettori, A.; Pompucci, G.; Paolini, B.; del Ciondolo, I.; Bressan, S.; Dundar, M.; Kenanoğlu, S.; Unfer, V.; Bertelli, M. Genetic Background, Nutrition and Obesity: A Review. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1751–1761. [Google Scholar] [CrossRef]
- Grzymisławska, M.; Puch, E.A.; Zawada, A.; Grzymisławski, M. Do Nutritional Behaviors Depend on Biological Sex and Cultural Gender? Adv. Clin. Exp. Med. 2020, 29, 165–172. [Google Scholar] [CrossRef]
- Mattes, R.; Foster, G.D. Food Environment and Obesity. Obesity 2014, 22, 2459–2461. [Google Scholar] [CrossRef]
- Clark, J.E. Taste and Flavour: Their Importance in Food Choice and Acceptance. Proc. Nutr. Soc. 1998, 57, 639–643. [Google Scholar] [CrossRef]
- Kabir, A.; Miah, S.; Islam, A. Factors Influencing Eating Behavior and Dietary Intake among Resident Students in a Public University in Bangladesh: A Qualitative Study. PLoS ONE 2018, 13, e198801. [Google Scholar] [CrossRef]
- Stok, F.M.; Renner, B.; Clarys, P.; Lien, N.; Lakerveld, J.; Deliens, T. Understanding Eating Behavior during the Transition from Adolescence to Young Adulthood: A Literature Review and Perspective on Future Research Directions. Nutrients 2018, 10, 667. [Google Scholar] [CrossRef]
- Barragán, R.; Coltell, O.; Portolés, O.; Asensio, E.M.; Sorlí, J.V.; Ortega-Azorín, C.; González, J.I.; Sáiz, C.; Fernández-Carrión, R.; Ordovas, J.M.; et al. Bitter, Sweet, Salty, Sour and Umami Taste Perception Decreases with Age: Sex-Specific Analysis, Modulation by Genetic Variants and Taste-Preference Associations in 18 to 80 Year-Old Subjects. Nutrients 2018, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Coltell, O.; Sorlí, J.V.; Asensio, E.M.; Fernández-Carrión, R.; Barragán, R.; Ortega-Azorín, C.; Estruch, R.; González, J.I.; Salas-Salvadó, J.; Lamon-Fava, S.; et al. Association between Taste Perception and Adiposity in Overweight or Obese Older Subjects with Metabolic Syndrome and Identification of Novel Taste-Related Genes. Am. J. Clin. Nutr. 2019, 109, 1709–1723. [Google Scholar] [CrossRef] [PubMed]
- Tepper, B.J.; Koelliker, Y.; Zhao, L.; Ullrich, N.V.; Lanzara, C.; D’Adamo, P.; Ferrara, A.; Ulivi, S.; Esposito, L.; Gasparini, P. Variation in the Bitter-Taste Receptor Gene TAS2R38, and Adiposity in a Genetically Isolated Population in Southern Italy. Obesity 2008, 16, 2289–2295. [Google Scholar] [CrossRef] [PubMed]
- Khataan, N.H.; Stewart, L.; Brenner, D.M.; Cornelis, M.C.; El-Sohemy, A. TAS2R38 Genotypes and Phenylthiocarbamide Bitter Taste Perception in a Population of Young Adults. J. Nutrigenet. Nutrigen. 2010, 2, 251–256. [Google Scholar] [CrossRef]
- Loos, R.J.F.; Yeo, G.S.H. The Bigger Picture of FTO: The First GWAS-Identified Obesity Gene. Nat. Rev. Endocrinol. 2014, 10, 51–61. [Google Scholar] [CrossRef]
- Hwang, L.D.; Lin, C.; Gharahkhani, P.; Cuellar-Partida, G.; Ong, J.S.; An, J.; Gordon, S.D.; Zhu, G.; Macgregor, S.; Lawlor, D.A.; et al. New Insight into Human Sweet Taste: A Genome-Wide Association Study of the Perception and Intake of Sweet Substances. Am. J. Clin. Nutr. 2019, 109, 1724–1737. [Google Scholar] [CrossRef]
- Apalasamy, Y.D.; Ming, M.F.; Rampal, S.; Bulgiba, A.; Mohamed, Z. Genetic Association of Snps in the Fto Gene and Predisposition to Obesity in Malaysian Malays. Braz. J. Med. Biol. Res. 2012, 45, 1119–1126. [Google Scholar] [CrossRef]
- Simchen, U.; Koebnick, C.; Hoyer, S.; Issanchou, S.; Zunft, H.J.F. Odour and Taste Sensitivity Is Associated with Body Weight and Extent of Misreporting of Body Weight. Eur. J. Clin. Nutr. 2006, 60, 698–705. [Google Scholar] [CrossRef]
- Skrandies, W.; Zschieschang, R. Olfactory and Gustatory Functions and Its Relation to Body Weight. Physiol. Behav. 2015, 142, 1–4. [Google Scholar] [CrossRef]
- Low, J.Y.Q.; Lacy, K.E.; McBride, R.; Keast, R.S.J. The Association between Sweet Taste Function, Anthropometry, and Dietary Intake in Adults. Nutrients 2016, 8, 241. [Google Scholar] [CrossRef]
- Fernandez-Garcia, J.C.; Alcaide, J.; Santiago-Fernandez, C.; Roca-Rodriguez, M.M.; Aguera, Z.; Baños, R.; Botella, C.; de La Torre, R.; Fernandez-Real, J.M.; Fruhbeck, G.; et al. An Increase in Visceral Fat Is Associated with a Decrease in the Taste and Olfactory Capacity. PLoS ONE 2017, 12, e171204. [Google Scholar] [CrossRef]
- Hardikar, S.; Höchenberger, R.; Villringer, A.; Ohla, K. Higher Sensitivity to Sweet and Salty Taste in Obese Compared to Lean Individuals. Appetite 2017, 111, 158–165. [Google Scholar] [CrossRef]
- Martelli, M.E.; Jacob, N.; Morais, M.A.; da-Cunha, D.T.; Corona, L.P.; Capitani, C.D.; Esteves, A.M. Taste Sensitivity throughout Age and the Relationship with the Sleep Quality. Sleep Sci. 2020, 13, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Szczygiel, E.J.; Cho, S.; Tucker, R.M. Multiple Dimensions of Sweet Taste Perception Altered after Sleep Curtailment. Nutrients 2019, 11, 2015. [Google Scholar] [CrossRef]
- Simon, S.L.; Field, J.; Miller, L.E.; DiFrancesco, M.; Beebe, D.W. Sweet/Dessert Foods Are More Appealing to Adolescents after Sleep Restriction. PLoS ONE 2015, 10, e115434. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.; Finlayson, G.; Dando, R. Sleep, Food Cravings and Taste. Appetite 2018, 125, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Li, J.; Cheng, F.W.; Cui, L.; Shu, R.; Wu, S.; Gao, X. Poor Sleep Quality Is Associated with Altered Taste Perception in Chinese Adults. J. Acad. Nutr. Diet. 2021, 121, 435–445. [Google Scholar] [CrossRef]
- Celis-Morales, C.; Lyall, D.M.; Guo, Y.; Steell, L.; Llanas, D.; Ward, J.; Mackay, D.F.; Biello, S.M.; Bailey, M.E.S.; Pell, J.P.; et al. Sleep Characteristics Modify the Association of Genetic Predisposition with Obesity and Anthropometric Measurements in 119,679 UK Biobank Participants. Am. J. Clin. Nutr. 2017, 105, 980–990. [Google Scholar] [CrossRef]
- Schröder, H.; Fitó, M.; Estruch, R.; Martínez-González, M.A.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventós, R.; Ros, E.; Salaverría, I.; Fiol, M.; et al. A Short Screener Is Valid for Assessing Mediterranean Diet Adherence among Older Spanish Men and Women. J. Nutr. 2011, 141, 1140–1145. [Google Scholar] [CrossRef]
- Zerón-Rugerio, M.F.; Cambras, T.; Izquierdo-Pulido, M. Social Jet Lag Associates Negatively with the Adherence to the Mediterranean Diet and Body Mass Index among Young Adults. Nutrients 2019, 11, 1756. [Google Scholar] [CrossRef]
- Corbalán-Tutau, M.D.; Madrid, J.A.; Garaulet, M. Timing and Duration of Sleep and Meals in Obese and Normal Weight Women. Association with Increase Blood Pressure. Appetite 2012, 59, 9–16. [Google Scholar] [CrossRef]
- Lopez-Minguez, J.; Dashti, H.S.; Madrid-Valero, J.J.; Madrid, J.A.; Saxena, R.; Scheer, F.A.J.L.; Ordoñana, J.R.; Garaulet, M. Heritability of the Timing of Food Intake. Clin. Nutr. 2019, 38, 767–773. [Google Scholar] [CrossRef]
- Huseinovic, E.; Winkvist, A.; Freisling, H.; Slimani, N.; Boeing, H.; Buckland, G.; Schwingshackl, L.; Olsen, A.; Tjonneland, A.; Stepien, M.; et al. Timing of Eating across Ten European Countries—Results from the European Prospective Investigation into Cancer and Nutrition (EPIC) Calibration Study. Public Health Nutr. 2019, 22, 324–335. [Google Scholar] [CrossRef]
- Castro-Diehl, C.; Wood, A.C.; Redline, S.; Reid, M.; Johnson, D.A.; Maras, J.E.; Jacobs, D.R.; Shea, S.; Crawford, A.; St-Onge, M.P. Mediterranean Diet Pattern and Sleep Duration and Insomnia Symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018, 41, zsy158. [Google Scholar] [CrossRef] [PubMed]
- Zuraikat, F.M.; Wood, R.A.; Barragán, R.; St-Onge, M.P. Sleep and Diet: Mounting Evidence of a Cyclical Relationship. Ann. Rev. Nutr. 2021, 41, 309–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, U.K.; Drayna, D. Genetics of Individual Differences in Bitter Taste Perception: Lessons from the PTC Gene. Clin. Genet. 2005, 67, 275–280. [Google Scholar] [CrossRef]
- Sandell, M.A.; Breslin, P.A.S. Variability in a Taste-Receptor Gene Determines Whether We Taste Toxins in Food. Curr. Biol. 2006, 16, PR792–PR794. [Google Scholar] [CrossRef] [PubMed]
- Wooding, S.P.; Ramirez, V.A.; Behrens, M. Bitter Taste Receptors: Genes, Evolution and Health. Evol. Med. Public Health 2021, 9, 431–447. [Google Scholar] [CrossRef] [PubMed]
- Gervis, J.E.; Fernández-Carrión, R.; Chui, K.K.H.; Ma, J.; Coltell, O.; Sorli, J.V.; Asensio, E.M.; Ortega-Azorín, C.; Pérez-Fidalgo, J.A.; Portolés, O.; et al. Associations between Taste Perception Profiles and Empirically Derived Dietary Patterns: An Exploratory Analysis among Older Adults with Metabolic Syndrome. Nutrients 2021, 14, 142. [Google Scholar] [CrossRef]
- Bell, K.I.; Tepper, B.J. Short-Term Vegetable Intake by Young Children Classified by 6-n-Propylthoiuracil Bitter-Taste Phenotype. Am. J. Clin. Nutr. 2006, 84, 245–251. [Google Scholar] [CrossRef]
- Yackinous, C.A.; Guinard, J.X. Relation between PROP (6-n-Propylthiouracil) Taster Status, Taste Anatomy and Dietary Intake Measures for Young Men and Women. Appetite 2002, 38, 201–209. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, S.A.; Feeney, E.L.; Scannell, A.G.M.; Markey, A.; Gibney, E.R. Bitter Taste Perception and Dietary Intake Patterns in Irish Children. J. Nutrigenet. Nutrigen. 2013, 6, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Garaulet, M.; Gómez-Abellán, P.; Alburquerque-Béjar, J.J.; Lee, Y.C.; Ordovás, J.M.; Scheer, F.A.J.L. Timing of Food Intake Predicts Weight Loss Effectiveness. Int. J. Obes. 2013, 37, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, T.; Tada, Y.; Hida, A.; Sunami, A.; Yokoyama, Y.; Yasuda, J.; Nakai, A.; Togo, F.; Kawano, Y. Effects of Feeding Schedule Changes on the Circadian Phase of the Cardiac Autonomic Nervous System and Serum Lipid Levels. Eur. J. Appl. Physiol. 2013, 113, 2603–2611. [Google Scholar] [CrossRef] [PubMed]
- Bandín, C.; Scheer, F.A.J.L.; Luque, A.J.; Ávila-Gandiá, V.; Zamora, S.; Madrid, J.A.; Gómez-Abellán, P.; Garaulet, M. Meal Timing Affects Glucose Tolerance, Substrate Oxidation and Circadian-Related Variables: A Randomized, Crossover Trial. Int. J. Obes. 2015, 39, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; van Cauter, E. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern Med. 2004, 141, 846–850. [Google Scholar] [CrossRef]
- de Castro, J.M. Heritability of Diurnal Changes in Food Intake in Free-Living Humans. Nutrition 2001, 17, 713–720. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Morris, C.J.; Shea, S.A. The Internal Circadian Clock Increases Hunger and Appetite in the Evening Independent of Food Intake and Other Behaviors. Obesity 2013, 21, 421–423. [Google Scholar] [CrossRef]
- Knutson, K.L.; Wu, D.; Patel, S.R.; Loredo, J.S.; Redline, S.; Cai, J.; Gallo, L.C.; Mossavar-Rahmani, Y.; Ramos, A.R.; Teng, Y.; et al. Association Between Sleep Timing, Obesity, Diabetes: The Hispanic Community Health Study/Study of Latinos (HCHS/SOL) Cohort Study. Sleep 2017, 40, zsx014. [Google Scholar] [CrossRef]
- Chaput, J.P.; Dutil, C.; Featherstone, R.; Ross, R.; Giangregorio, L.; Saunders, T.J.; Janssen, I.; Poitras, V.J.; Kho, M.E.; Ross-White, A.; et al. Sleep Timing, Sleep Consistency, and Health in Adults: A Systematic Review. Appl. Physiol. Nutr. Metab. 2020, 45, S232–S247. [Google Scholar] [CrossRef] [PubMed]
- Girtman, K.L.; Baylin, A.; O’Brien, L.M.; Jansen, E.C. Later Sleep Timing and Social Jetlag Are Related to Increased Inflammation in a Population with a High Proportion of OSA: Findings from the Cleveland Family Study. J. Clin. Sleep Med. 2022, 18, 2179–2187. [Google Scholar] [CrossRef]
- Zhai, Z.; Liu, X.; Zhang, H.; Dong, X.; He, Y.; Niu, M.; Pan, M.; Wang, C.; Wang, X.; Li, Y. Associations of Midpoint of Sleep and Night Sleep Duration with Type 2 Diabetes Mellitus in Chinese Rural Population: The Henan Rural Cohort Study. BMC Public Health 2021, 21, PR792–PR794. [Google Scholar] [CrossRef]
- Vetter, C.; Devore, E.E.; Ramin, C.A.; Speizer, F.E.; Willett, W.C.; Schernhammer, E.S. Mismatch of Sleep and Work Timing and Risk of Type 2 Diabetes. Diabetes Care 2015, 38, 1707–1713. [Google Scholar] [CrossRef]
- Kanerva, N.; Kronholm, E.; Partonen, T.; Ovaskainen, M.L.; Kaartinen, N.E.; Konttinen, H.; Broms, U.; Männistö, S. Tendency toward Eveningness Is Associated with Unhealthy Dietary Habits. Chronobiol. Int. 2012, 29, 920–927. [Google Scholar] [CrossRef]
- Kleiser, C.; Wawro, N.; Stelmach-Mardas, M.; Boeing, H.; Gedrich, K.; Himmerich, H.; Linseisen, J. Are Sleep Duration, Midpoint of Sleep and Sleep Quality Associated with Dietary Intake among Bavarian Adults? Eur. J. Clin. Nutr. 2017, 71, 631–637. [Google Scholar] [CrossRef]
- Garcia-Bailo, B.; Toguri, C.; Eny, K.M.; El-Sohemy, A. Genetic Variation in Taste and Its Influence on Food Selection. OMICS 2009, 13, 69–80. [Google Scholar] [CrossRef]
- McCaffery, J.M.; Papandonatos, G.D.; Peter, I.; Huggins, G.S.; Raynor, H.A.; Delahanty, L.M.; Cheskin, L.J.; Balasubramanyam, A.; Wagenknecht, L.E.; Wing, R.R. Obesity Susceptibility Loci and Dietary Intake in the Look AHEAD Trial. Am. J. Clin. Nutr. 2012, 95, 1477–1486. [Google Scholar] [CrossRef]
- Brunkwall, L.; Ericson, U.; Hellstrand, S.; Gullberg, B.; Orho-Melander, M.; Sonestedt, E. Genetic Variation in the Fat Mass and Obesity-Associated Gene (FTO) in Association with Food Preferences in Healthy Adults. Food Nutr. Res. 2013, 57, 20028. [Google Scholar] [CrossRef]
- Ortega, F.J.; Agüera, Z.; Sabater, M.; Moreno-Navarrete, J.M.; Alonso-Ledesma, I.; Xifra, G.; Botas, P.; Delgado, E.; Jimenez-Murcia, S.; Fernández-García, J.C.; et al. Genetic Variations of the Bitter Taste Receptor TAS2R38 Are Associated with Obesity and Impact on Single Immune Traits. Mol. Nutr. Food Res. 2016, 60, 1673–1683. [Google Scholar] [CrossRef]
- Chupeerach, C.; Tapanee, P.; On-Nom, N.; Temviriyanukul, P.; Chantong, B.; Reeder, N.; Adegoye, G.A.; Tolar-Peterson, T. The Influence of TAS2R38 Bitter Taste Gene Polymorphisms on Obesity Risk in Three Racially Diverse Groups. Biomedicine 2021, 11, 43–49. [Google Scholar] [CrossRef]
- Corella, D.; Ortega-Azorín, C.; Sorlí, J.V.; Covas, M.I.; Carrasco, P.; Salas-Salvadó, J.; Martínez-González, M.Á.; Arós, F.; Lapetra, J.; Serra-Majem, L.; et al. Statistical and Biological Gene-Lifestyle Interactions of MC4R and FTO with Diet and Physical Activity on Obesity: New Effects on Alcohol Consumption. PLoS ONE 2012, 7, e52344. [Google Scholar] [CrossRef] [PubMed]
- Frayling, T.M.; Timpson, N.J.; Weedon, M.N.; Zeggini, E.; Freathy, R.M.; Lindgren, C.M.; Perry, J.R.B.; Elliott, K.S.; Lango, H.; Rayner, N.W.; et al. A Common Variant in the FTO Gene Is Associated with Body Mass Index and Predisposes to Childhood and Adult Obesity. Science 2007, 316, 889–894. [Google Scholar] [CrossRef]
- Scuteri, A.; Sanna, S.; Chen, W.M.; Uda, M.; Albai, G.; Strait, J.; Najjar, S.; Nagaraja, R.; Orrú, M.; Usala, G.; et al. Genome-Wide Association Scan Shows Genetic Variants in the FTO Gene Are Associated with Obesity-Related Traits. PLoS Genet. 2007, 3, 1200–1210. [Google Scholar] [CrossRef]
- Garaulet, M.; Lee, Y.C.; Shen, J.; Parnell, L.D.; Arnett, D.K.; Tsai, M.Y.; Lai, C.Q.; Ordovas, J.M. CLOCK Genetic Variation and Metabolic Syndrome Risk: Modulation by Monounsaturated Fatty Acids. Am. J. Clin. Nutr. 2009, 90, 1466–1475. [Google Scholar] [CrossRef] [PubMed]
- Zerón-rugerio, M.F.; Longo-silva, G.; Hernáez, Á.; Ortega-regules, A.E.; Cambras, T.; Izquierdo-pulido, M. The Elapsed Time between Dinner and the Midpoint of Sleep Is Associated with Adiposity in Young Women. Nutrients 2020, 12, 410. [Google Scholar] [CrossRef]
- McHill, A.W.; Phillips, A.J.K.; Czeisler, C.A.; Keating, L.; Yee, K.; Barger, L.K.; Garaulet, M.; Scheer, F.A.J.L.; Klerman, E.B. Later Circadian Timing of Food Intake Is Associated with Increased Body Fat. Am. J. Clin. Nutr. 2017, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Minguez, J.; Saxena, R.; Bandín, C.; Scheer, F.A.; Garaulet, M. Late Dinner Impairs Glucose Tolerance in MTNR1B Risk Allele Carriers: A Randomized, Cross-over Study. Clin. Nutr. 2018, 37, 1133–1140. [Google Scholar] [CrossRef]
- Prats-Puig, A.; Grau-Cabrera, P.; Riera-Pérez, E.; Cortés-Marina, R.; Fortea, E.; Soriano-Rodríguez, P.; de Zegher, F.; Ibánez, L.; Bassols, J.; López-Bermejo, A. Variations in the Obesity Genes FTO, TMEM18 and NRXN3 Influence the Vulnerability of Children to Weight Gain Induced by Short Sleep Duration. Int. J. Obes. 2013, 37, 182–187. [Google Scholar] [CrossRef]
| Total (n = 412) | Male (n = 141) | Female (n = 271) | p | |
|---|---|---|---|---|
| Age (years) | 46.5 ± 13.9 | 45.6 ± 14.8 | 47.0 ± 13.4 | 0.333 |
| BMI (kg/m2) | 27.9 ± 5.2 | 29.0 ± 4.9 | 27.3 ± 5.3 | 0.002 |
| Waist circumference (cm) | 92.5 ± 15.1 | 101.0 ± 15.1 | 88.2 ± 13.2 | <0.001 |
| SBP (mm Hg) | 125.6 ± 17.2 | 131.1 ± 15.5 | 122.1 ± 17.1 | <0.001 |
| DBP (mm Hg) | 78.8 ± 10.1 | 82.0 ± 11.2 | 77.1 ± 9.0 | <0.001 |
| Total cholesterol (mg/dL) | 213.6 ± 41.0 | 207.8 ± 40.3 | 216.7 ± 40.1 | 0.036 |
| LDL cholesterol (mg/dL) | 139.5 ± 32.6 | 139.9 ± 32.6 | 139.3 ± 32.6 | 0.857 |
| HDL cholesterol (mg/dL) | 59.7 ± 14.2 | 51.2 ± 10.3 | 64.2 ± 13.9 | <0.001 |
| Triglycerides (mg/dL) | 112.4 ± 79.6 | 127.8 ± 91.9 | 104.4 ± 71.3 | 0.004 |
| Fasting glucose (mg/dL) | 95.0 ± 19.5 | 98.2 ± 23.4 | 93.4 ± 17.0 | 0.017 |
| Adherence to MedDiet | 8.5 ± 2.2 | 8.5 ± 2.0 | 8.5 ± 2.2 | 0.869 |
| Obesity cases (%) | 30.9 | 37.1 | 27.8 | 0.038 |
| Type 2 diabetes (%) | 4.5 | 5.8 | 3.7 | 0.235 |
| Current smokers (%) | 19.0 | 15.7 | 20.7 | 0.142 |
| Low physical activity (%) | 32 | 25.6 | 35.2 | 0.032 |
| Bitter (PTC) | 2.0 ± 1.6 | 1.6 ± 1.5 | 2.2 ± 1.6 | <0.001 |
| Sweet | 1.9 ± 1.1 | 1.7 ± 0.9 | 2.0 ± 1.2 | 0.005 |
| Salty | 2.6 ± 1.3 | 2.3 ± 1.3 | 2.7 ± 1.3 | 0.009 |
| Sour | 2.7 ± 1.3 | 2.4 ± 1.3 | 2.9 ± 1.4 | <0.001 |
| Umami | 2.0 ± 1.4 | 1.9 ± 1.3 | 2.0 ± 1.4 | 0.744 |
| Total (n = 412) | Male (n = 141) | Female (n = 271) | p | |
|---|---|---|---|---|
| Sleep duration (h) | 7.8 ± 0.9 | 7.7 ± 1.0 | 7.8 ± 0.8 | 0.542 |
| Waketime (hour:min) | 7:10 ± 0:53 | 7:05 ± 0:54 | 7:13 ± 0:52 | 0.255 |
| Sleep midpoint (hour:min) | 4:13 ± 0:45 | 04:18 ± 0:54 | 04:10 ± 0:40 | 0.233 |
| Social jetlag | 1.4 ± 1.0 | 1.3 ± 1.0 | 1.4 ± 1.0 | 0.427 |
| Total (n = 302) | Male (n = 110) | Female (n = 192) | p | |
|---|---|---|---|---|
| Eating window (h) | 13.3 ± 1.0 | 13.3 ± 1.0 | 13.3 ± 1.0 | 0.785 |
| Eating midpoint (hour:min) | 14:53 ± 0:34 | 14:54 ± 0:37 | 14:50 ± 0:32 | 0.114 |
| Eating jetlag | 1.0 ± 0.7 | 1.0 ± 0.8 | 1.0 ± 0.6 | 0.830 |
| Breakfast time (hour:min) | 8:10 ± 0:50 | 8:06 ± 0:49 | 8:11 ± 0:50 | 0.488 |
| Mid-morning snack (hour:min) | 10:53 ± 0:33 | 10:38 ± 0:35 | 11:01 ± 0:29 | <0.001 |
| Lunch time (hour:min) | 14:39 ± 0:35 | 14:34 ± 0:32 | 14:42 ± 0:36 | 0.041 |
| Evening tea time (hour:min) | 18:07 ± 0:35 | 18:10 ± 0:36 | 18:05 ± 0:5 | 0.464 |
| Dinner time (hour:min) | 21:31 ± 0:34 | 21:35 ± 0:35 | 21:28 ± 0:35 | 0.080 |
| Taste (Predictor) | Eating Pattern (Outcome) | β ± SE | p1 | p2 |
|---|---|---|---|---|
| Bitter (PTC) | Eating window | 0.03 ± 0.04 | 0.380 | 0.476 |
| Eating midpoint | −0.05 ± 0.02 | 0.009 | 0.001 | |
| Eating jetlag | 0.05 ± 0.03 | 0.042 | 0.501 | |
| Breakfast time | −0.06 ± 0.03 | 0.057 | 0.043 | |
| Mid-morning snack | 0.05 ± 0.03 | 0.126 | 0.678 | |
| Lunch time | −0.03 ± 0.02 | 0.141 | 0.105 | |
| Afternoon teatime | −0.02 ± 0.03 | 0.577 | 0.523 | |
| Dinner time | −0.04 ± 0.02 | 0.061 | 0.009 | |
| Adherence to MedDiet | −0.08 ± 0.07 | 0.058 | 0.249 | |
| Sweet | Eating window | −0.08 ± 0.05 | 0.140 | 0.081 |
| Eating midpoint | 0.08 ± 0.03 | 0.011 | 0.030 | |
| Eating jetlag | 0.13 ± 0.04 | <0.001 | 0.031 | |
| Breakfast time | 0.14 ± 0.05 | 0.002 | 0.002 | |
| Mid-morning snack | 0.08 ± 0.05 | 0.092 | 0.610 | |
| Lunch time | 0.01 ± 0.03 | 0.661 | 0.711 | |
| Afternoon teatime | 0.08 ± 0.04 | 0.071 | 0.100 | |
| Dinner time | 0.04 ± 0.03 | 0.190 | 0.430 | |
| Adherence to MedDiet | 0.23 ± 0.10 | 0.106 | 0.020 | |
| Salty | Eating window | −0.02 ± 0.04 | 0.567 | 0.477 |
| Eating midpoint | 0.04 ± 0.02 | 0.087 | 0.147 | |
| Eating jetlag | 0.07 ± 0.03 | 0.024 | 0.178 | |
| Breakfast time | 0.07 ± 0.04 | 0.063 | 0.063 | |
| Mid-morning snack | 0.01 ± 0.04 | 0.723 | 0.337 | |
| Lunch time | 0.03 ± 0.03 | 0.267 | 0.315 | |
| Afternoon teatime | 0.06 ± 0.04 | 0.128 | 0.148 | |
| Dinner time | 0.03 ± 0.02 | 0.308 | 0.469 | |
| Adherence to MedDiet | 0.10 ± 0.08 | 0.433 | 0.233 | |
| Sour | Eating window | −0.01 ± 0.04 | 0.786 | 0.630 |
| Eating midpoint | 0.04 ± 0.03 | 0.086 | 0.207 | |
| Eating jetlag | 0.09 ± 0.03 | 0.005 | 0.164 | |
| Breakfast time | 0.07 ± 0.04 | 0.048 | 0.048 | |
| Mid-morning snack | 0.05 ± 0.04 | 0.168 | 0.928 | |
| Lunch time | 0.04 ± 0.03 | 0.080 | 0.090 | |
| Afternoon teatime | 0.02 ± 0.04 | 0.562 | 0.672 | |
| Dinner time | 0.03 ± 0.03 | 0.257 | 0.552 | |
| Adherence to MedDiet | 0.15 ± 0.08 | 0.267 | 0.067 | |
| Umami | Eating window | −0.03 ± 0.04 | 0.502 | 0.365 |
| Eating midpoint | 0.05 ± 0.02 | 0.030 | 0.127 | |
| Eating jetlag | 0.03 ± 0.03 | 0.270 | 0.539 | |
| Breakfast time | 0.08 ± 0.04 | 0.036 | 0.031 | |
| Mid-morning snack | 0.08 ± 0.04 | 0.037 | 0.261 | |
| Lunch time | −0.01 ± 0.02 | 0.654 | 0.759 | |
| Afternoon teatime | 0.02 ± 0.04 | 0.654 | 0.832 | |
| Dinner time | 0.03 ± 0.02 | 0.221 | 0.656 | |
| Adherence to MedDiet | 0.11 ± 0.08 | 0.464 | 0.159 |
| Gene (Predictor) | Eating Pattern (Outcome) | β ± SE | p1 | p2 |
|---|---|---|---|---|
| TAS2R38-rs713598 | Eating window | 0.13 ± 0.09 | 0.155 | 0.163 |
| Eating midpoint | −0.13 ± 0.05 | 0.011 | 0.008 | |
| Eating jetlag | 0.00 ± 0.06 | 0.988 | 0.831 | |
| Breakfast time | −0.17 ± 0.08 | 0.029 | 0.029 | |
| Mid-morning snack | 0.12 ± 0.08 | 0.126 | 0.265 | |
| Lunch time | −0.07 ± 0.05 | 0.204 | 0.205 | |
| Afternoon teatime | 0.05 ± 0.08 | 0.554 | 0.495 | |
| Dinner time | −0.06 ± 0.05 | 0.277 | 0.218 | |
| Adherence to MedDiet | −0.09 ± 0.17 | 0.580 | 0.707 | |
| FTO-rs9939609 | Eating window | −0.14 ± 0.08 | 0.076 | 0.063 |
| Eating midpoint | 0.04 ± 0.05 | 0.442 | 0.669 | |
| Eating jetlag | −0.1 ± 0.06 | 0.813 | 0.365 | |
| Breakfast time | 0.09 ± 0.07 | 0.210 | 0.197 | |
| Mid-morning snack | 0.11 ± 0.06 | 0.084 | 0.336 | |
| Lunch time | −0.11 ± 0.05 | 0.014 | 0.021 | |
| Afternoon teatime | −0.13 ± 0.07 | 0.046 | 0.038 | |
| Dinner time | −0.05 ± 0.05 | 0.319 | 0.146 | |
| Adherence to MedDiet | −0.16 ± 0.15 | 0.300 | 0.471 | |
| CLOCK-rs4580704 | Eating window | 0.04 ± 0.08 | 0.627 | 0.684 |
| Eating midpoint | −0.03 ± 0.05 | 0.491 | 0.281 | |
| Eating jetlag | 0.07 ± 0.06 | 0.254 | 0.459 | |
| Breakfast time | −0.02 ± 0.07 | 0.759 | 0.787 | |
| Mid-morning snack | −0.05 ± 0.08 | 0.519 | 0.536 | |
| Lunch time | 0.02 ± 0.05 | 0.653 | 0.522 | |
| Afternoon teatime | −0.07 ± 0.07 | 0.332 | 0.268 | |
| Dinner time | 0.00 ± 0.05 | 0.928 | 0.747 | |
| Adherence to MedDiet | 0.09 ± 0.17 | 0.576 | 0.435 |
| Taste (Predictor) | Sleep Pattern (Outcome) | β ± SE | p1 | p2 |
|---|---|---|---|---|
| Bitter (PTC) | Waketime | −0.06 ± 0.03 | 0.020 | <0.001 |
| Total Sleep Duration | 0.02 ± 0.03 | 0.594 | 0.875 | |
| Midpoint of sleep | −0.06 ± 0.03 | 0.034 | 0.009 | |
| Social jetlag | 0.06 ± 0.04 | 0.110 | 0.766 | |
| Sweet | Waketime | 0.06 ± 0.04 | 0.128 | 0.465 |
| Total Sleep Duration | −0.01 ± 0.05 | 0.811 | 0.426 | |
| Midpoint of sleep | 0.01 ± 0.04 | 0.894 | 0.990 | |
| Social jetlag | 0.10 ± 0.06 | 0.070 | 0.341 | |
| Salty | Waketime | −0.01 ± 0.03 | 0.850 | 0.425 |
| Total Sleep Duration | −0.05 ± 0.04 | 0.256 | 0.157 | |
| Midpoint of sleep | −0.02 ± 0.04 | 0.642 | 0.635 | |
| Social jetlag | 0.13 ± 0.04 | 0.004 | 0.009 | |
| Sour | Waketime | 0.03 ± 0.03 | 0.374 | 0.984 |
| Total Sleep Duration | 0.00 ± 0.04 | 0.994 | 0.586 | |
| Midpoint of sleep | 0.02 ± 0.04 | 0.483 | 0.617 | |
| Social jetlag | 0.18 ± 0.04 | <0.001 | 0.002 | |
| Umami | Waketime | 0.01 ± 0.03 | 0.780 | 0.575 |
| Total Sleep Duration | −0.05 ± 0.04 | 0.254 | 0.127 | |
| Midpoint of sleep | 0.04 ± 0.04 | 0.252 | 0.393 | |
| Social jetlag | 0.09 ± 0.05 | 0.051 | 0.258 |
| Gene (Predictor) | Sleep Pattern (Outcome) | β ± SE | p1 | p2 |
|---|---|---|---|---|
| TAS2R38-rs713598 | Waketime | −0.14 ± 0.07 | 0.036 | 0.024 |
| Total Sleep Duration | 0.01 ± 0.08 | 0.933 | 0.783 | |
| Midpoint of sleep | −0.14 ± 0.09 | 0.118 | 0.019 | |
| Social jetlag | 0.02 ± 0.09 | 0.865 | 0.592 | |
| FTO-rs9939609 | Waketime | 0.15 ± 0.06 | 0.015 | 0.039 |
| Total Sleep Duration | 0.08 ± 0.07 | 0.304 | 0.295 | |
| Midpoint of sleep | 0.18 ± 0.06 | 0.005 | 0.008 | |
| Social jetlag | −0.15 ± 0.08 | 0.061 | 0.028 | |
| CLOCK-rs4580704 | Waketime | 0.03 ± 0.07 | 0.603 | 0.787 |
| Total Sleep Duration | −0.07 ± 0.08 | 0.433 | 0.398 | |
| Midpoint of sleep | 0.11 ± 0.07 | 0.147 | 0.194 | |
| Social jetlag | 0.07 ± 0.09 | 0.425 | 0.570 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barragán, R.; Fernández-Carrión, R.; Asensio-Márquez, E.M.; Ortega-Azorín, C.; Álvarez-Sala, A.; Pérez-Fidalgo, A.; Sorlí, J.V.; Portolés, O.; González-Monje, I.; St-Onge, M.P.; et al. Timing of Meals and Sleep in the Mediterranean Population: The Effect of Taste, Genetics, Environmental Determinants, and Interactions on Obesity Phenotypes. Nutrients 2023, 15, 708. https://doi.org/10.3390/nu15030708
Barragán R, Fernández-Carrión R, Asensio-Márquez EM, Ortega-Azorín C, Álvarez-Sala A, Pérez-Fidalgo A, Sorlí JV, Portolés O, González-Monje I, St-Onge MP, et al. Timing of Meals and Sleep in the Mediterranean Population: The Effect of Taste, Genetics, Environmental Determinants, and Interactions on Obesity Phenotypes. Nutrients. 2023; 15(3):708. https://doi.org/10.3390/nu15030708
Chicago/Turabian StyleBarragán, Rocío, Rebeca Fernández-Carrión, Eva María Asensio-Márquez, Carolina Ortega-Azorín, Andrea Álvarez-Sala, Alejandro Pérez-Fidalgo, José Vicente Sorlí, Olga Portolés, Inmaculada González-Monje, Marie Pierre St-Onge, and et al. 2023. "Timing of Meals and Sleep in the Mediterranean Population: The Effect of Taste, Genetics, Environmental Determinants, and Interactions on Obesity Phenotypes" Nutrients 15, no. 3: 708. https://doi.org/10.3390/nu15030708
APA StyleBarragán, R., Fernández-Carrión, R., Asensio-Márquez, E. M., Ortega-Azorín, C., Álvarez-Sala, A., Pérez-Fidalgo, A., Sorlí, J. V., Portolés, O., González-Monje, I., St-Onge, M. P., & Corella, D. (2023). Timing of Meals and Sleep in the Mediterranean Population: The Effect of Taste, Genetics, Environmental Determinants, and Interactions on Obesity Phenotypes. Nutrients, 15(3), 708. https://doi.org/10.3390/nu15030708











