Low Free Triiodothyronine as a More Sensitive Predictor of Survival Than Total Testosterone among Dialysis Men
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Correlation Analysis
3.3. Implications on Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, V.S.; Kathpalia, S.C.; Henriquez, C. Endocrine Abnormalities Associated With Chronic Renal Failure. Med. Clin. N. Am. 1978, 62, 1341–1361. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Qureshi, A.R.; Nakashima, A.; Arver, S.; Parini, P.; Lindholm, B.; Bárány, P.; Heimbürger, O.; Stenvinkel, P. Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol. Dial. Transplant. 2010, 26, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Mallamaci, F.; Tripepi, G.; Cutrupi, S.; Pizzini, P. Low triiodothyronine and survival in end-stage renal disease. Kidney Int. 2006, 70, 523–528. [Google Scholar] [CrossRef]
- Meuwese, C.L.; Dekker, F.W.; Lindholm, B.; Qureshi, A.R.; Heimburger, O.; Barany, P.; Stenvinkel, P.; Carrero, J.J. Baseline Levels and Trimestral Variation of Triiodothyronine and Thyroxine and Their Association with Mortality in Maintenance Hemodialysis Patients. Clin. J. Am. Soc. Nephrol. 2012, 7, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.I.; Nam, J.Y.; Shin, S.K.; Kang, E.W. Low Triiodothyronine Syndrome and Long-Term Cardiovascular Outcome in Incident Peritoneal Dialysis Patients. Clin. J. Am. Soc. Nephrol. 2015, 10, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Lee, L.-C.; Wang, W.-J. The association between serum testosterone and mortality among elderly men on hemodialysis. J. Clin. Lab. Anal. 2018, 32, e22394. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Hoermann, R.; Fui, M.N.T.; Zajac, J.D.; Ierino, F.L.; Roberts, M.A. Sex steroids levels in chronic kidney disease and kidney transplant recipients: Associations with disease severity and prediction of mortality. Clin. Endocrinol. 2014, 82, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Qureshi, A.R.; Parini, P.; Arver, S.; Lindholm, B.; Bárány, P.; Heimbürger, O.; Stenvinkel, P. Low Serum Testosterone Increases Mortality Risk among Male Dialysis Patients. J. Am. Soc. Nephrol. 2009, 20, 613–620. [Google Scholar] [CrossRef]
- Carrero, J.J.; Qureshi, A.R.; Axelsson, J.; Yilmaz, M.I.; Rehnmark, S.; Witt, M.R.; Bárány, P.; Heimbürger, O.; Suliman, M.E.; Alvestrand, A.; et al. Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J. Intern. Med. 2007, 262, 690–701. [Google Scholar] [CrossRef]
- Song, S.H.; Kwak, I.S.; Lee, N.W.; Kang, Y.H.; Seong, E.Y.; Park, J.S. The prevalence of low triiodothyronine according to the stage of chronic kidney disease in subjects with a normal thyroid-stimulating hormone. Nephrol. Dial. Transplant. 2008, 24, 1534–1538. [Google Scholar] [CrossRef]
- Meuwese, C.L.; Dekkers, O.; Stenvinkel, P.; Dekker, F.; Carrero, J.J. Nonthyroidal illness and the cardiorenal syndrome. Nat. Rev. Nephrol. 2013, 9, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Díez, J.J. Thyroid dysfunction and kidney disease. Eur. J. Endocrinol. 2009, 160, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Wilber, J.F.; Utiger, R.D. The effect of glucocorticoids on thyrotropin secretion. J. Clin. Investig. 1969, 48, 2096–2103. [Google Scholar] [CrossRef] [PubMed]
- Wiersinga, W.M.; Touber, J.L. The influence of beta-adrenoceptor blocking agents on plasma thyroxine and triiodothyronine. J. Clin. Endocrinol. Metab. 1977, 45, 293–298. [Google Scholar] [CrossRef]
- Mastorci, F.; Sabatino, L.; Vassalle, C.; Pingitore, A. Cardioprotection and Thyroid Hormones in the Clinical Setting of Heart Failure. Front. Endocrinol. 2020, 10, 927. [Google Scholar] [CrossRef]
- Miyashita, K.; Murakami, M.; Iriuchijima, T.; Takeuchi, T.; Mori, M. Regulation of rat liver type 1 iodothyronine deiodinase mRNA levels by testosterone. Mol. Cell. Endocrinol. 1995, 115, 161–167. [Google Scholar] [CrossRef]
- Napolitano, G.; Bonomini, M.; Bomba, G.; Bucci, I.; Todisco, V.; Albertazzi, A.; Monaco, F. Thyroid function and plasma selenium in chronic uremic patients on hemodi-alysis treat- ment. Biol. Trace Elem. Res. 1996, 55, 221–230. [Google Scholar] [CrossRef]
- Lim, V.S.; Flanigan, M.J.; Zavala, D.C.; Freeman, R.M. Protective adaptation of low serum triiodothyronine in patients with chronic renal failure. Kidney Int. 1985, 28, 541–549. [Google Scholar] [CrossRef]
- Klein, I.; Ojamaa, K. Thyroid hormone and the cardiovascular system. N. Engl. J. Med. 2001, 344, 501–509. [Google Scholar] [CrossRef]
- Trivieri, M.G.; Oudit, G.Y.; Sah, R.; Kerfant, B.-G.; Sun, H.; Gramolini, A.O.; Pan, Y.; Wickenden, A.D.; Croteau, W.; de Escobar, G.M.; et al. Cardiac-specific elevations in thyroid hormone enhance contractility and prevent pressure overload-induced cardiac dysfunction. Proc. Natl. Acad. Sci. USA 2006, 103, 6043–6048. [Google Scholar] [CrossRef]
- Tatar, E.; Kircelli, F.; Asci, G.; Carrero, J.J.; Gungor, O.; Demirci, M.S.; Ozbek, S.S.; Ceylan, N.; Ozkahya, M.; Toz, H.; et al. Associations of triiodothyronine levels with carotid aterosclerosis and arterial stiffness in hemodialisysis patients. Clin. J. Am. Soc. Nephrol. 2011, 6, 2240–2246. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.I.; Sonmez, A.; Karaman, M.; Ay, S.A.; Saglam, M.; Yaman, H.; Kilic, S.; Eyileten, T.; Caglar, K.; Oguz, Y.; et al. Low triiodothyronine alters flow-mediated vasodilatation in advanced nondi-abetic kidney disease. Am. J. Nephrol. 2011, 33, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Tripepi, G.; Cutrupi, S.; Pizzini, P.; Mallamaci, F. Low Triiodothyronine: A New Facet of Inflammation in End-Stage Renal Disease. J. Am. Soc. Nephrol. 2005, 16, 2789–2795. [Google Scholar] [CrossRef] [PubMed]
- Zoccali, C.; Benedetto, F.; Mallamaci, F.; Tripepi, G.; Cutrupi, S.; Pizzini, P.; Malatino, L.S.; Bonanno, G.; Seminara, G. Low triiodothyronine and cardiomyopathy in patients with end-stage renal disease. J. Hypertens. 2006, 24, 2039–2046. [Google Scholar] [CrossRef]
- Tatar, E.; Kircelli, F.; Ok, E. The contribution of thyroid dysfunction on cardiovascular disease in patients with chronic kidney disease. Atherosclerosis 2013, 227, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Enia, G.; Panuccio, V.; Cutrupi, S.; Pizzini, P.; Tripepi, G.; Mallamaci, F.; Zoccali, C. Subclinical hypothyroidism is linked to micro-inflammation and predicts death in continuous ambulatory peritoneal dialysis. Nephrol. Dial. Transplant. 2006, 22, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Ozen, K.P.; Asci, G.; Gungor, O.; Carrero, J.J.; Kircelli, F.; Tatar, E.; Ok, E.S.; Ozkahya, M.; Toz, H.; Cirit, M.; et al. Nutritional State Alters the Association between Free Triiodothyronine Levels and Mortality in Hemodialysis Patients. Am. J. Nephrol. 2011, 33, 305–312. [Google Scholar] [CrossRef]
- Fernández-Reyes, M.J.; Diez, J.J.; Collado, A.; Iglesias, P.; Bajo, M.A.; Estrada, P.; Del Peso, G.; Heras, M.; Molina, A.; Selgas, R. Are low concentrations of serum triiodothyronine a good marker for long-term mortality in hemodialysis patients? Clin. Nephrol. 2010, 73, 238–240. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Shoji, T.; Miyashima, M.; Nagata, Y.; Kakutani, Y.; Ochi, A.; Morioka, T.; Nakatani, S.; Mori, K.; Tsujimoto, Y.; et al. Low Free Triiodothyronine Level as a Predictor of Cardiovascular Events and All-Cause Mortality in Patients Undergoing Hemodialysis: The DREAM Cohort. J. Atheroscler. Thromb. 2021, 28, 1071–1082. [Google Scholar] [CrossRef]
- Xu, H.; Brusselaers, N.; Lindholm, B.; Zoccali, C.; Carrero, J.J. Thyroid Function Test Derangements and Mortality in Dialysis Patients: A Systematic Review and Meta-analysis. Am. J. Kidney Dis. 2016, 68, 923–932. [Google Scholar] [CrossRef]
- Nakashima, A.; Ohkido, I.; Yokoyama, K.; Mafune, A.; Urashima, M.; Yokoo, T. Associations Between Low Serum Testosterone and All-Cause Mortality and Infection-Related Hospitalization in Male Hemodialysis Patients: A Prospective Cohort Study. Kidney Int. Rep. 2017, 2, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Gungor, O.; Kircelli, F.; Carrero, J.J.; Asci, G.; Toz, H.; Tatar, E.; Hur, E.; Sever, M.S.; Arinsoy, T.; Ok, E. Endogenous testosterone and mortality in male hemodialysis patients: Is it the result of aging? Clin. J. Am. Soc. Nephrol. CJASN 2010, 5, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ravel, V.A.; You, A.S.; Streja, E.; Rivara, M.B.; Potukuchi, P.K.; Brunelli, S.M.; Kovesdy, C.P.; Kalantar-Zadeh, K.; Rhee, C.M. Association between Testosterone and Mortality Risk among U.S. Males Receiving Dialysis. Am. J. Nephrol. 2017, 46, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Kojo, G.; Yoshida, T.; Ohkawa, S.; Odamaki, M.; Kato, A.; Takita, T.; Maruyama, Y.; Kumagai, H. Association of serum total testosterone concentration with skeletal muscle mass in men under hemodialysis. Int. Urol. Nephrol. 2013, 46, 985–991. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Bárány, P.; Yilmaz, M.I.; Qureshi, A.R.; Sonmez, A.; Heimbürger, O.; Ozgurtas, T.; Yenicesu, M.; Lindholm, B.; Stenvinkel, P. Testosterone defieciency is a cause of anemia and reduced responsiveness to eryth-ropoiesis-stimulating agents in men with chronic kidney disease. Nephrol. Dial. Transplant. 2012, 27, 709–715. [Google Scholar] [CrossRef]
- Doumouchtsis, K.K.; Kostakis, A.I.; Doumouchtsis, S.; Grapsa, E.I.; Passalidou, I.A.; Tziamalis, M.P.; Poulakou, M.V.; Vlachos, I.; Perrea, D.N. The effect of sexual hormone abnormalities on proximal femur bone mineral density in hemodialysis patients and the possible role of RANKL. Hemodial. Int. 2008, 12, 100–107. [Google Scholar] [CrossRef]
- De Araújo, I.C.; Kamimura, M.A.; Draibe, S.A.; Canziani, M.E.F.; Manfredi, S.R.; Avesani, C.M.; Sesso, R.; Cuppari, L. Nutritional Parameters and Mortality in Incident Hemodialysis Patients. J. Ren. Nutr. 2006, 16, 27–35. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P.; Cuppari, L.; Ikizler, T.A.; Kalantar-Zadeh, K.; Kaysen, G.; Mitch, W.E.; Price, S.R.; Wanner, C.; Wang, A.Y.; et al. Etiology of the Protein-Energy Wasting Syndrome in Chronic Kidney Disease: A Consensus Statement From the International Society of Renal Nutrition and Metabolism (ISRNM). J. Ren. Nutr. 2013, 23, 77–90. [Google Scholar] [CrossRef]
- Garibotto, G.; Picciotto, D.; Verzola, D. Testosterone deficiency, frailty and muscle wasting in CKD: A converging paradigm? Nephrol. Dial. Transplant. 2019, 34, 723–726. [Google Scholar] [CrossRef]
- Yilmaz, M.I.; Sonmez, A.; Qureshi, A.R.; Saglam, M.; Stenvinkel, P.; Yaman, H.; Eyileten, T.; Caglar, K.; Oguz, Y.; Taslipinar, A.; et al. Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with non- dialysis chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2011, 6, 1617–1625. [Google Scholar] [CrossRef]
- Niemczyk, S.; Niemczyk, L.; Szamotulska, K.; Bartoszewicz, Z.; Romejko-Ciepielewska, K.; Gomółka, M.; Saracyn, M.; Matuszkiewicz-Rowińska, J. Is Free Testosterone Concentration a Prognostic Factor of Survival in Chronic Renal Failure (CRF)? Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2015, 21, 3401–3408. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.K.; Stenvinkel, P.; Lin, M.; Hemmelgarn, B.; Thadhani, R.; Klarenbach, S.; Chan, C.; Zimmerman, D.; Cembrowski, G.; Strippoli, G.; et al. Serum Testosterone Levels and Clinical Outcomes in Male Hemodialysis Patients. Am. J. Kidney Dis. 2014, 63, 268–275. [Google Scholar] [CrossRef] [PubMed]
- De Groot, L.J. Dangerous dogmas in medicine: The nonthyroidal illness syndrome. J. Clin. Endocrinol. Metab. 1999, 84, 151–164. [Google Scholar] [CrossRef]
- Meuwese, C.L.; Carrero, J.J.; Cabezas-Rodríguez, I.; Heimburger, O.; Barany, P.; Lindholm, B.; Qureshi, A.R.; Ripsweden, J.; Dekker, F.W.; Stenvinkel, P. Nonthyroidal illness: A risk factor for coronary calcification and arterial stiffness in patients undergoing peritoneal dialysis? J. Intern. Med. 2013, 274, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Tatar, E.; Demirci, M.S.; Kircelli, F.; Gungor, O.; Yaprak, M.; Asci, G.; Başçi, A.; Ozkahya, M.; Ok, E. The association between thyroid hormones and arterial stiffness in peritoneal dialysis patients. Int. Urol. Nephrol. 2011, 44, 601–606. [Google Scholar] [CrossRef]
- Mizuno, I.; Takahashi, Y.; Okimura, Y.; Kaji, H.; Chihara, K. Upregulation of the klotho gene expression by thyroid hormone and during adipose differentiation in 3T3-L1 adipocytes. Life Sci. 2001, 68, 2917–2923. [Google Scholar] [CrossRef]
- Sato, Y.; Nakamura, R.; Satoh, M.; Fujishita, K.; Mori, S.; Ishida, S.; Yamaguchi, T.; Inoue, K.; Nagao, T.; Ohno, Y. Thyroid Hormone Targets Matrix Gla Protein Gene Associated with Vascular Smooth Muscle Calcification. Circ. Res. 2005, 97, 550–557. [Google Scholar] [CrossRef]
- Zoccali, C. The endothelium as a target in renal diseases. J. Nephrol. 2007, 20, S39–S44. [Google Scholar]
- Basu, G.; Mohapatra, A. Interactions between thyroid disorders and kidney disease. Indian J. Endocrinol. Metab. 2012, 16, 204–213. [Google Scholar] [CrossRef]
- Meuwese, C.L.; Carrero, J.J. Chronic kidney disease and hypothalamic-pituitary axis dysfunction: The chicken or the egg? Arch. Med. Res. 2013, 44, 591–600. [Google Scholar] [CrossRef]
- Carrero, J.J.; Stenvinkel, P. Inflammation in end-stage renal disease- what have we learned in 10 years? Semin. Dial. 2010, 23, 498–509. [Google Scholar] [CrossRef] [PubMed]
- Fukata, J.; Imura, H.; Nakao, K. Cytokines as mediators in the regulation of the hypothalamic-pituitary-adrenocortical function. J. Endocrinol. Investig. 1994, 17, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.; Kennedy, R. Cytokines and hypothalamic-pituitary function. Cytokine 1993, 5, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Carrero, J.J.; Stenvinkel, P. Persistent Inflammation as a Catalyst for Other Risk Factors in Chronic Kidney Disease: A Hypothesis Proposal. Clin. J. Am. Soc. Nephrol. 2009, 4, S49–S55. [Google Scholar] [CrossRef]
- Warner, M.H.; Beckett, G.J. Mechanisms behind the non-thyroidal illness syndrome: An update. J. Endocrinol. 2009, 205, 1–13. [Google Scholar] [CrossRef]
- Axelsson, J.; Qureshi, A.R.; Divino-Filho, J.C.; Bárány, P.; Heimbürger, O.; Lindholm, B.; Stenvinkel, P. Are insulin-like growth factor and its binding proteins 1 and 3 clinically useful as markers of malnutrition, sarcopenia and inflammation in end-stage renal disease? Eur. J. Clin. Nutr. 2006, 60, 718–726. [Google Scholar] [CrossRef]
| HD | PD | p * | |
|---|---|---|---|
| N = 31 | N = 17 | ||
| Age (years, range) | 61.4 ± 10.0; 42–84 | 59.2 ± 12.2; 41–77 | 0.606 |
| Duration of dialysis (years, range) | 2.8 ± 2.7; 0.3–12.8 | 1.7 ± 1.6; 0.3–6.8 | 0.010 |
| Prior CVD, % | 74.2% | 64.7% | 0.534 |
| DM, % | 61.3% | 47.0% | 0.391 |
| Hypertension, % | 100.0% | 100.0% | 1 |
| Total testosterone (ng/mL, range) | 3.1 ± 1.3; 1.1–8.0 | 3.7 ± 1.3; 1.7–6.4 | 0.070 |
| TSH (uIU/mL, range) | 1.7 ± 1.1; 0.4–5.6 | 2.2 ± 1.2; 0.8–5.7 | 0.132 |
| fT3 (pmol/L, range) | 4.0 ± 0.9; 1.8–6.8 | 4.0 ± 0.7; 2.1–4.9 | 0.608 |
| fT4 (pmol/L, range) | 14.8 ± 2.4; 11.3–20.8 | 16.4 ± 2.4; 13.3–20.7 | 0.038 |
| Age | TT | Dialysis Duration | ||
|---|---|---|---|---|
| TSH | r | −0.175 | −0.068 | −0.147 |
| p | 0.347 | 0.717 | 0.429 | |
| fT3 | r | 0.053 | 0.463 | −0.029 |
| p | 0.779 | 0.009 | 0.877 | |
| fT4 | r | −0.038 | −0.073 | −0.356 |
| p | 0.841 | 0.702 | 0.054 | |
| Parameter | Non Survivors | Survivors | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SD | Median | n | Mean ± SD | Median | ||
| Age (years) | 9 | 62 ± 11 | 59 | 39 | 60 ± 11 | 63 | 0.728 |
| Dialysis duration (years) | 9 | 2.5 ± 2.2 | 1.6 | 39 | 2.3 ± 2.5 | 1.6 | 0.755 |
| Total testosterone (ng/mL) | 9 | 2.8 ± 0.9 | 3.1 | 39 | 3.4 ± 1.4 | 3.3 | 0.350 |
| TSH (uIU/mL) | 9 | 2.3 ± 1.5 | 2.1 | 39 | 1.8 ± 1.1 | 1.7 | 0.348 |
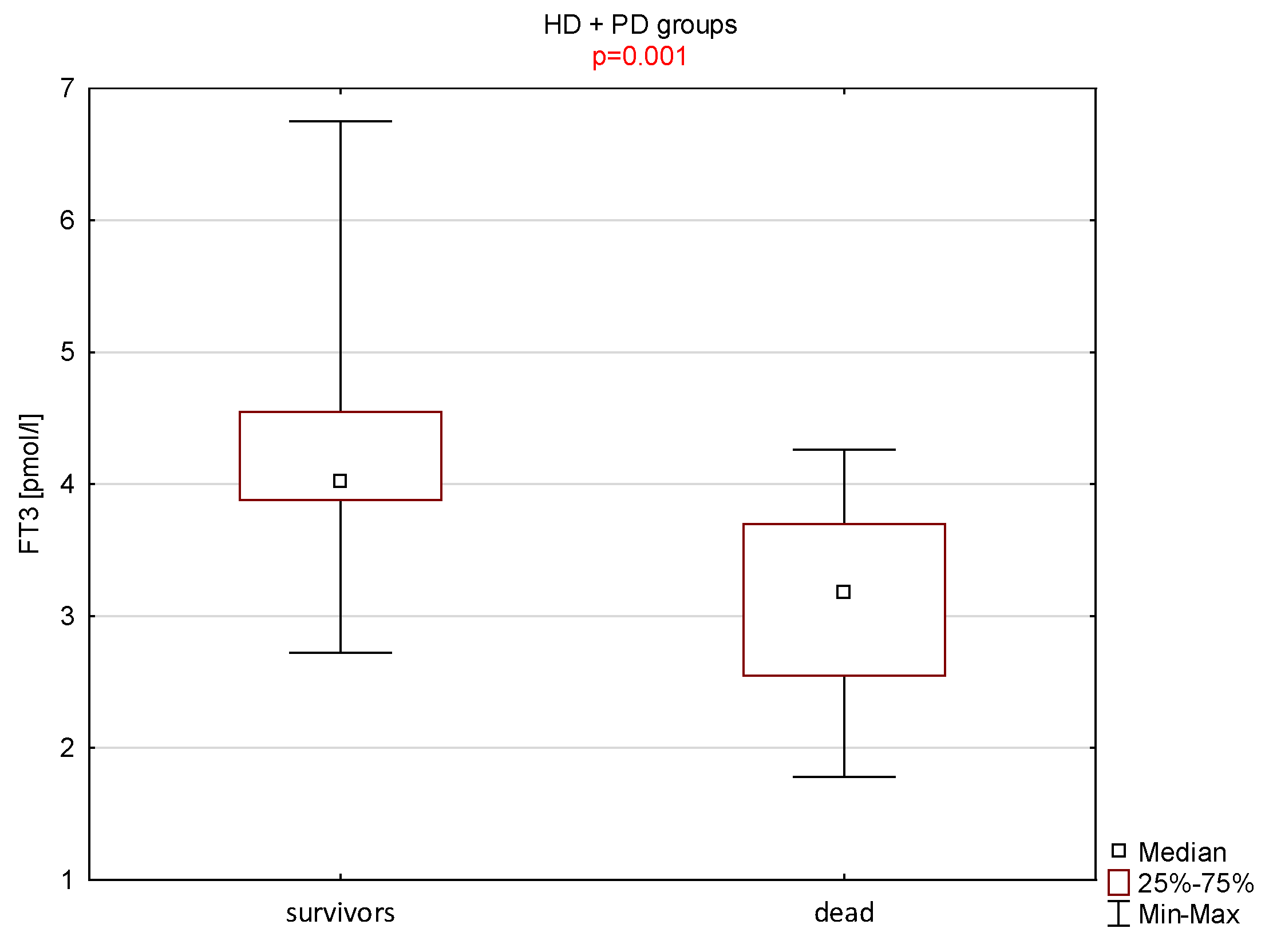
| fT3 (pmol/L) | 9 | 3.1 ± 0.8 | 3.2 | 39 | 4.2 ± 0.7 | 4.0 | 0.001 |
| fT4 (pmol/L) | 9 | 16.1 ± 2.6 | 16.2 | 39 | 15.2 ± 2.4 | 15.1 | 0.395 |
| Parameter | Cut-Off Value | Sensitivity | Specificity | AUC | Significance-p |
|---|---|---|---|---|---|
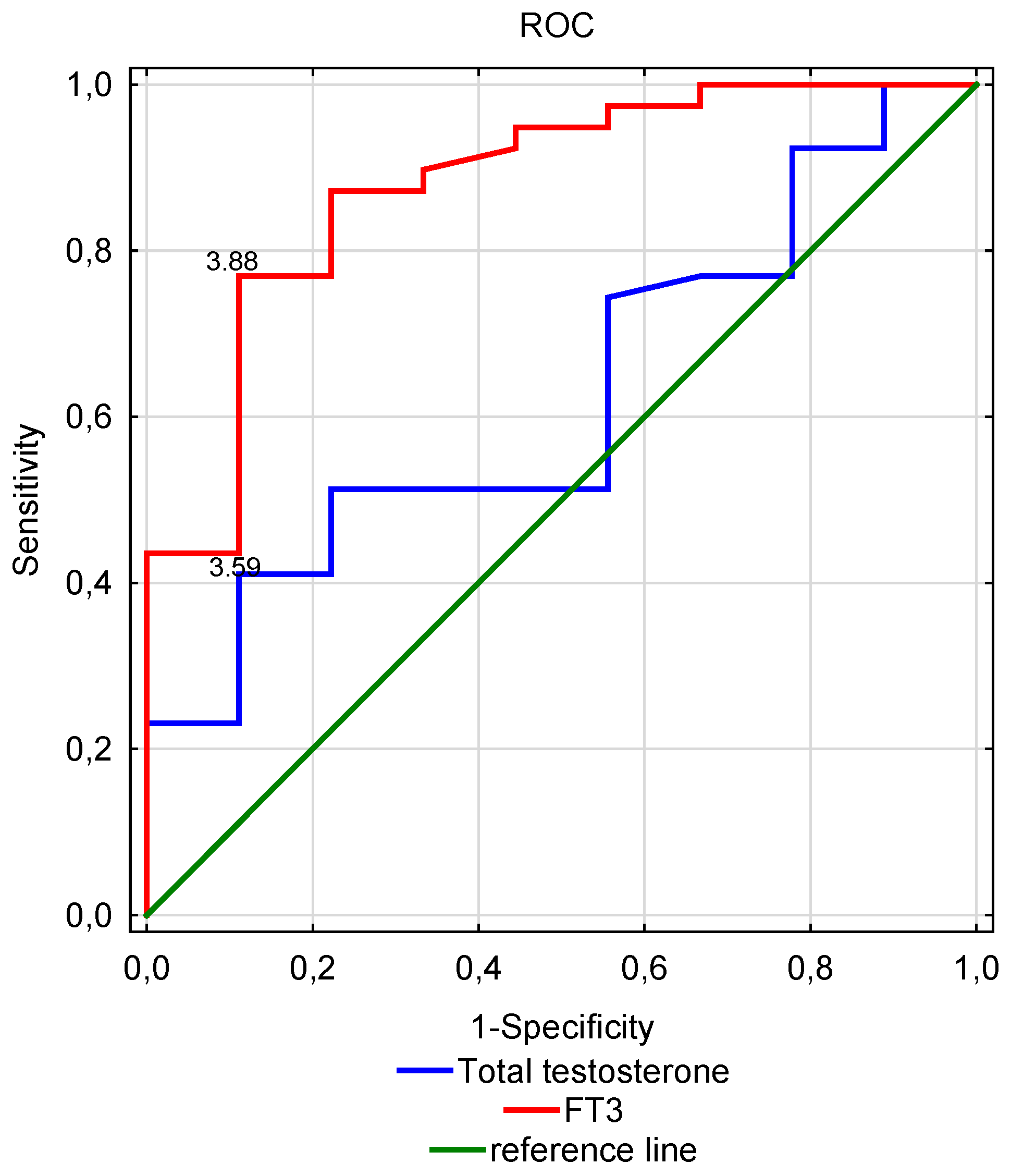
| Total testosterone (ng/mL) | 3.59 | 0.41 | 0.12 | 0.625 | 0.188 |
| TSH (uIU/mL) | 2.23 | 0.77 | 0.56 | 0.603 | 0.326 |
| fT3 (pmol/L) | 3.88 | 0.77 | 0.11 | 0.879 | <0.001 |
| fT4 (pmol/L) | 15.54 | 0.56 | 0.25 | 0.599 | 0.375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leśniak, K.; Rymarz, A.; Sobol, M.; Niemczyk, S. Low Free Triiodothyronine as a More Sensitive Predictor of Survival Than Total Testosterone among Dialysis Men. Nutrients 2023, 15, 595. https://doi.org/10.3390/nu15030595
Leśniak K, Rymarz A, Sobol M, Niemczyk S. Low Free Triiodothyronine as a More Sensitive Predictor of Survival Than Total Testosterone among Dialysis Men. Nutrients. 2023; 15(3):595. https://doi.org/10.3390/nu15030595
Chicago/Turabian StyleLeśniak, Ksymena, Aleksandra Rymarz, Maria Sobol, and Stanisław Niemczyk. 2023. "Low Free Triiodothyronine as a More Sensitive Predictor of Survival Than Total Testosterone among Dialysis Men" Nutrients 15, no. 3: 595. https://doi.org/10.3390/nu15030595
APA StyleLeśniak, K., Rymarz, A., Sobol, M., & Niemczyk, S. (2023). Low Free Triiodothyronine as a More Sensitive Predictor of Survival Than Total Testosterone among Dialysis Men. Nutrients, 15(3), 595. https://doi.org/10.3390/nu15030595









