Dietary Responses of Dementia-Related Genes Encoding Metabolic Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. MD Genes
2.2. Gene Expression Data and Transcription Analysis
2.3. Correlation between MD Gene Expression and Dietary and Lifestyle Factors
2.4. Gene–Diet Interactions
3. Results
3.1. MD Genes as Aging Genes, as Rate-Limited Enzymes
3.1.1. Aging Genes
3.1.2. Rate-Limited Enzymes
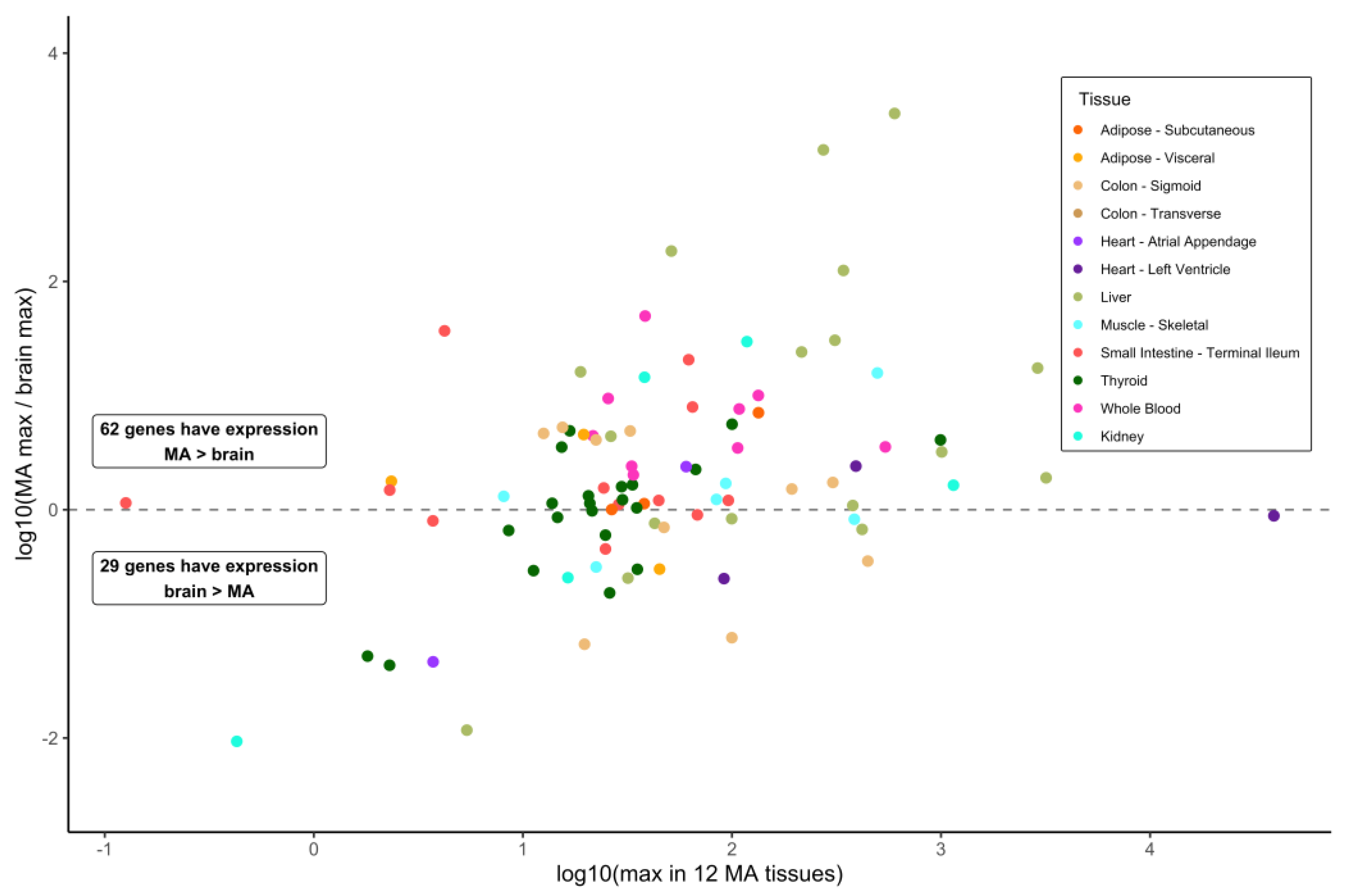
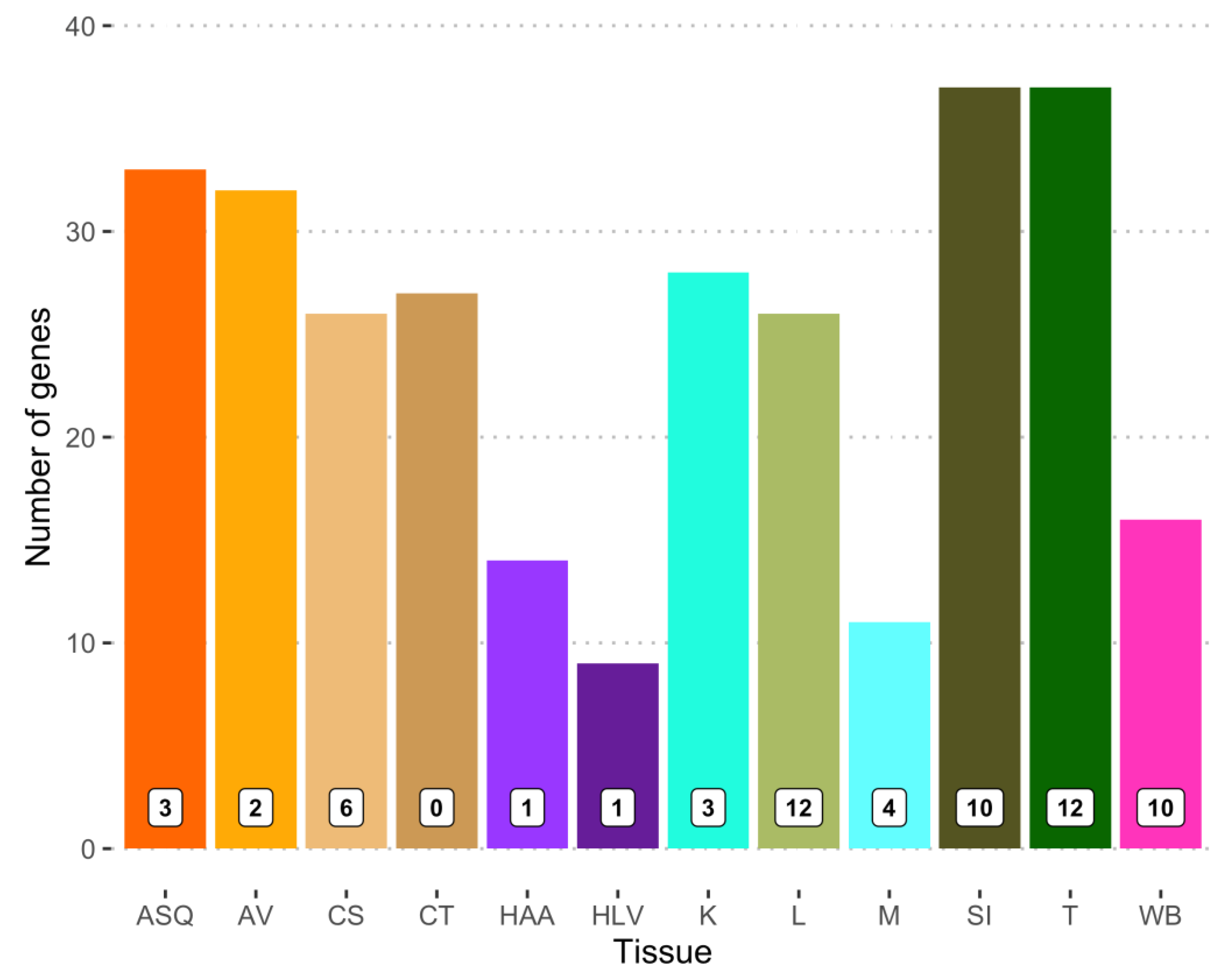
3.2. Gene Expression in Brain Sections and Selected Metabolically Active Tissues
3.3. Correlations of MD Gene Expression in Blood with Dietary Intake, Age, and Sex
3.3.1. Effects of Dietary Intake on MD Gene Expression
3.3.2. Age and Sex Effects on MD Gene Expression
3.4. eQTL Signals in Blood and the Allele-Specific Response to Dietary Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grand, J.H.; Caspar, S.; MacDonald, S.W. Clinical features and multidisciplinary approaches to dementia care. J. Multidiscip. Healthc. 2011, 4, 125–147. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Feldman, H.; Fillit, H.; Sano, M.; Schmitt, F.; Aisen, P.; Leibman, C.; Mucha, L.; Ryan, J.M.; Sullivan, S.D.; et al. Dependence as a unifying construct in defining Alzheimer’s disease severity. Alzheimers Dement. 2010, 6, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimers Dement. 2022, 18, 700–789. [Google Scholar] [CrossRef]
- Navarrete, E.; Prospéro, O.; Hudson, R.; Guevara, R. Enfermedades neurodegenerativas que cursan con demencia. Gac. Med. Mex. 2000, 136, 573–584. [Google Scholar] [PubMed]
- Petit, D.; Montplaisir, J.; Boeve, B.F. Chapter 91—Alzheimer’s disease and other dementias. In Principles and Practice of Sleep Medicine, 5th ed.; Kryger, M.H., Roth, T., Dement, W.C., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2011; pp. 1038–1047. ISBN 9781416066453. [Google Scholar] [CrossRef]
- Kramarow, E.A.; Tejada-Vera, B. Dementia Mortality in the United States, 2000–2017. Natl. Vital Stat. Rep. 2019, 68, 1–29. [Google Scholar]
- GBD 2019 Collaborators. Global mortality from dementia: Application of a new method and results from the Global Burden of Disease Study. Alzheimers Dement. 2021, 7, e12200. [Google Scholar] [CrossRef]
- Stokes, A.C.; Weiss, J.; Lundberg, D.J.; Xie, W.; Kim, J.K.; Preston, S.H.; Crimmins, E.M. Estimates of the association of dementia with US mortality levels using linked survey and mortality records. JAMA Neurol. 2020, 77, 1543–1550. [Google Scholar] [CrossRef]
- Hou, X.H.; Feng, L.; Zhang, C.; Cao, X.P.; Tan, L.; Yu, J.T. Models for predicting risk of dementia: A systematic review. J. Neurol Neurosurg. Psychiatry 2019, 90, 373–379. [Google Scholar] [CrossRef]
- McCullagh, C.D.; Craig, D.; McIlroy, S.P.; Passmore, A.P. Risk factors for dementia. Adv. Psychiatr. Treat. 2018, 7, 24–31. [Google Scholar] [CrossRef]
- van der Flier, W.M.; Scheltens, P. Epidemiology and risk factors of dementia. J. Neurol. Neurosurg. Psychiatry 2005, 76 (Suppl. 5), v2–v7. [Google Scholar] [CrossRef]
- Palacios, N.; Lee, J.S.; Scott, T.; Kelly, R.S.; Bhupathiraju, S.N.; Bigornia, S.J.; Tucker, K.L. Circulating plasma metabolites and cognitive function in a Puerto Rican cohort. J. Alzheimers Dis. 2020, 76, 1267–1280. [Google Scholar] [CrossRef] [PubMed]
- Eubank, J.M.; Oberlin, D.J.; Alto, A.; Sahyoun, N.R.; Asongwed, E.; Monroe-Lord, L.; Harrison, E.A. Effects of lifestyle factors on cognition in minority population of older adults: A review. Front. Nutr. 2022, 9, 841070. [Google Scholar] [CrossRef] [PubMed]
- Kornblith, E.; Bahorik, A.; Boscardin, W.J.; Xia, F.; Barnes, D.E.; Yaffe, K. Association of race and ethnicity with incidence of dementia among older adults. JAMA 2022, 327, 1488–1495. [Google Scholar] [CrossRef]
- Tahmi, M.; Palta, P.; Luchsinger, J.A. Metabolic syndrome and cognitive function. Curr. Cardiol. Rep. 2021, 23, 180. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and human aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef]
- Yu, W.W.; Randhawa, A.K.; Blair, S.N.; Sui, X.; Kuk, J.L. Age- and sex-specific all-cause mortality risk greatest in metabolic syndrome combinations with elevated blood pressure from 7 U.S. cohorts. PLoS ONE 2019, 14, e0218307. [Google Scholar] [CrossRef]
- Tangen, G.G.; Robinson, H.S. Measuring physical performance in highly active older adults: Associations with age and gender? Aging Clin. Exp. Res. 2020, 32, 229–237. [Google Scholar] [CrossRef]
- Smiley, A.; King, D.; Bidulescu, A. The association between sleep duration and metabolic syndrome: The NHANES 2013/2014. Nutrients 2019, 11, 2582. [Google Scholar] [CrossRef]
- Landrum, M.J.; Chitipiralla, S.; Brown, G.R.; Chen, C.; Gu, B.; Hart, J.; Hoffman, D.; Jang, W.; Kaur, K.; Liu, C.; et al. ClinVar: Improvements to accessing data. Nucleic Acids Res. 2020, 48, D835–D844. [Google Scholar] [CrossRef]
- Buniello, A.; MacArthur, J.A.L.; Cerezo, M.; Harris, L.W.; Hayhurst, J.; Malangone, C.; McMahon, A.; Morales, J.; Mountjoy, E.; Sollis, E.; et al. The NHGRI-EBI GWAS Catalog of published genome-wide association studies, targeted arrays and summary statistics. Nucleic Acids Res. 2019, 47, D1005–D1012. [Google Scholar] [CrossRef]
- Robinson, P.K. Enzymes: Principles and biotechnological applications. Essays Biochem. 2015, 59, 1–41. [Google Scholar] [CrossRef]
- Gildawie, K.R.; Galli, R.L.; Shukitt-Hale, B.; Carey, A.N. Protective effects of foods containing flavonoids on age-related cognitive decline. Curr. Nutr. Rep. 2018, 7, 39–48. [Google Scholar] [CrossRef]
- Shinjyo, N.; Kita, K. Infection and immunometabolism in the central nervous system: A possible mechanistic link between metabolic imbalance and dementia. Front. Cell Neurosci. 2021, 15, 765217. [Google Scholar] [CrossRef] [PubMed]
- Mietelska-Porowska, A.; Domańska, J.; Want, A.; Więckowska-Gacek, A.; Chutorański, D.; Koperski, M.; Wojda, U. Induction of brain insulin resistance and Alzheimer’s molecular changes by Western diet. Int. J. Mol. Sci. 2022, 23, 4744. [Google Scholar] [CrossRef]
- Melo van Lent, D.; Gokingco, H.; Short, M.I.; Yuan, C.; Jacques, P.F.; Romero, J.R.; DeCarli, C.S.; Beiser, A.S.; Seshadri, S.; Himali, J.J.; et al. Higher Dietary Inflammatory Index scores are associated with brain MRI markers of brain aging: Results from the Framingham Heart Study Offspring cohort. Alzheimers Dement. 2022, 6, 12685. [Google Scholar] [CrossRef]
- Vu, T.H.T.; Beck, T.; Bennett, D.A.; Schneider, J.A.; Hayden, K.M.; Shadyab, A.H.; Rajan, K.B.; Morris, M.C.; Cornelis, M.C. Adherence to MIND diet, genetic susceptibility, and incident dementia in three US cohorts. Nutrients 2022, 14, 2759. [Google Scholar] [CrossRef]
- Melo van Lent, D.; O’Donnell, A.; Beiser, A.S.; Vasan, R.S.; DeCarli, C.S.; Scarmeas, N.; Wagner, M.; Jacques, P.F.; Seshadri, S.; Himali, J.J.; et al. Mind diet adherence and cognitive performance in the Framingham Heart Study. J. Alzheimers Dis. 2021, 82, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Moat, S.J.; George, R.S.; Carling, R.S. Use of dried blood spot specimens to monitor patients with inherited metabolic disorders. Int. J. Neonatal. Screen. 2020, 6, 26. [Google Scholar] [CrossRef]
- Duarte, J.M.N.; Schuck, P.F.; Wenk, G.L.; Ferreira, G.C. Metabolic disturbances in diseases with neurological involvement. Aging Dis. 2013, 5, 238–255. [Google Scholar] [CrossRef]
- Pietz, J.; Rupp, A.; Ebinger, F.; Rating, D.; Mayatepek, E.; Boesch, C.; Kreis, R. Cerebral energy metabolism in phenylketonuria: Findings by quantitative in vivo 31P MR spectroscopy. Pediatr. Res. 2003, 53, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.d.C.; Viegas, C.M.; Schuck, P.F.; Latini, A.; Dutra-Filho, C.S.; Wyse, A.T.S.; Wannmacher, C.M.D.; Vargas, C.R.; Wajner, M. Glutaric acid moderately compromises energy metabolism in rat brain. Int. J. Dev. Neurosci. 2005, 23, 687–693. [Google Scholar] [CrossRef]
- van Wegberg, A.M.J.; MacDonald, A.; Ahring, K.; Bélanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Giżewska, M.; et al. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet. J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef]
- Strauss, K.A.; Williams, K.B.; Carson, V.J.; Poskitt, L.; Bowser, L.E.; Young, M.; Robinson, D.L.; Hendrickson, C.; Beiler, K.; Taylor, C.M.; et al. Glutaric acidemia type 1: Treatment and outcome of 168 patients over three decades. Mol. Genet. Metab. 2020, 131, 325–340. [Google Scholar] [CrossRef]
- Montfort, M.; Chabás, A.; Vilageliu, L.; Grinberg, D. Functional analysis of 13 GBA mutant alleles identified in Gaucher disease patients: Pathogenic changes and “modifier” polymorphisms. Hum. Mutat. 2004, 23, 567–575. [Google Scholar] [CrossRef]
- Van Kampen, J.M.; Baranowski, D.C.; Robertson, H.A.; Shaw, C.A.; Kay, D.G. The progressive BSSG rat model of Parkinson’s: Recapitulating multiple key features of the human disease. PLoS ONE 2015, 10, e0139694. [Google Scholar] [CrossRef]
- Gannon, O.J.; Robison, L.S.; Salinero, A.E.; Abi-Ghanem, C.; Mansour, F.M.; Kelly, R.D.; Tyagi, A.; Brawley, R.R.; Ogg, J.D.; Zuloaga, K.L. High-fat diet exacerbates cognitive decline in mouse models of Alzheimer’s disease and mixed dementia in a sex-dependent manner. J. Neuroinflammation 2022, 19, 110. [Google Scholar] [CrossRef]
- Townsend, R.F.; Woodside, J.V.; Prinelli, F.; O’Neill, R.F.; McEvoy, C.T. Associations between dietary patterns and neuroimaging markers: A systematic review. Front. Nutr. 2022, 9, 806006. [Google Scholar] [CrossRef]
- Train the Brain Consortium. Randomized trial on the effects of a combined physical/cognitive training in aged MCI subjects: The Train the Brain study. Sci. Rep. 2017, 7, 39471. [Google Scholar] [CrossRef] [PubMed]
- Balbim, G.M.; Falck, R.S.; Barha, C.K.; Starkey, S.Y.; Bullock, A.; Davis, J.C.; Liu-Ambrose, T. Effects of exercise training on the cognitive function of older adults with different types of dementia: A systematic review and meta-analysis. Br. J. Sports Med. 2022, 56, 933–940. [Google Scholar] [CrossRef] [PubMed]
- Penninkilampi, R.; Casey, A.N.; Fiatarone Singh, M.; Brodaty, H. The association between social engagement, loneliness, and risk of dementia: A systematic review and meta-analysis. J. Alzheimers Dis. 2018, 66, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Rolls, E.; Cheng, W.; Kang, J.; Dong, G.; Xie, C.; Zhao, X.M.; Sahakian, B.; Feng, J. Associations of social isolation and loneliness with later dementia. Neurology 2022, 99, e164–e175. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, C.C.; Grady, C.R.; Pisitkun, T.; Parulekar, J.; Knepper, M.A. From 20th century metabolic wall charts to 21st century systems biology: Database of mammalian metabolic enzymes. Am. J. Physiol. Renal. Physiol. 2017, 312, F533–F542. [Google Scholar] [CrossRef]
- Bairoch, A. The ENZYME database in 2000. Nucleic Acids Res. 2000, 28, 304–305. [Google Scholar] [CrossRef] [PubMed]
- Online Mendelian Inheritance in Man, OMIM®. McKusick-Nathans Institute of Genetic Medicine, Johns Hopkins University (Baltimore, MD). Available online: https://omim.org/ (accessed on 9 April 2021).
- Zhao, M.; Chen, X.; Gao, G.; Tao, L.; Wei, L. RLEdb: A database of rate-limiting enzymes and their regulation in human, rat, mouse, yeast and E. coli. Cell Res. 2009, 19, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.Q.; Parnell, L.D.; Lee, Y.C.; Zeng, H.; Smith, C.E.; McKeown, N.M.; Ordovás, J.M. The impact of alcoholic drinks and dietary factors on epigenetic markers associated with triglyceride levels. Clin. Epigenetics 2022. submitted. [Google Scholar]
- Lai, C.Q.; Parnell, L.D.; Smith, C.E.; Guo, T.; Sayols-Baixeras, S.; Aslibekyan, S.; Tiwari, H.K.; Irvin, M.R.; Bender, C.; Fei, D.; et al. Carbohydrate and fat intake associated with risk of metabolic diseases through epigenetics of CPT1A. Am. J. Clin. Nutr. 2020, 112, 1200–1211. [Google Scholar] [CrossRef]
- Tacutu, R.; Thornton, D.; Johnson, E.; Budovsky, A.; Barardo, D.; Craig, T.; Diana, E.; Lehmann, G.; Toren, D.; Wang, J.; et al. Human Ageing Genomic Resources: New and updated databases. Nucleic Acids Res. 2018, 46, D1083–D1090. [Google Scholar] [CrossRef]
- Tanaka, T.; Basisty, N.; Fantoni, G.; Candia, J.; Moore, A.Z.; Biancotto, A.; Schilling, B.; Bandinelli, S.; Ferrucci, L. Plasma proteomic biomarker signature of age predicts health and life span. Elife 2020, 9, e61073. [Google Scholar] [CrossRef]
- Parnell, L.D.; McCaffrey, K.S.; Brooks, A.W.; Smith, C.E.; Lai, C.Q.; Christensen, J.J.; Wiley, C.D.; Ordovás, J.M. Rate-limiting enzymes in cardiometabolic health and aging in humans. Lifestyle Genom. 2022. submitted. [Google Scholar]
- Parnell, L.D.; Blokker, B.A.; Dashti, H.S.; Nesbeth, P.D.; Cooper, B.E.; Ma, Y.; Lee, Y.C.; Hou, R.; Lai, C.Q.; Richardson, K.; et al. CardioGxE, a catalog of gene-environment interactions for cardiometabolic traits. BioData Min. 2014, 7, 21. [Google Scholar] [CrossRef]
- Kramer, W.; Stengelin, S.; Baringhaus, K.H.; Enhsen, A.; Heuer, H.; Becker, W.; Corsiero, D.; Girbig, F.; Noll, R.; Weyland, C. Substrate specificity of the ileal and the hepatic Na(+)/bile acid cotransporters of the rabbit. I. Transport studies with membrane vesicles and cell lines expressing the cloned transporters. J. Lipid Res. 1999, 40, 1604–1617. [Google Scholar] [CrossRef]
- Ordovás, J.M.; Ferguson, L.R.; Tai, E.S.; Mathers, J.C. Personalised nutrition and health. BMJ 2018, 2018, 361. [Google Scholar] [CrossRef]
- Kondoh, H.; Teruya, T.; Kameda, M.; Yanagida, M. Decline of ergothioneine in frailty and cognition impairment. FEBS Lett. 2022, 596, 1270–1278. [Google Scholar] [CrossRef] [PubMed]
- Minocha, T.; Birla, H.; Obaid, A.A.; Rai, V.; Sushma, P.; Shivamallu, C.; Moustafa, M.; Al-Shehri, M.; Al-Emam, A.; Tikhonova, M.A.; et al. Flavonoids as promising neuroprotectants and their therapeutic potential against Alzheimer’s disease. Oxid. Med. Cell Longev. 2022, 2022, 6038996. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Y.; Readhead, B.; Chen, K.; Su, Y.; Reiman, E.M.; Dudley, J.T. Longitudinal data in peripheral blood confirm that PM20D1 is a quantitative trait locus (QTL) for Alzheimer’s disease and implicate its dynamic role in disease progression. Clin. Epigenetics 2020, 12, 189. [Google Scholar] [CrossRef]
- Jiménez-Jiménez, F.J.; Alonso-Navarro, H.; García-Martín, E.; Agúndez, J.A.G. Cerebrospinal and blood levels of amino acids as potential biomarkers for Parkinson’s disease: Review and meta-analysis. Eur. J. Neurol. 2020, 27, 2336–2347. [Google Scholar] [CrossRef]
- Parikh, N.S.; Kamel, H.; Zhang, C.; Kumar, S.; Rosenblatt, R.; Spincemaille, P.; Gupta, A.; Cohen, D.E.; de Leon, M.J.; Gottesman, R.F.; et al. Association between liver fibrosis and incident dementia in the UK Biobank study. Eur. J. Neurol. 2022, 29, 2622–2630. [Google Scholar] [CrossRef] [PubMed]
- Keene, C.D.; Rodrigues, C.M.P.; Eich, T.; Chhabra, M.S.; Steer, C.J.; Low, W.C. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10671–10676. [Google Scholar] [CrossRef] [PubMed]
- Cuevas, E.; Burks, S.; Raymick, J.; Robinson, B.; Gómez-Crisóstomo, N.P.; Escudero-Lourdes, C.; Guzman Lopez, A.G.; Chigurupati, S.; Hanig, J.; Ferguson, S.A.; et al. Tauroursodeoxycholic acid (TUDCA) is neuroprotective in a chronic mouse model of Parkinson’s disease. Nutr. Neurosci. 2022, 25, 1374–1391. [Google Scholar] [CrossRef]
- Dionísio, P.A.; Amaral, J.D.; Ribeiro, M.F.; Lo, A.C.; D’Hooge, R.; Rodrigues, C.M.P. Amyloid-β pathology is attenuated by tauroursodeoxycholic acid treatment in APP/PS1 mice after disease onset. Neurobiol. Aging 2015, 36, 228–240. [Google Scholar] [CrossRef]
- Kiser, C.; Gonul, C.P.; Olcum, M.; Genc, S. Inhibitory effects of sulforaphane on NLRP3 inflammasome activation. Mol. Immunol. 2021, 140, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.; Islam, T.; Zaman, T.; Shahjaman, M.; Karim, M.R.; Huq, F.; Quinn, J.M.W.; Holsinger, R.M.D.; Gov, E.; Moni, M.A. Identification of molecular signatures and pathways to identify novel therapeutic targets in Alzheimer’s disease: Insights from a systems biomedicine perspective. Genomics 2020, 112, 1290–1299. [Google Scholar] [CrossRef] [PubMed]
- Giannos, P.; Prokopidis, K.; Raleigh, S.M.; Kelaiditi, E.; Hill, M. Altered mitochondrial microenvironment at the spotlight of musculoskeletal aging and Alzheimer’s disease. Sci. Rep. 2022, 12, 11290. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Shi, Y.; Liu, W.; Liu, S.; Sun, M.Z. Taurine improves neuron injuries and cognitive impairment in a mouse Parkinson’s disease model through inhibition of microglial activation. Neurotoxicology 2021, 83, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Abuirmeileh, A.N.; Abuhamdah, S.M.; Ashraf, A.; Alzoubi, K.H. Protective effect of caffeine and/or taurine on the 6-hydroxydopamine-induced rat model of Parkinson’s disease: Behavioral and neurochemical evidence. Restor. Neurol. Neurosci. 2021, 39, 149–157. [Google Scholar] [CrossRef]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. A diet rich in taurine, cysteine, folate, B12 and betaine may lessen risk for Alzheimer’s disease by boosting brain synthesis of hydrogen sulfide. Med. Hypotheses 2019, 132, 109356. [Google Scholar] [CrossRef]
- Graeser, A.C.; Boesch-Saadatmandi, C.; Lippmann, J.; Wagner, A.E.; Huebbe, P.; Storm, N.; Höppner, W.; Wiswedel, I.; Gardemann, A.; Minihane, A.M.; et al. Nrf2-dependent gene expression is affected by the proatherogenic apoE4 genotype-studies in targeted gene replacement mice. J. Mol. Med. 2011, 89, 1027–1035. [Google Scholar] [CrossRef]
- Loke, W.M.; Proudfoot, J.M.; Hodgson, J.M.; McKinley, A.J.; Hime, N.; Magat, M.; Stocker, R.; Croft, K.D. Specific dietary polyphenols attenuate atherosclerosis in apolipoprotein E-knockout mice by alleviating inflammation and endothelial dysfunction. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 749–757. [Google Scholar] [CrossRef]
- Cheng, T.; Wang, W.; Li, Q.; Han, X.; Xing, J.; Qi, C.; Lan, X.; Wan, J.; Potts, A.; Guan, F.; et al. Cerebroprotection of flavanol (-)-epicatechin after traumatic brain injury via Nrf2-dependent and -independent pathways. Free Radic. Biol. Med. 2016, 92, 15–28. [Google Scholar] [CrossRef]
- Pallauf, K.; Duckstein, N.; Hasler, M.; Klotz, L.O.; Rimbach, G. Flavonoids as putative inducers of the transcription factors Nrf2, FoxO, and PPARγ. Oxid. Med. Cell Longev. 2017, 2017, 4397340. [Google Scholar] [CrossRef]
- Schipper, H.M.; Song, W.; Tavitian, A.; Cressatti, M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 2019, 172, 40–70. [Google Scholar] [CrossRef]
- Ohgaki, R.; van IJzendoorn, S.C.; Matsushita, M.; Hoekstra, D.; Kanazawa, H. Organellar Na+/H+ exchangers: Novel players in organelle pH regulation and their emerging functions. Biochemistry 2011, 50, 443–450. [Google Scholar] [CrossRef]
- Sun, W.L.; Li, X.Y.; Dou, H.Y.; Wang, X.D.; Li, J.D.; Shen, L.; Ji, H.F. Myricetin supplementation decreases hepatic lipid synthesis and inflammation by modulating gut microbiota. Cell Rep. 2021, 36, 109641. [Google Scholar] [CrossRef]
- Rühlemann, M.C.; Degenhardt, F.; Thingholm, L.B.; Wang, J.; Skiecevičienė, J.; Rausch, P.; Hov, J.R.; Lieb, W.; Karlsen, T.H.; Laudes, M.; et al. Application of the distance-based F test in an mGWAS investigating β diversity of intestinal microbiota identifies variants in SLC9A8 (NHE8) and 3 other loci. Gut Microbes 2018, 9, 68–75. [Google Scholar] [CrossRef]
- Karunakaran, U.; Elumalai, S.; Moon, J.S.; Jeon, J.H.; Kim, N.D.; Park, K.G.; Won, K.C.; Leem, J.; Lee, I.K. Myricetin protects against high glucose-induced β-cell apoptosis by attenuating endoplasmic reticulum stress via inactivation of cyclin-dependent kinase Diabetes Metab. J. 2019, 43, 192–205. [Google Scholar] [CrossRef]
- Wu, L.; Guo, T.; Deng, R.; Liu, L.; Yu, Y. Apigenin ameliorates insulin resistance and lipid accumulation by endoplasmic reticulum stress and SREBP-1c/SREBP-2 pathway in palmitate-induced HepG2 cells and high-fat diet-fed mice. J. Pharmacol. Exp. Ther. 2021, 377, 146–156. [Google Scholar] [CrossRef]
- Han, J.; Chitu, V.; Stanley, E.R.; Wszolek, Z.K.; Karrenbauer, V.D.; Harris, R.A. Inhibition of colony stimulating factor-1 receptor (CSF-1R) as a potential therapeutic strategy for neurodegenerative diseases: Opportunities and challenges. Cell Mol. Life Sci. 2022, 79, 219. [Google Scholar] [CrossRef] [PubMed]
- Elmore, M.R.P.; Hohsfield, L.A.; Kramár, E.A.; Soreq, L.; Lee, R.J.; Pham, S.T.; Najafi, A.R.; Spangenberg, E.E.; Wood, M.A.; West, B.L.; et al. Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 2018, 17, e12832. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Ma, M.; Fan, X.; Li, M.; Liu, Q.; Liu, X.; Xu, G. Down-regulation of IGF-1/IGF-1R in hippocampus of rats with vascular dementia. Neurosci. Lett. 2012, 513, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.I.; Zhou, Y.; Eissa, I.H.; Wang, Y.; Zhang, J.; Jiang, L.; Hu, W.; Qi, J.; Chen, Z. Tetradecyl 2,3-dihydroxybenzoate alleviates oligodendrocyte damage following chronic cerebral hypoperfusion through IGF-1 receptor. Neurochem. Int. 2020, 138, 104749. [Google Scholar] [CrossRef] [PubMed]
- Gontier, G.; George, C.; Chaker, Z.; Holzenberger, M.; Aïd, S. Blocking IGF signaling in adult neurons alleviates Alzheimer’s disease pathology through amyloid-β clearance. J. Neurosci. 2015, 35, 11500–11513. [Google Scholar] [CrossRef]
- Yamada, M.; Kasagi, F.; Sasaki, H.; Masunari, N.; Mimori, Y.; Suzuki, G. Association between dementia and midlife risk factors: The Radiation Effects Research Foundation Adult Health Study. J. Am. Geriatr. Soc. 2003, 51, 410–414. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ohara, T.; Ninomiya, T.; Hata, J.; Yoshida, D.; Mukai, N.; Nagata, M.; Uchida, K.; Shirota, T.; Kitazono, T.; et al. Milk and dairy consumption and risk of dementia in an elderly Japanese population: The Hisayama Study. J. Am. Geriatr. Soc. 2014, 62, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Jia, X.; Zhang, J.; Huang, F.; Wang, H.; Zhang, B.; Wang, L.; Jiang, H.; Wang, Z. Diet-cognition associations differ in mild cognitive impairment subtypes. Nutrients 2021, 13, 1341. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, R.F.; Janssen, J.A.; Brugts, M.P.; van Duijn, C.M.; Hofman, A.; Koudstaal, P.J.; Ikram, M.A. Insulin-like growth factor-I receptor stimulating activity is associated with dementia. J. Alzheimers Dis. 2014, 42, 137–142. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, P.; Huang, R.; Wang, C.; Sun, L.; Lan, B.; He, Y.; Zhao, H.; Gao, Y. PINK1: The guard of mitochondria. Life Sci. 2020, 259, 118247. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, H.X.; Luo, Y.; Cui, J.G.; Talukder, M.; Li, J.L. Lycopene mitigates DEHP-induced hepatic mitochondrial quality control disorder via regulating SIRT1/PINK1/mitophagy axis and mitochondrial unfolded protein response. Environ. Pollut. 2022, 292 Pt. B, 118390. [Google Scholar] [CrossRef]
- Crowe-White, K.M.; Phillips, T.A.; Ellis, A.C. Lycopene and cognitive function. J. Nutr. Sci. 2019, 8, e20. [Google Scholar] [CrossRef] [PubMed]
- Maki, R.A.; Tyurin, V.A.; Lyon, R.C.; Hamilton, R.L.; DeKosky, S.T.; Kagan, V.E.; Reynolds, W.F. Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory deficits in a mouse model of Alzheimer disease. J. Biol. Chem. 2009, 284, 3158–3169. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Castellano-Escuder, P.; Carmona, F.; Lefèvre-Arbogast, S.; Low, D.Y.; Du Preez, A.; Ruigrok, S.R.; Manach, C.; Urpi-Sarda, M.; Korosi, A.; et al. Food and microbiota metabolites associate with cognitive decline in older subjects: A 12-year prospective study. Mol. Nutr. Food Res. 2021, 65, e2100606. [Google Scholar] [CrossRef] [PubMed]
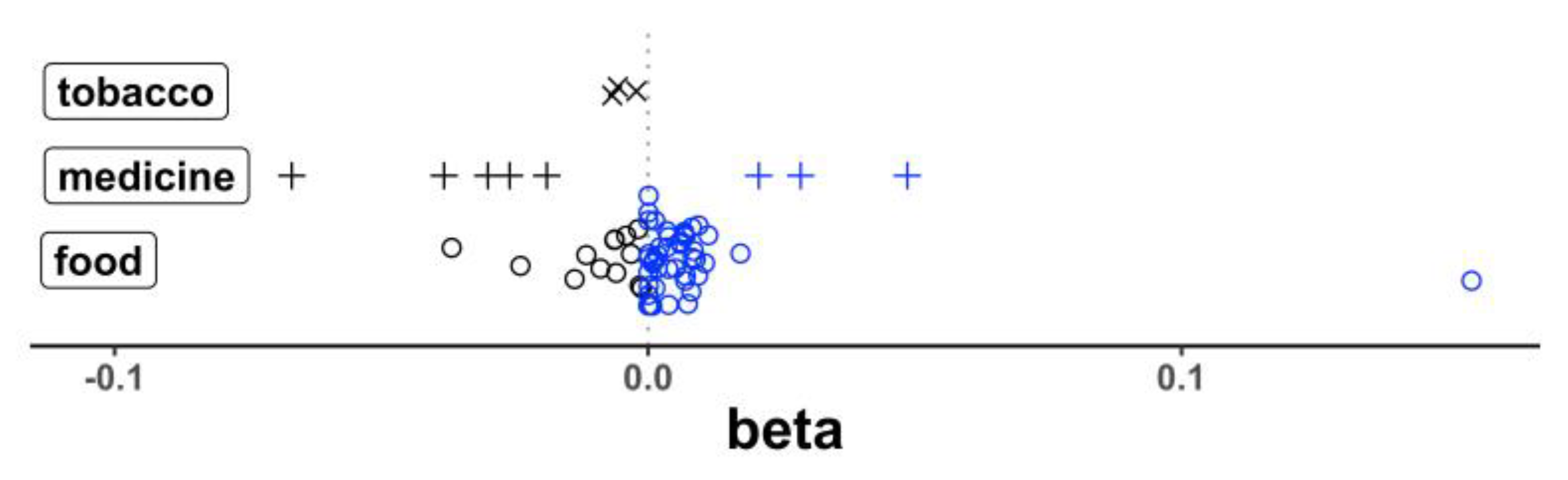
| Gene | Dietary Factor | p | Beta | SE * |
|---|---|---|---|---|
| HMOX1 | Type 2 diabetes medication | 1.09 × 10−6 | −0.067 | 1.37 × 10−2 |
| HMOX1 | Manganese | 1.47 × 10−4 | 0.017 | 4.55 × 10−3 |
| HMOX1 | Gallocatechin | 7.83 × 10−5 | 0.0089 | 2.25 × 10−3 |
| HMOX1 | Alcohol | 3.92 × 10−5 | 0.0081 | 1.96 × 10−3 |
| HMOX1 | Bananas | 2.79 × 10−4 | 0.0069 | 1.91 × 10−3 |
| HMOX1 | Theaflavin, total | 7.71 × 10−5 | 0.0068 | 1.71 × 10−3 |
| HMOX1 | Quercetin | 5.57 × 10−5 | 0.0038 | 9.43 × 10−4 |
| HMOX1 | Tea | 7.73 × 10−5 | 0.0036 | 9.15 × 10−4 |
| HMOX1 | Epicatechin 3-gallate | 7.91 × 10−5 | 0.0018 | 4.61 × 10−4 |
| HMOX1 | Epigallocatechin | 9.16 × 10−5 | 0.0013 | 3.41 × 10−4 |
| HMOX1 | Flavonoids, total | 4.63 × 10−5 | 7.74 × 10−5 | 1.90 × 10−5 |
| HMOX1 | Folate, total | 1.43 × 10−4 | 5.64 × 10−5 | 1.48 × 10−5 |
| IGF1R | Dairy protein | 2.92 × 10−4 | −0.0016 | 4.40 × 10−4 |
| MPO | Alcohol | 1.76 × 10−4 | 0.0014 | 3.63 × 10−4 |
| PLCG2 | Cigarettes, number per day | 5.98 × 10−5 | −0.0022 | 5.47 × 10−4 |
| PTGS2 | Alcohol | 5.82 × 10−6 | −0.0089 | 1.96 × 10−3 |
| SLC10A2 | Broccoli | 2.09 × 10−4 | 0.0082 | 2.21 × 10−3 |
| SLC9A8 | Pie, ready-made | 2.80 × 10−4 | −0.024 | 6.57 × 10−3 |
| SLC9A8 | Myricetin | 1.46 × 10−4 | 0.0095 | 2.49 × 10−3 |
| SLC9A8 | Apigenin | 7.28 × 10−5 | 0.0094 | 2.36 × 10−3 |
| SLC9A8 | Red wine | 8.84 × 10−6 | 0.0035 | 7.82 × 10−4 |
| UQCRC1 | Taurine | 1.47 × 10−4 | 0.154 | 4.06 × 10−2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parnell, L.D.; Magadmi, R.; Zwanger, S.; Shukitt-Hale, B.; Lai, C.-Q.; Ordovás, J.M. Dietary Responses of Dementia-Related Genes Encoding Metabolic Enzymes. Nutrients 2023, 15, 644. https://doi.org/10.3390/nu15030644
Parnell LD, Magadmi R, Zwanger S, Shukitt-Hale B, Lai C-Q, Ordovás JM. Dietary Responses of Dementia-Related Genes Encoding Metabolic Enzymes. Nutrients. 2023; 15(3):644. https://doi.org/10.3390/nu15030644
Chicago/Turabian StyleParnell, Laurence D, Rozana Magadmi, Sloane Zwanger, Barbara Shukitt-Hale, Chao-Qiang Lai, and José M Ordovás. 2023. "Dietary Responses of Dementia-Related Genes Encoding Metabolic Enzymes" Nutrients 15, no. 3: 644. https://doi.org/10.3390/nu15030644
APA StyleParnell, L. D., Magadmi, R., Zwanger, S., Shukitt-Hale, B., Lai, C.-Q., & Ordovás, J. M. (2023). Dietary Responses of Dementia-Related Genes Encoding Metabolic Enzymes. Nutrients, 15(3), 644. https://doi.org/10.3390/nu15030644







