The Influence of SARS-CoV-2 Pandemic on the Diagnosis of Celiac Disease and Clinical Practice in Pediatric Gastroenterology
Abstract
1. Introduction
2. Materials and Methods
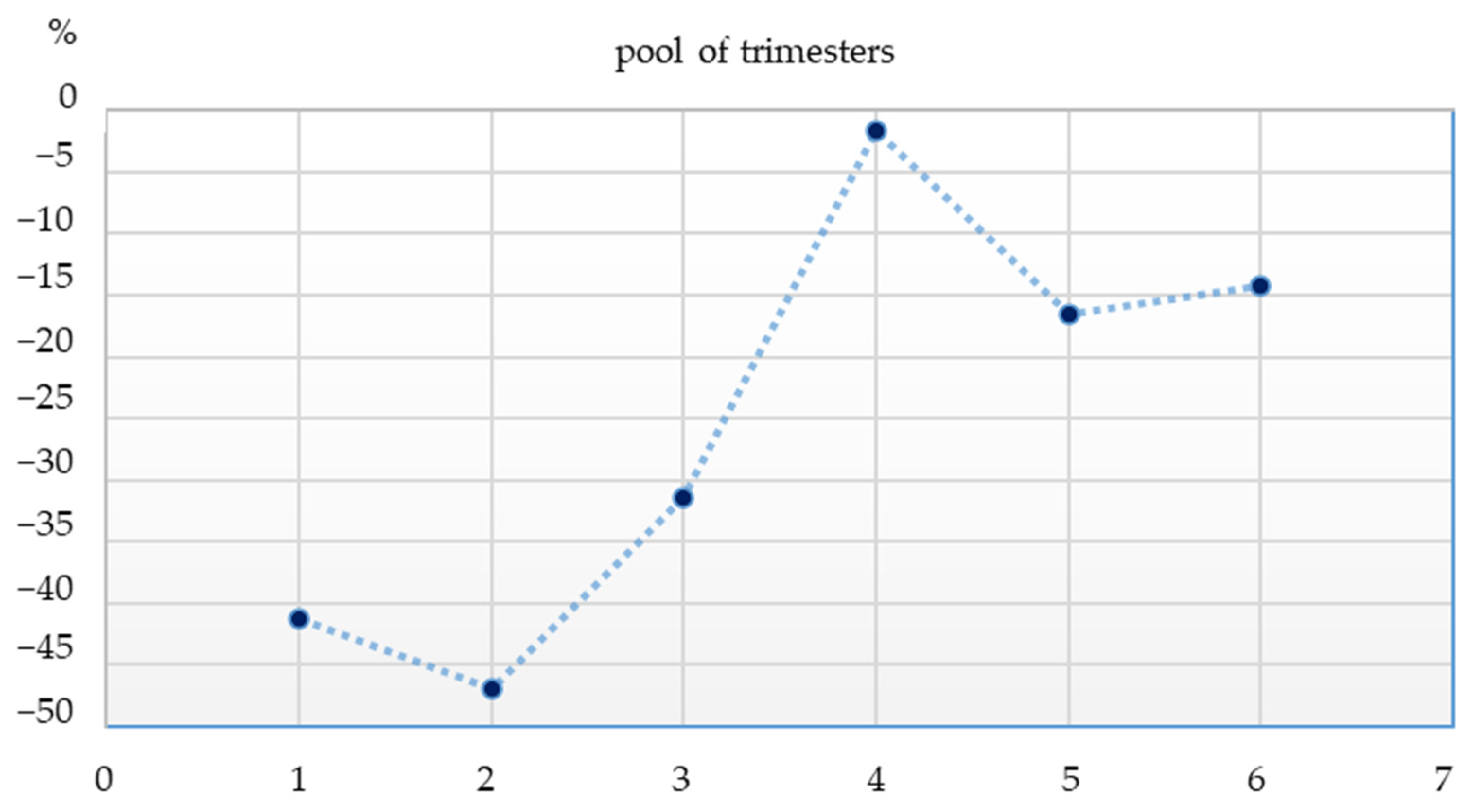
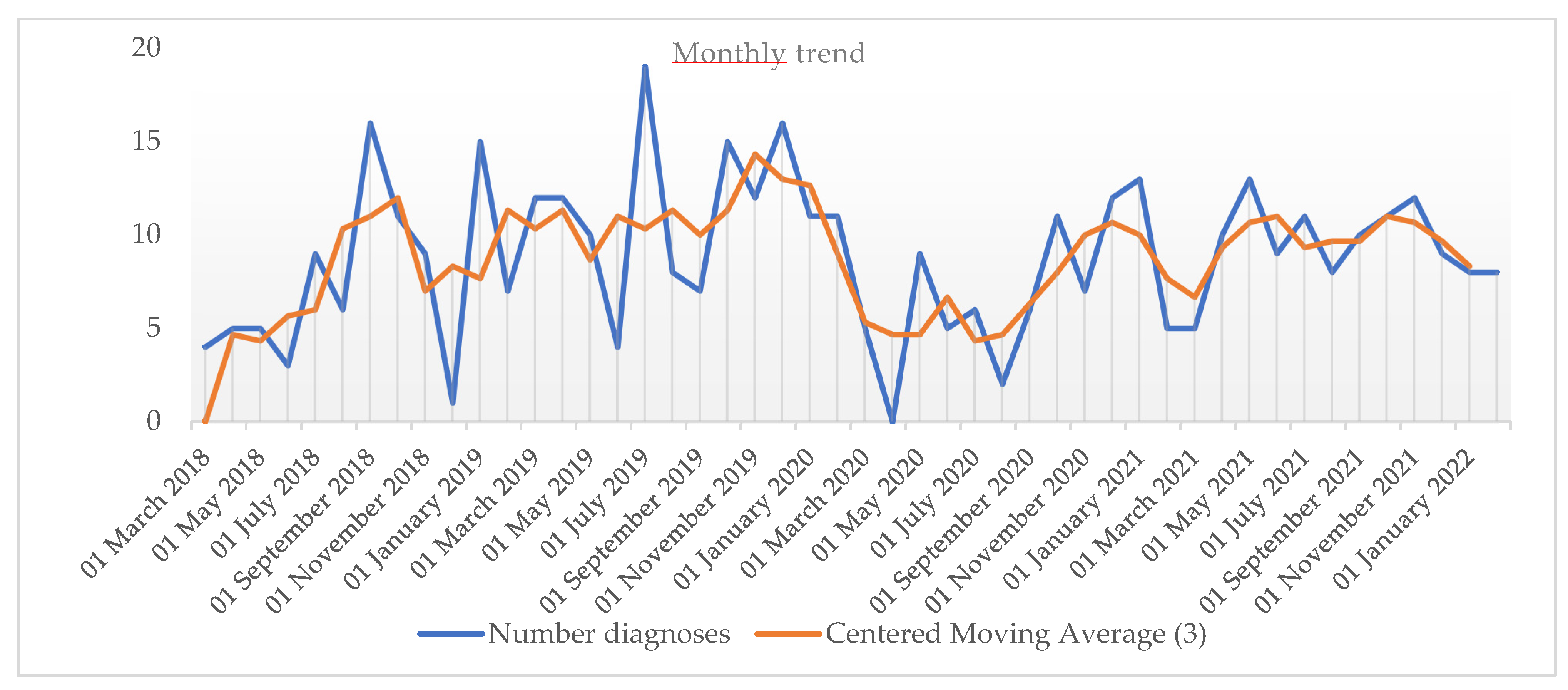
3. Results
4. Discussion
4.1. The Influence of SARS-CoV-2 Pandemic on Pediatric Gastroenterology Clinical Practice
4.2. The Influence of SARS-CoV-2 Pandemic on the Frequency of New Diagnoses of CD in a Pediatric Gastroenterology Tertiary Center
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smatti, M.K.; Cyprian, F.S.; Nasrallah, G.K.; Al Thani, A.A.; Almishal, R.O.; Yassine, H.M. Viruses and Autoimmunity: A Review on the Potential Interaction and Molecular Mechanisms. Viruses 2019, 11, 762. [Google Scholar] [CrossRef] [PubMed]
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Plot, L.; Amital, H. Infectious associations of Celiac disease. Autoimmun. Rev. 2009, 8, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, B.L.; Yu, J.; Tanaka, C.; Longhurst, C.A.; Kim, J.J. Incidence of New-Onset Type 1 Diabetes Among US Children During the COVID-19 Global Pandemic. JAMA Pediatr. 2022, 176, 414–415. [Google Scholar] [CrossRef]
- Verdoni, L.; Mazza, A.; Gervasoni, A.; Martelli, L.; Ruggeri, M.; Ciuffreda, M.; Bonanomi, E.; D’Antiga, L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: An observational cohort study. Lancet 2020, 395, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Viner, R.M.; Mytton, O.T.; Bonell, C.; Melendez-Torres, G.J.; Ward, J.; Hudson, L.; Waddington, C.; Thomas, J.; Russell, S.; van der Klis, F.; et al. Susceptibility to SARS-CoV-2 Infection Among Children and Adolescents Compared with Adults: A Systematic Review and Meta-analysis. JAMA Pediatr. 2021, 175, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Dowell, A.C.; Butler, M.S.; Jinks, E.; Tut, G.; Lancaster, T.; Sylla, P.; Begum, J.; Bruton, R.; Pearce, H.; Verma, K.; et al. Children develop robust and sustained cross-reactive spike-specific immune responses to SARS-CoV-2 infection. Nat. Immunol. 2022, 23, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Gokden, Y.; Hot, S.; Adas, M.; Ogutmen Koc, D.; Atak, S.; Hot, A.B. Celiac disease and COVID-19 pandemic: Should we worry? Acta Gastroenterol. Belg. 2020, 83, 517–525. [Google Scholar] [PubMed]
- Uche-Anya, E.; Husby, S.; Kaplan, G.G.; Underwood, F.E.; Green, P.H.R.; Lebwohl, B. An International Reporting Registry of Patients with Celiac Disease and COVID-19: Initial Results From SECURE-CELIAC. Clin. Gastroenterol. Hepatol. 2021, 19, 2435–2437.e4. [Google Scholar] [CrossRef] [PubMed]
- Gasbarrini, G.; Dionisi, T.; Corazza, G.R.; Aronico, N.; Cammarota, G.; Ianiro, G.; De Vitis, I.; Candelli, M.; Mancarella, F.A.; Simeoni, S.; et al. COVID-19 in celiac disease: A multicentric retrospective cohort study. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4400–4404. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Alimenti, E.; Maimaris, S.; Nicolardi, M.L.; Manzella La Barbera, F.; Baiardi, P.; Biagi, F. Prevalence, incidence and clinical features of SARS-CoV-2 infection in adult coeliac patients. Eur. J. Gastroenterol. Hepatol. 2021, 33, 1361–1366. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.P.; Lau, W.S.; Khan, Z.H.; Heaton, P.A. Letter: No-biopsy pathway for diagnosing adult coeliac disease. Aliment Pharmacol. Ther. 2021, 53, 357–358. [Google Scholar] [CrossRef] [PubMed]
- Penny, H.A.; Raju, S.A.; Lau, M.S.; Marks, L.J.; Baggus, E.M.; Bai, J.C.; Bassotti, G.; Bontkes, H.J.; Carroccio, A.; Danciu, M.; et al. Accuracy of a no-biopsy approach for the diagnosis of coeliac disease across different adult cohorts. Gut 2021, 70, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Elli, L.; Barisani, D.; Vaira, V.; Bardella, M.T.; Topa, M.; Vecchi, M.; Doneda, L.; Scricciolo, A.; Lombardo, V.; Roncoroni, L. How to manage celiac disease and gluten-free diet during the COVID-19 era: Proposals from a tertiary referral center in a high-incidence scenario. BMC Gastroenterol. 2020, 20, 87. [Google Scholar] [CrossRef]
- Gralnek, I.M.; Hassan, C.; Beilenhoff, U.; Antonelli, G.; Ebigbo, A.; Pellisè, M.; Arvanitakis, M.; Bhandari, P.; Bisschops, R.; Van Hooft, J.E.; et al. ESGE and ESGENA Position Statement on gastrointestinal endoscopy and the COVID-19 pandemic. Endoscopy 2020, 52, 483–490. [Google Scholar] [CrossRef]
- Trovato, C.M.; Montuori, M.; Cucchiara, S.; Oliva, S. ESPGHAN ‘biopsy-sparing’ guidelines for celiac disease in children with low antitransglutaminase during COVID-19. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1523–1526. [Google Scholar] [CrossRef]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.; Kurppa, K.; Mearin, M.L.; Ribes-Koninckx, C.; Shamir, R.; Troncone, R.; Auricchio, R.; Castillejo, G.; et al. European Society Paediatric Gastroenterology, Hepatology and Nutrition Guidelines for Diagnosing Coeliac Disease 2020. J. Pediatr. Gastroenterol. Nutr. 2020, 70, 141–156. [Google Scholar] [CrossRef]
- Tripathi, K.; Godoy Brewer, G.; Thu Nguyen, M.; Singh, Y.; Saleh Ismail, M.; Sauk, J.S.; Parian, A.M.; Limketkai, B.N. COVID-19 and Outcomes in Patients with Inflammatory Bowel Disease: Systematic Review and Meta-Analysis. Inflamm. Bowel Dis. 2022, 28, 1265–1279. [Google Scholar] [CrossRef]
- Maida, M.; Sferrazza, S.; Savarino, E.; Ricciardiello, L.; Repici, A.; Morisco, F.; Furnari, M.; Fuccio, L.; Morreale, G.C.; Vitello, A.; et al. Impact of the COVID-19 pandemic on Gastroenterology Divisions in Italy: A national survey. Dig. Liver Dis. 2020, 52, 808–815. [Google Scholar] [CrossRef]
- Catassi, G.N.; Vallorani, M.; Cerioni, F.; Lionetti, E.; Catassi, C. A negative fallout of COVID-19 lockdown in Italy: Life-threatening delay in the diagnosis of celiac disease. Dig. Liver Dis. 2020, 52, 1092–1093. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.H.; Rakkar, J.; Au, A.K.; Fuhrman, D.; Clark, R.S.B.; Horvat, C.M. Trends in US Pediatric Hospital Admissions in 2020 Compared with the Decade Before the COVID-19 Pandemic. JAMA Netw. Open 2021, 4, e2037227. [Google Scholar] [CrossRef] [PubMed]
- Nourazari, S.; Davis, S.R.; Granovsky, R.; Austin, R.; Straff, D.J.; Joseph, J.W.; Sanchez, L.D. Decreased hospital admissions through emergency departments during the COVID-19 pandemic. Am. J. Emerg. Med. 2021, 42, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Pines, J.M.; Zocchi, M.S.; Black, B.S.; Carlson, J.N.; Celedon, P.; Moghtaderi, A.; Venkat, A.; US Acute Care Solutions Research Group. Characterizing pediatric emergency department visits during the COVID-19 pandemic. Am. J. Emerg. Med. 2021, 41, 201–204. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Keshvari, M.; Mirnasuri, S.; Yon, D.K.; Lee, S.W.; Il Shin, J.; Smith, L. The global impact of COVID-19 pandemic on the incidence of pediatric new-onset type 1 diabetes and ketoacidosis: A systematic review and meta-analysis. J. Med. Virol. 2022, 94, 5112–5127. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.Y.; Ramakrishna, S.; Long, A.H.; Phillips, C.A.; Montiel-Esparza, R.; Diorio, C.J.; Bailey, L.C.; Maude, S.L.; Aplenc, R.; Batra, V.; et al. Delayed cancer diagnoses and high mortality in children during the COVID-19 pandemic. Pediatr. Blood Cancer 2020, 67, e28427. [Google Scholar] [CrossRef] [PubMed]
- Federazione Italiana delle Società delle Malattie dell’Apparato Digerente. COVID-19: Consigli FISMAD per l’assistenza ai pazienti con malattie dell’apparato digerente e per gli operatori sanitari in Gastroenterologia. Available online: https://fismad.it/wp-content/uploads/2020/03/COVID-19_FISMAD.pdf (accessed on 10 November 2022).
- Kriem, J.; Rahhal, R. COVID-19 pandemic and challenges in pediatric gastroenterology practice. World J. Gastroenterol. 2020, 26, 5387–5394. [Google Scholar] [CrossRef]
- Lebwohl, B.; Rubio-Tapia, A. Epidemiology, Presentation, and Diagnosis of Celiac Disease. Gastroenterology 2021, 160, 63–75. [Google Scholar] [CrossRef]
- Lechtman, N.; Shamir, R.; Cohen, S.; Chodick, G.; Kariv, R.; Supino-Rosin, L.; Weintraub, Y.; Yerushalmy-Feler, A.; Ben Tov, A. Increased incidence of coeliac disease autoimmunity rate in Israel: A 9-year analysis of population-based data. Aliment Pharmacol. Ther. 2021, 53, 696–703. [Google Scholar] [CrossRef]
- Gatti, S.; Lionetti, E.; Balanzoni, L.; Verma, A.K.; Galeazzi, T.; Gesuita, R.; Scattolo, N.; Cinquetti, M.; Fasano, A.; Catassi, C.; et al. Increased Prevalence of Celiac Disease in School-age Children in Italy. Clin. Gastroenterol. Hepatol. 2020, 18, 596–603. [Google Scholar] [CrossRef]
- Renzo, S.; Scarallo, L.; Antoniello, L.M.; Bramuzzo, M.; Chiaro, A.; Cisarò, F.; Contini, A.C.I.; De Angelis, G.L.; De Angelis, P.; Di Nardo, G.; et al. Impact of COVID-19 pandemic on pediatric endoscopy: A multicenter study on behalf of the SIGENP Endoscopy Working Group. Dig. Liver Dis. 2022, 54, 572–579. [Google Scholar] [CrossRef]
- Lazzerini, M.; Barbi, E.; Apicella, A.; Marchetti, F.; Cardinale, F.; Trobia, G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc. Health 2020, 4, e10–e11. [Google Scholar] [CrossRef]
- Morales, J.S.; Valenzuela, P.L.; Castillo-García, A.; Butragueño, J.; Jiménez-Pavón, D.; Carrera-Bastos, P.; Lucia, A. The Exposome and Immune Health in Times of the COVID-19 Pandemic. Nutrients 2021, 14, 24. [Google Scholar] [CrossRef]
- Vojdani, A.; Vojdani, E.; Rosenberg, A.Z.; Shoenfeld, Y. The Role of Exposomes in the Pathophysiology of Autoimmune Diseases II: Pathogens. Pathophysiology 2022, 29, 243–280. [Google Scholar] [CrossRef] [PubMed]
- Myléus, A.; Hernell, O.; Gothefors, L.; Hammarström, M.L.; Persson, L.Å.; Stenlund, H.; Ivarsson, A. Early infections are associated with increased risk for celiac disease: An incident case-referent study. BMC Pediatr. 2012, 12, 194. [Google Scholar] [CrossRef]
- Canova, C.; Zabeo, V.; Pitter, G.; Romor, P.; Baldovin, T.; Zanotti, R.; Simonato, L. Association of maternal education, early infections, and antibiotic use with celiac disease: A population-based birth cohort study in northeastern Italy. Am. J. Epidemiol. 2014, 180, 76–85. [Google Scholar] [CrossRef]
- Kemppainen, K.M.; Lynch, K.F.; Liu, E.; Lönnrot, M.; Simell, V.; Briese, T.; Koletzko, S.; Hagopian, W.; Rewers, M.; She, J.X.; et al. Factors That Increase Risk of Celiac Disease Autoimmunity After a Gastrointestinal Infection in Early Life. Clin. Gastroenterol. Hepatol. 2017, 15, 694–702.e5. [Google Scholar] [CrossRef]
- Tye-Din, J.A.; Galipeau, H.J.; Agardh, D. Celiac Disease: A Review of Current Concepts in Pathogenesis, Prevention, and Novel Therapies. Front. Pediatr. 2018, 6, 350. [Google Scholar] [CrossRef] [PubMed]
- Kothandaraman, N.; Rengaraj, A.; Xue, B.; Yew, W.S.; Velan, S.S.; Karnani, N.; Leow, M.K.S. COVID-19 endocrinopathy with hindsight from SARS. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E139–E150. [Google Scholar] [CrossRef] [PubMed]
- Louis, T.J.; Qasem, A.; Abdelli, L.S.; Naser, S.A. Extra-Pulmonary Complications in SARS-CoV-2 Infection: A Comprehensive Multi Organ-System Review. Microorganisms 2022, 10, 153. [Google Scholar] [CrossRef]
- Jiang, L.; Tang, K.; Irfan, O.; Li, X.; Zhang, E.; Bhutta, Z. Epidemiology, Clinical Features, and Outcomes of Multisystem Inflammatory Syndrome in Children (MIS-C) and Adolescents-a Live Systematic Review and Meta-analysis. Curr. Pediatr. Rep. 2022, 10, 19–30. [Google Scholar] [CrossRef]
- Dendrou, C.A.; Petersen, J.; Rossjohn, J.; Fugger, L. HLA variation and disease. Nat. Rev. Immunol. 2018, 18, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Castelli, E.C.; de Castro, M.V.; Naslavsky, M.S.; Scliar, M.O.; Silva, N.S.B.; Pereira, R.N.; Ciriaco, V.A.O.; Castro, C.F.B.; Mendes-Junior, C.T.; Silveira, E.S.; et al. MUC22, HLA-A, and HLA-DOB variants and COVID-19 in resilient super-agers from Brazil. Front. Immunol. 2022, 13, 975918. [Google Scholar] [CrossRef] [PubMed]
- Greco, N.; Meacci, A.; Mora, B.; Vestri, A.; Picarelli, A. Coeliac disease in the COVID-19 pandemic: Does HLA have a protective effect? Ann. Med. 2022, 54, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Stephens, H.A.; Klaythong, R.; Sirikong, M.; Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Endy, T.P.; Libraty, D.H.; Nisalak, A.; Innis, B.L.; et al. HLA-A and -B allele associations with secondary dengue virus infections correlate with disease severity and the infecting viral serotype in ethnic Thais. Tissue Antigens 2002, 60, 309–318. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, K.S.; Fowke, K.R.; Kimani, J.; Dunand, V.A.; Nagelkerke, N.J.; Ball, T.B.; Oyugi, J.; Njagi, E.; Gaur, L.K.; Brunham, R.C.; et al. Influence of HLA supertypes on susceptibility and resistance to human immunodeficiency virus type 1 infection. J. Infect. Dis. 2000, 181, 1581–1589. [Google Scholar] [CrossRef]
- Andrianou, X.D.; Konstantinou, C.; Rodríguez-Flores, M.A.; Papadopoulos, F.; Makris, K.C. Population-wide measures due to the COVID-19 pandemic and exposome changes in the general population of Cyprus in March-May 2020. BMC Public Health 2022, 22, 2279. [Google Scholar] [CrossRef]
- Monzani, A.; Lionetti, E.; Felici, E.; Fransos, L.; Azzolina, D.; Rabbone, I.; Catassi, C. Adherence to the Gluten-Free Diet during the Lockdown for COVID-19 Pandemic: A Web-Based Survey of Italian Subjects with Celiac Disease. Nutrients 2020, 12, 3467. [Google Scholar] [CrossRef]
- Lionetti, E.; Catassi, C.; Lionetti, P. COVID-19 e la malattia celiaca. Giorn. Gastr. Epatol. Nutr. Ped. 2020, XII, 76–80. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crocco, M.; Calvi, A.; Canzoneri, F.; Malerba, F.; Zampatti, N.; Chiaro, A.; Arrigo, S.; Gandullia, P.; Proietti, S.; Bonassi, S. The Influence of SARS-CoV-2 Pandemic on the Diagnosis of Celiac Disease and Clinical Practice in Pediatric Gastroenterology. Nutrients 2023, 15, 559. https://doi.org/10.3390/nu15030559
Crocco M, Calvi A, Canzoneri F, Malerba F, Zampatti N, Chiaro A, Arrigo S, Gandullia P, Proietti S, Bonassi S. The Influence of SARS-CoV-2 Pandemic on the Diagnosis of Celiac Disease and Clinical Practice in Pediatric Gastroenterology. Nutrients. 2023; 15(3):559. https://doi.org/10.3390/nu15030559
Chicago/Turabian StyleCrocco, Marco, Angela Calvi, Francesca Canzoneri, Federica Malerba, Noemi Zampatti, Andrea Chiaro, Serena Arrigo, Paolo Gandullia, Stefania Proietti, and Stefano Bonassi. 2023. "The Influence of SARS-CoV-2 Pandemic on the Diagnosis of Celiac Disease and Clinical Practice in Pediatric Gastroenterology" Nutrients 15, no. 3: 559. https://doi.org/10.3390/nu15030559
APA StyleCrocco, M., Calvi, A., Canzoneri, F., Malerba, F., Zampatti, N., Chiaro, A., Arrigo, S., Gandullia, P., Proietti, S., & Bonassi, S. (2023). The Influence of SARS-CoV-2 Pandemic on the Diagnosis of Celiac Disease and Clinical Practice in Pediatric Gastroenterology. Nutrients, 15(3), 559. https://doi.org/10.3390/nu15030559






