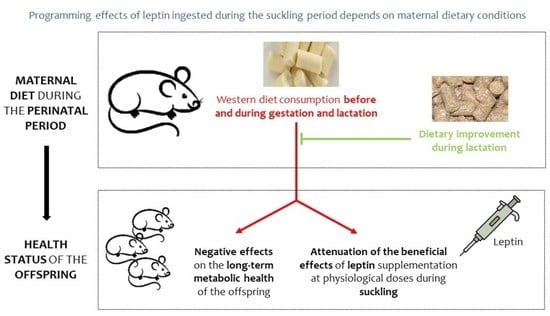
Influence of Maternal Metabolic Status and Diet during the Perinatal Period on the Metabolic Programming by Leptin Ingested during the Suckling Period in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Determination of Blood Parameters under Fed/Fasting Conditions
2.3. Extraction and Quantification of Hepatic Lipid Content
2.4. RNA Extraction
2.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.6. Statistical Analysis
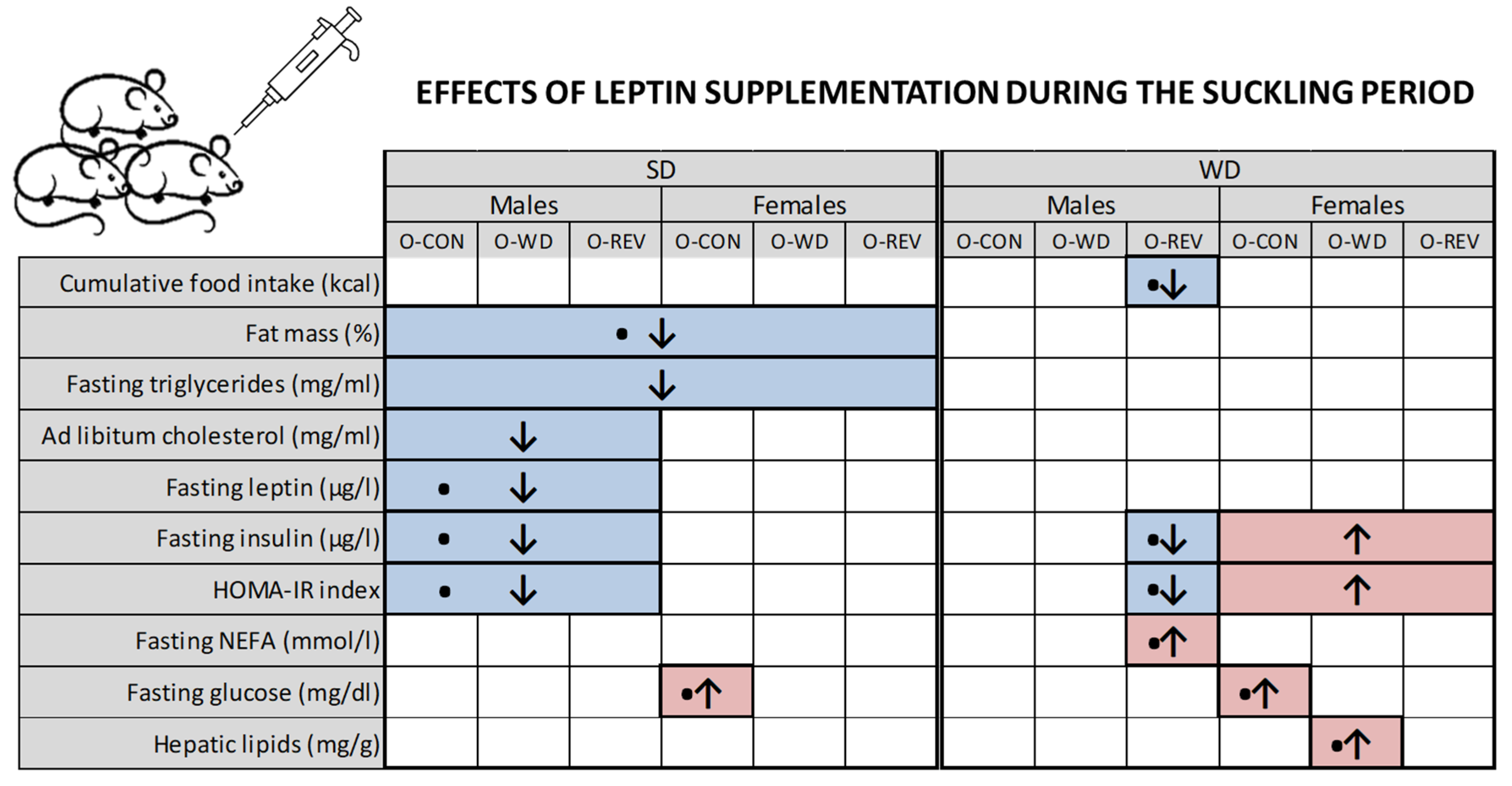
3. Results
3.1. Food Intake and Body Weight-Related Parameters
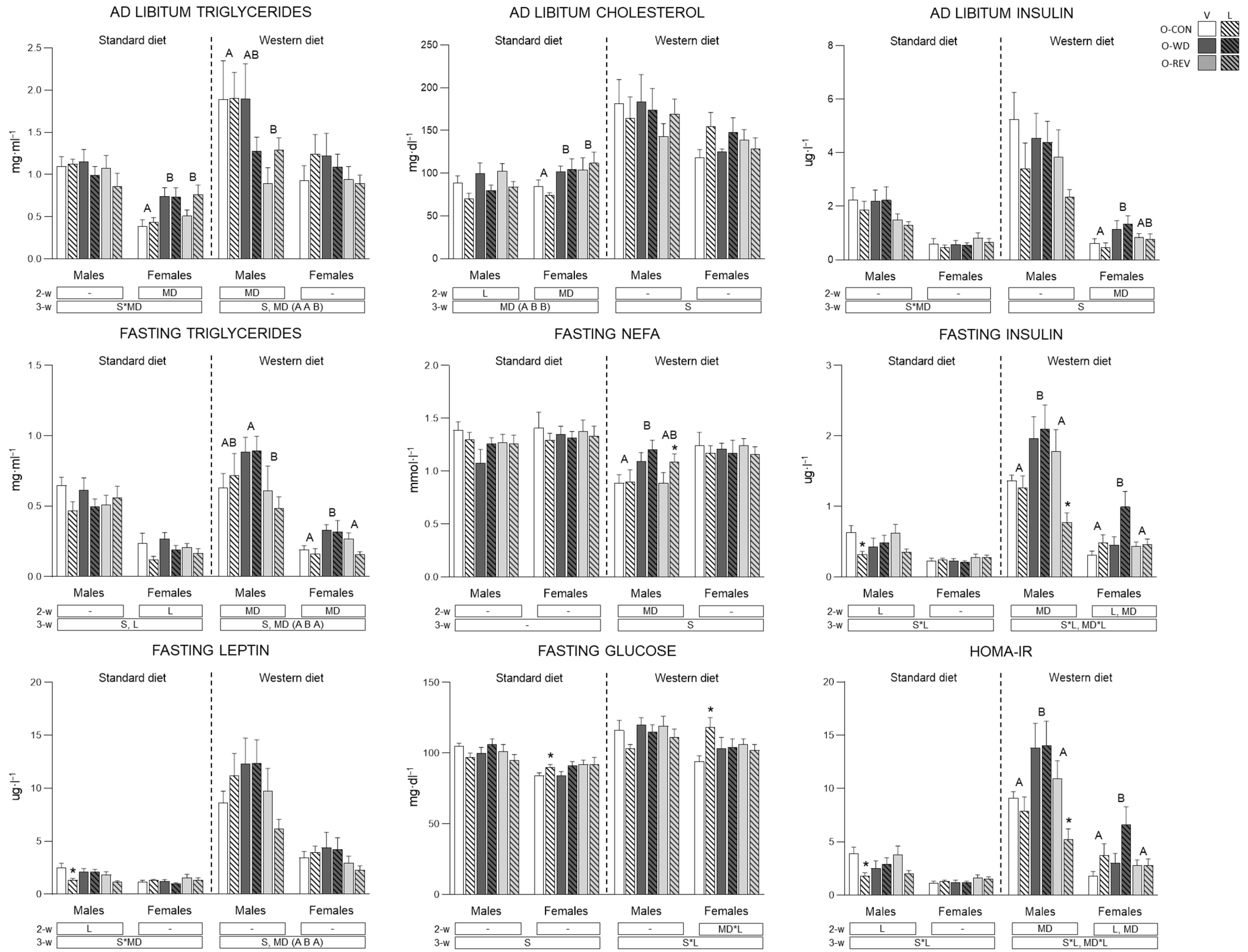
3.2. Circulating Parameters
3.3. Hepatic Lipid Content
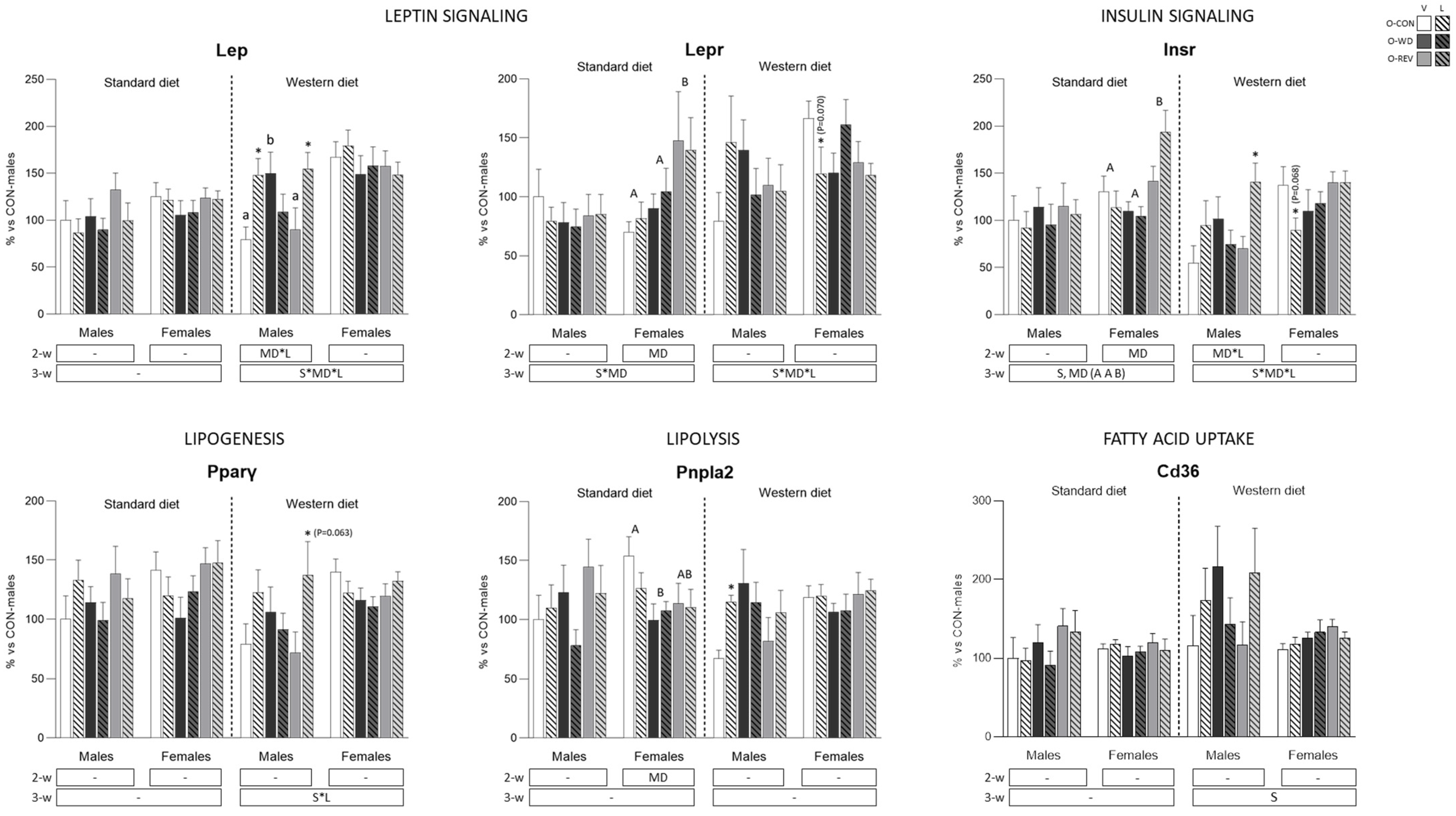
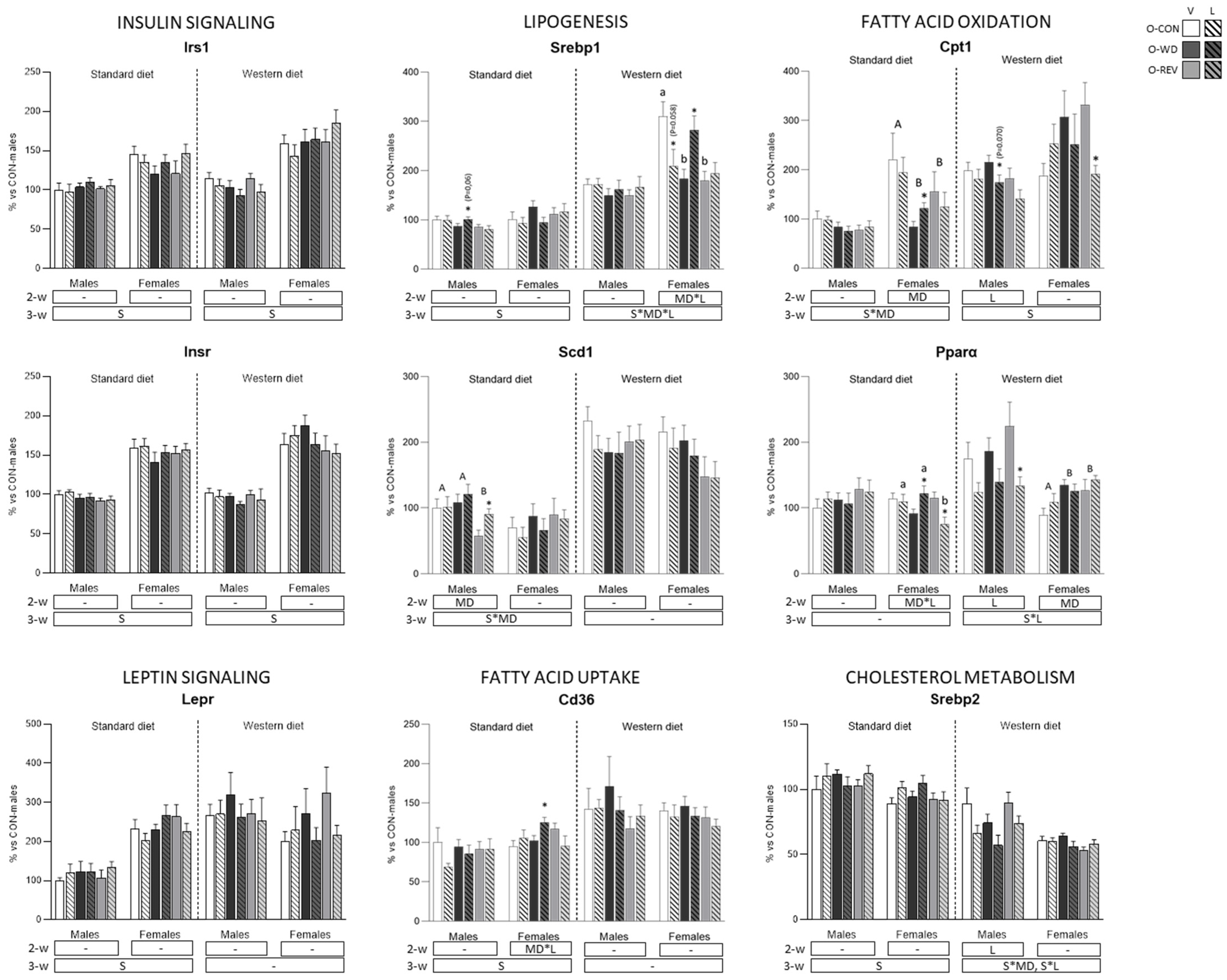
3.4. Expression of Energy Metabolism-Related Genes in rWAT and Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barker, D.J.P. The Fetal Origins of Diseases of Old Age. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 3), S3–S9. [Google Scholar]
- Lyons, K.E.; Ryan, C.A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. Breast Milk, a Source of Beneficial Microbes and Associated Benefits for Infant Health. Nutrients 2020, 12, 1039. [Google Scholar] [CrossRef] [PubMed]
- Gorski, J.N.; Dunn-Meynell, A.A.; Hartman, T.G.; Levin, B.E. Postnatal Environment Overrides Genetic and Prenatal Factors Influencing Offspring Obesity and Insulin Resistance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R768–R778. [Google Scholar] [CrossRef] [PubMed]
- Pomar, C.A.; Van Nes, R.; Sánchez, J.; Picó, C.; Keijer, J.; Palou, A. Maternal Consumption of a Cafeteria Diet during Lactation in Rats Leads the Offspring to a Thin-Outside-Fat-inside Phenotype. Int. J. Obes. 2017, 41, 1279–1287. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.M.; Bueno, F.G.; Schamber, C.R.; de Mello, J.C.P.; de Oliveira, J.C.; Francisco, F.A.; Moreira, V.M.; Junior, M.D.F.; Pedrino, G.R.; de Freitas Mathias, P.C.; et al. Maternal Diet-Induced Obesity during Suckling Period Programs Offspring Obese Phenotype and Hypothalamic Leptin/Insulin Resistance. J. Nutr. Biochem. 2018, 61, 24–32. [Google Scholar] [CrossRef]
- Bayol, S.A.; Farrington, S.J.; Stickland, N.C. A Maternal “junk Food” Diet in Pregnancy and Lactation Promotes an Exacerbated Taste for “Junk Food” and a Greater Propensity for Obesity in Rat Offspring. Br. J. Nutr. 2007, 98, 843–851. [Google Scholar] [CrossRef]
- Bayol, S.A.; Simbi, B.H.; Bertrand, J.A.; Stickland, N.C. Offspring from Mothers Fed a “junk Food” Diet in Pregnancy and Lactation Exhibit Exacerbated Adiposity That Is More Pronounced in Females. J. Physiol. 2008, 586, 3219–3230. [Google Scholar] [CrossRef]
- Bayol, S.A.; Simbi, B.H.; Stickland, N.C. A Maternal Cafeteria Diet during Gestation and Lactation Promotes Adiposity and Impairs Skeletal Muscle Development and Metabolism in Rat Offspring at Weaning. J. Physiol. 2005, 567, 951–961. [Google Scholar] [CrossRef]
- Pomar, C.A.; Castillo, P.; Palou, M.; Palou, A.; Picó, C. Implementation of a Healthy Diet to Lactating Rats Attenuates the Early Detrimental Programming Effects in the Offspring Born to Obese Dams. Putative Relationship with Milk Hormone Levels. J. Nutr. Biochem. 2022, 107, 109043. [Google Scholar] [CrossRef]
- Castillo, P.; Kuda, O.; Kopecky, J.; Pomar, C.A.; Palou, A.; Palou, M.; Picó, C. Reverting to a Healthy Diet during Lactation Normalizes Maternal Milk Lipid Content of Diet-Induced Obese Rats and Prevents Early Alterations in the Plasma Lipidome of the Offspring. Mol. Nutr. Food Res. 2022, 66, 2200204. [Google Scholar] [CrossRef]
- Martin-Gronert, M.S.; Ozanne, S.E. Maternal Nutrition during Pregnancy and Health of the Offspring. Biochem. Soc. Trans. 2006, 34, 779–782. [Google Scholar] [CrossRef]
- Ip, S.; Chung, M.; Raman, G.; Trikalinos, T.A.; Lau, J. A Summary of the Agency for Healthcare Research and Quality’s Evidence Report on Breastfeeding in Developed Countries. Breastfeed. Med. 2009, 4 (Suppl. 1), S17. [Google Scholar] [CrossRef] [PubMed]
- Palou, M.; Picó, C.; Palou, A. Leptin as a Breast Milk Component for the Prevention of Obesity. Nutr. Rev. 2018, 76, 875–892. [Google Scholar] [CrossRef] [PubMed]
- Badillo-Suárez, P.A.; Rodríguez-Cruz, M.; Nieves-Morales, X. Impact of Metabolic Hormones Secreted in Human Breast Milk on Nutritional Programming in Childhood Obesity. J. Mammary Gland Biol. Neoplasia 2017, 22, 171–191. [Google Scholar] [CrossRef] [PubMed]
- Picó, C.; Oliver, P.; Sánchez, J.; Miralles, O.; Caimari, A.; Priego, T.; Palou, A. The Intake of Physiological Doses of Leptin during Lactation in Rats Prevents Obesity in Later Life. Int. J. Obes. 2007, 31, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, J.; Priego, T.; Palou, M.; Tobaruela, A.; Palou, A.; Picó, C. Oral Supplementation with Physiological Doses of Leptin during Lactation in Rats Improves Insulin Sensitivity and Affects Food Preferences Later in Life. Endocrinology 2008, 149, 733–740. [Google Scholar] [CrossRef]
- Priego, T.; Sánchez, J.; Palou, A.; Picó, C. Leptin Intake during the Suckling Period Improves the Metabolic Response of Adipose Tissue to a High-Fat Diet. Int. J. Obes. 2010, 34, 809–819. [Google Scholar] [CrossRef]
- Konieczna, J.; García, A.P.; Sánchez, J.; Palou, M.; Palou, A.; Picó, C. Oral Leptin Treatment in Suckling Rats Ameliorates Detrimental Effects in Hypothalamic Structure and Function Caused by Maternal Caloric Restriction during Gestation. PLoS ONE 2013, 8, e81906. [Google Scholar] [CrossRef]
- Konieczna, J.; Palou, M.; Sánchez, J.; Picó, C.; Palou, A. Leptin Intake in Suckling Rats Restores Altered T3 Levels and Markers of Adipose Tissue Sympathetic Drive and Function Caused by Gestational Calorie Restriction. Int. J. Obes. 2015, 39, 959–966. [Google Scholar] [CrossRef]
- Szostaczuk, N.; Priego, T.; Palou, M.; Palou, A.; Picó, C. Oral Leptin Supplementation throughout Lactation in Rats Prevents Later Metabolic Alterations Caused by Gestational Calorie Restriction. Int. J. Obes. 2017, 41, 360–371. [Google Scholar] [CrossRef]
- Kanguru, L.; McCaw-Binns, A.; Bell, J.; Yonger-Coleman, N.; Wilks, R.; Hussein, J. The Burden of Obesity in Women of Reproductive Age and in Pregnancy in a Middle-Income Setting: A Population Based Study from Jamaica. PLoS ONE 2017, 12, e0188677. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis Model Assessment: Insulin Resistance and β-Cell Function from Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Caimari, A.; Oliver, P.; Palou, A. Adipose Triglyceride Lipase Expression and Fasting Regulation Are Differently Affected by Cold Exposure in Adipose Tissues of Lean and Obese Zucker Rats. J. Nutr. Biochem. 2012, 23, 1041–1050. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Seki, Y.; Vuguin, P.M.; Du, X.Q.; Fiallo, A.; Glenn, A.S.; Singer, S.; Breuhahn, K.; Katz, E.B.; Charron, M.J. High-Fat Intake during Pregnancy and Lactation Exacerbates High-Fat Diet-Induced Complications in Male Offspring in Mice. Endocrinology 2013, 154, 3565–3576. [Google Scholar] [CrossRef]
- Pomar, C.A.; Picó, C.; Palou, A.; Sánchez, J. Maternal Consumption of a Cafeteria Diet during Lactation Leads to Altered Diet-Induced Thermogenesis in Descendants after Exposure to a Western Diet in Adulthood. Nutrients 2022, 14, 1958. [Google Scholar] [CrossRef]
- Picó, C.; Palou, M.; Pomar, C.A.; Palou, A. Benefits of Breastfeeding in Infant Health: A Role for Milk Signaling Peptides. In Molecular Nutrition: Mother and Infant; Academic Press: Cambridge, MA, USA, 2021; pp. 29–56. [Google Scholar] [CrossRef]
- Ellsworth, L.; Perng, W.; Harman, E.; Das, A.; Pennathur, S.; Gregg, B. Impact of Maternal Overweight and Obesity on Milk Composition and Infant Growth. Matern. Child Nutr. 2020, 16, e12979. [Google Scholar] [CrossRef]
- Castro, H.; Pomar, C.A.; Palou, A.; Picó, C.; Sánchez, J. Offspring Predisposition to Obesity Due to Maternal-Diet-Induced Obesity in Rats Is Preventable by Dietary Normalization before Mating. Mol. Nutr. Food Res. 2017, 61, 1473–1478. [Google Scholar] [CrossRef]
- Priego, T.; Sánchez, J.; Picó, C.; Palou, A. Sex-Differential Expression of Metabolism-Related Genes in Response to a High-Fat Diet. Obesity 2008, 16, 819–826. [Google Scholar] [CrossRef]
- Salinero, A.E.; Anderson, B.M.; Zuloaga, K.L. Sex Differences in the Metabolic Effects of Diet-Induced Obesity Vary by Age of Onset. Int. J. Obes. 2018, 42, 1088–1091. [Google Scholar] [CrossRef]
- Arcones, A.C.; Cruces-Sande, M.; Ramos, P.; Mayor, F.; Murga, C. Sex Differences in High Fat Diet-Induced Metabolic Alterations Correlate with Changes in the Modulation of GRK2 Levels. Cells 2019, 8, 1464. [Google Scholar] [CrossRef]
- Cignarelli, A.; Genchi, V.A.; Perrini, S.; Natalicchio, A.; Laviola, L.; Giorgino, F. Insulin and Insulin Receptors in Adipose Tissue Development. Int. J. Mol. Sci. 2019, 20, 759. [Google Scholar] [CrossRef] [PubMed]
- Laviola, L.; Perrini, S.; Cignarelli, A.; Giorgino, F. Insulin Signalling in Human Adipose Tissue. Arch. Physiol. Biochem. 2006, 112, 82–88. [Google Scholar] [CrossRef] [PubMed]
- De la Cruz-Color, L.; Hernández-Nazará, Z.H.; Maldonado-González, M.; Navarro-Muñíz, E.; Domínguez-Rosales, J.A.; Torres-Baranda, J.R.; Ruelas-Cinco, E.D.C.; Ramírez-Meza, S.M.; Ruíz-Madrigal, B. Association of the PNPLA2, SCD1 and Leptin Expression with Fat Distribution in Liver and Adipose Tissue from Obese Subjects. Exp. Clin. Endocrinol. Diabetes 2020, 128, 715–722. [Google Scholar] [CrossRef] [PubMed]
- Jeon, T.I.; Osborne, T.F. SREBPs: Metabolic Integrators in Physiology and Metabolism. Trends Endocrinol. Metab. 2012, 23, 65–72. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, P.; Pomar, C.A.; Palou, A.; Palou, M.; Picó, C. Influence of Maternal Metabolic Status and Diet during the Perinatal Period on the Metabolic Programming by Leptin Ingested during the Suckling Period in Rats. Nutrients 2023, 15, 570. https://doi.org/10.3390/nu15030570
Castillo P, Pomar CA, Palou A, Palou M, Picó C. Influence of Maternal Metabolic Status and Diet during the Perinatal Period on the Metabolic Programming by Leptin Ingested during the Suckling Period in Rats. Nutrients. 2023; 15(3):570. https://doi.org/10.3390/nu15030570
Chicago/Turabian StyleCastillo, Pedro, Catalina Amadora Pomar, Andreu Palou, Mariona Palou, and Catalina Picó. 2023. "Influence of Maternal Metabolic Status and Diet during the Perinatal Period on the Metabolic Programming by Leptin Ingested during the Suckling Period in Rats" Nutrients 15, no. 3: 570. https://doi.org/10.3390/nu15030570
APA StyleCastillo, P., Pomar, C. A., Palou, A., Palou, M., & Picó, C. (2023). Influence of Maternal Metabolic Status and Diet during the Perinatal Period on the Metabolic Programming by Leptin Ingested during the Suckling Period in Rats. Nutrients, 15(3), 570. https://doi.org/10.3390/nu15030570