Medical Nutrition Therapy in Critically Ill Patients with COVID-19—A Single-Center Observational Study
Abstract
1. Introduction
2. Materials and Methods
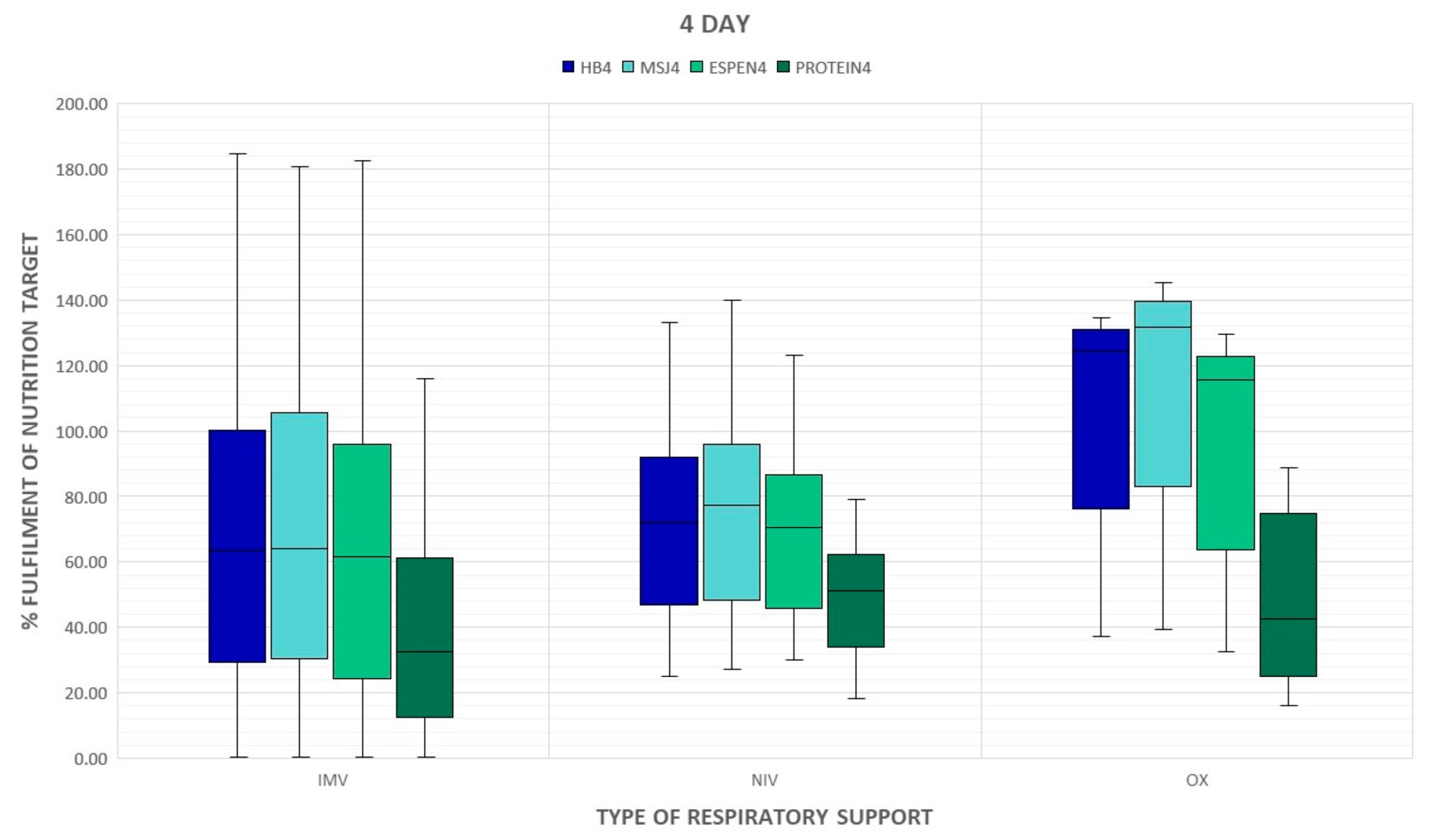
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fish, M.; Rynne, J.; Jennings, A.; Lam, C.; Lamikanra, A.A.; Ratcliff, J.; Cellone-Trevelin, S.; Timms, E.; Jiriha, J.; Tosi, I.; et al. Coronavirus disease 2019 subphenotypes and differential treatment response to convalescent plasma in critically ill adults: Secondary analyses of a randomized clinical trial. Intensive Care Med. 2022, 48, 1525–1538. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Putowski, Z.; Czok, M.; Liberski, I.S.; Krzych, J. Basics of Mechanical Ventilation for Non-Aneasthetists. Part 2: Clinical Aspects. Adv. Respir. Med. 2020, 88, 580–589. [Google Scholar] [CrossRef]
- Putowski, Z.; Szczepańska, A.; Czok, M.; Krzych, Ł.J. Veno-Venous Extracorporeal Membrane Oxygenation in COVID-19—Where Are We Now? Int. J. Environ. Res. Public Health 2021, 18, 1173. [Google Scholar] [CrossRef]
- Barazzoni, R.; Bischoff, S.C.; Breda, J.; Wickramasinghe, K.; Krznaric, Z.; Nitzan, D.; Pirlich, M.; Singer, P. ESPEN expert statements and practical guidance for nutritional management of individuals with SARS-CoV-2 infection. Clin. Nutr. 2020, 39, 1631–1638. [Google Scholar] [CrossRef]
- Bedock, D.; Couffignal, J.; Lassen, P.B.; Soares, L.; Mathian, A.; Fadlallah, J.; Amoura, Z.; Oppert, J.-M.; Faucher, P. Evolution of Nutritional Status after Early Nutritional Management in COVID-19 Hospitalized Patients. Nutrients 2021, 13, 2276. [Google Scholar] [CrossRef]
- Kokoszka-Bargieł, I.; Cyprys, P.; Madeja, P.; Rutkowska, K.; Wajda-Pokrontka, M.; Madowicz, J.; Knapik, P. Factors influencing death in COVID-19 patients treated in the ICU: A single-centre, cross-sectional study. Anaesthesiol Intensive Ther. 2022, 54, 132–140. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Mifflin, M.D.; St Jeor, S.T.; Hill, L.A.; Scott, B.J.; Daugherty, S.A.; Koh, Y.O. A new predictive equation for resting energy expenditure in healthy individuals. Am. J. Clin. Nutr. 1990, 51, 241–247. [Google Scholar] [CrossRef]
- Roza, A.M.; Shizgal, H.M. The Harris Benedict equation reevaluated: Resting energy requirements and the body cell mass. Am. J. Clin. Nutr. 1984, 40, 168–182. [Google Scholar] [CrossRef]
- Reeves, A.; White, H.; Sosnowski, K.; Tran, K.; Jones, M.; Palmer, M. Energy and protein intakes of hospitalised patients with acute respiratory failure receiving non-invasive ventilation. Clin. Nutr. 2014, 33, 1068–1073. [Google Scholar] [CrossRef]
- Thomas, S.; Alexander, C.; Cassady, B.A. Nutrition risk prevalence and nutrition care recommendations for hospitalized and critically-ill patients with COVID-19. Clin. Nutr. ESPEN 2021, 44, 38–49. [Google Scholar] [CrossRef]
- Zusman, O.; Theilla, M.; Cohen, J.; Kagan, I.; Bendavid, I.; Singer, P. Resting energy expenditure, calorie and protein consumption in critically ill patients: A retrospective cohort study. Crit. Care 2016, 20, 1–8. [Google Scholar] [CrossRef]
- Dvir, D.; Cohen, J.; Singer, P. Computerized energy balance and complications in critically ill patients: An observational study. Clin. Nutr. 2006, 25, 37–44. [Google Scholar] [CrossRef]
- Choi, E.Y.; Park, D.-A.; Park, J. Calorie Intake of Enteral Nutrition and Clinical Outcomes in Acutely Critically Ill Patients: A me-ta-analysis of randomized controlled trials. J. Parenter. Enter. Nutr. 2014, 39, 291–300. [Google Scholar] [CrossRef]
- Al-Dorzi, H.M.; Albarrak, A.; Ferwana, M.; Murad, M.H.; Arabi, Y.M. Lower versus higher dose of enteral caloric intake in adult critically ill patients: A systematic review and meta-analysis. Crit. Care 2016, 20, 1–19. [Google Scholar] [CrossRef]
- Marik, P.E.; Hooper, M.H. Normocaloric versus hypocaloric feeding on the outcomes of ICU patients: A systematic review and meta-analysis. Intensiv. Care Med. 2016, 42, 316–323. [Google Scholar] [CrossRef]
- Song, J.H.; Lee, H.S.; Kim, S.Y.; Kim, E.Y.; Jung, J.Y.; Kang, Y.A.; Park, M.S.; Kim, Y.S.; Kim, S.K.; Chang, J.; et al. The influence of protein provision in the early phase of intensive care on clinical outcomes for critically ill patients on mechanical ventilation. Asia Pac. J. Clin. Nutr. 2017, 26, 234–240. [Google Scholar] [CrossRef]
- Allingstrup, M.J.; Esmailzadeh, N.; Knudsen, A.W.; Espersen, K.; Jensen, T.H.; Wiis, J.; Perner, A.; Kondrup, J. Provision of protein and energy in relation to measured requirements in intensive care patients. Clin. Nutr. 2012, 31, 462–468. [Google Scholar] [CrossRef]
- Nicolo, M.; Heyland, D.K.; Chittams, J.; Sammarco, T.; Compher, C. Clinical Outcomes Related to Protein Delivery in a Critically Ill Population: A Multicenter, Multinational Observation Study. J. Parenter. Enter. Nutr. 2016, 40, 45–51. [Google Scholar] [CrossRef]
- Matejovic, M.; Huet, O.; Dams, K.; Elke, G.; Alonso, C.V.; Csomos, A.; Krzych, J.; Tetamo, R.; Puthucheary, Z.; Rooyackers, O.; et al. Medical nutrition therapy and clinical outcomes in critically ill adults: A European multinational, prospective observational cohort study (EuroPN). Crit. Care 2022, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.; Dumont, N.; Clemente, R.; Allan, K.; Downer, C.; Mitchell, A. Critical care: Meeting protein requirements without overfeeding energy. Clin. Nutr. ESPEN 2016, 11, e55–e62. [Google Scholar] [CrossRef]
- Czuczwar, M.; Potręć, B. Nutritional intervention in the intensive care unit according to recent guidelines. Anaesthesiol. Intensive Ther. 2021, 2018, 1–7. [Google Scholar]
- Chapple, L.S.; Weinel, L.; Ridley, E.J.; Jones, D.; Chapman, M.J.; Peake, S.L. Clinical Sequelae From Overfeeding in Enterally Fed Critically Ill Adults: Where Is the Evidence? J. Parenter. Enter. Nutr. 2020, 44, 980–991. [Google Scholar] [CrossRef] [PubMed]
- Weijs, P.J.; Looijaard, W.G.; Beishuizen, A.; Girbes, A.R.; Straaten, H.M.O.-V. Early high protein intake is associated with low mortality and energy overfeeding with high mortality in non-septic mechanically ventilated critically ill patients. Crit. Care 2014, 18, 701. [Google Scholar] [CrossRef]
- Langer, T.; Brioni, M.; Guzzardella, A.; Carlesso, E.; Cabrini, L.; Castelli, G.; Dalla Corte, F.; De Robertis, E.; Favarato, M.; Forastieri, A.; et al. Prone position in intubated, mechanically ventilated patients with COVID-19: A multi-centric study of more than 1000 patients. Crit. Care 2021, 25, 128. [Google Scholar] [CrossRef]
- McAuley, D.; Giles, S.; Fichter, H.; Perkins, G.; Gao, F. What is the optimal duration of ventilation in the prone position in acute lung injury and acute respiratory distress syndrome? Intensive Care Med. 2002, 28, 414–418. [Google Scholar] [CrossRef]
- Munshi, L.; Del Sorbo, L.; Adhikari, N.K.J.; Hodgson, C.L.; Wunsch, H.; Meade, M.O.; Uleryk, E.; Mancebo, J.; Pesenti, A.; Ranieri, V.M.; et al. Prone Position for Acute Respiratory Distress Syndrome. A Systematic Review and Meta-Analysis. Ann. Am. Thorac. Soc. 2017, 14 (Suppl. S4), S280–S288. [Google Scholar] [CrossRef]
- Bruni, A.; Garofalo, E.; Grande, L.; Auletta, G.; Cubello, D.; Greco, M.; Lombardo, N.; Garieri, P.; Papaleo, A.; Doldo, P.; et al. Nursing issues in enteral nutrition during prone position in critically ill patients: A systematic review of the literature. Intensive Crit. Care Nurs. 2020, 60, 102899. [Google Scholar] [CrossRef]
- de Paula, J.A.; Rabito, E.I.; Justino, S.R.; Leite, L.S.; Dantas, D.; da Silva, J.S.M.; Maffini, L.F.; Júnior, O.R. Administration of enteral nutrition and gastrointestinal complications in Covid-19 critical patients in prone position. Clin. Nutr. Open Sci. 2022, 45, 80–90. [Google Scholar] [CrossRef]



| Category | No. (%) or Median (Q1–Q3) |
|---|---|
| Males/Females | 47 (65%)/25 (35%) |
| BMI [kg m−2] | 29 (25–34) |
| APACHE II [points] | 18 (14–25) |
| SAPS II [points] | 38.5 (30–49.5) |
| NRS score [points] | 4 (3–5) |
| CFS score [points] | 4 (3–5) |
| Albumin concentration [g dL−1] | 2.8 (2.5–3.0) |
| C-Reactive Protein concentration [mg dL−1] | 101 (48,1–150) |
| D-dimer concentration [ng mL−1] | 2739 (1449–6952.5) |
| Lactate dehydrogenase level [U L−1] | 559 (352–715.15) |
| Total lymphocyte count [103 µL−1] | 0.6 (0.39–0.89) |
| SOFA score [points] | 9 (5–12) |
| Acute respiratory failure with oxygen support | 74 (100%) |
| Oxygenation Index < 100 [mmHg] | 11 (15%) |
| Oxygenation Index 100–300 [mmHg] | 57 (79%) |
| Oxygenation Index > 300 [mmHg] | 4 (6%) |
| Acute circulatory failure with catecholamine support | 54 (75%) |
| Acute renal failure with renal replacement therapy | 11 (15%) |
| Day | Formula Used to Assess the Calorie Target | % of BMR Calorie Intake—Median (Q1–Q3) | <70% | 70–100% | >100% | n |
|---|---|---|---|---|---|---|
| 1 | HB | 24 (6–44) | 69(96%) | 3 (4%) | 0(0%) | 72 |
| MsJ | 24 (6–43) | 69(96%) | 3 (4%) | 0(0%) | ||
| 20 kcal/kg BW | 23 (6,47) | 69 (96%) | 3 (4%) | 0 (0%) | ||
| 2 | HB | 64 (33–86) | 41 (57%) | 18 (25%) | 13 (18%) | 72 |
| MsJ | 67 (36–91) | 38 (53%) | 20 (28%) | 14 (19%) | ||
| 20 kcal/kg BW | 60 (30–90) | 44 (61%) | 19 (26.5%) | 9 (12.5%) | ||
| 3 | HB | 57 (35–93) | 43 (62%) | 9 (13%) | 17 (25%) | 69 |
| MsJ | 61 (37–98) | 61 (37–98) | 61 (37–98) | 61 (37–98) | ||
| 20 kcal/kg BW | 43 (63%) | 43 (63%) | 43 (63%) | 43 (63%) | ||
| 4 | HB | 72 (34–113) | 72 (34–113) | 72 (34–113) | 72 (34–113) | 66 |
| MsJ | 33 (50%) | 33 (50%) | 33 (50%) | 33 (50%) | ||
| 20 kcal/kg BW | 16 (24%) | 16 (24%) | 16 (24%) | 16 (24%) | ||
| 5 | HB | 54 (33–102) | 54 (33–102) | 54 (33–102) | 54 (33–102) | 60 |
| MsJ | 33 (55%) | 33 (55%) | 33 (55%) | 33 (55%) | ||
| 20 kcal/kg BW | 11 (18%) | 11 (18%) | 11 (18%) | 11 (18%) | ||
| 6 | HB | 62 (42–92) | 62 (42–92) | 62 (42–92) | 62 (42–92) | 53 |
| MsJ | 30 (57%) | 30 (57%) | 30 (57%) | 30 (57%) | ||
| 20 kcal/kg BW | 11 (21%) | 11 (21%) | 11 (21%) | 11 (21%) | ||
| 7 | HB | 69 (48–99) | 69 (48–99) | 69 (48–99) | 69 (48–99) | 51 |
| MsJ | 27 (53%) | 27 (53%) | 27 (53%) | 27 (53%) | ||
| 20 kcal/kg BW | 14 (27%) | 14 (27%) | 14 (27%) | 14 (27%) |
| Day | % of Protein Intake—Median (Q1–Q3) | n |
|---|---|---|
| Day 1 | 11 (0–27) | 72 |
| Day 2 | 35 (15–57) | 72 |
| Day 3 | 34 (14–61) | 69 |
| Day 4 | 40 (19–61) | 66 |
| Day 5 | 38 (12,59) | 60 |
| Day 6 | 40 (22–63) | 53 |
| Day 7 | 43 (29–65) | 51 |
| Energy–BH | Energy–MsJ | Energy–20 kcal/kg BW | Protein Intake | ||
|---|---|---|---|---|---|
| Day 4 | APACHE II | r = −0.08 (p > 0.05) | r = −0.07 (p > 0.05) | r = −0.06 (p > 0.05) | r = −0.07 (p > 0.05) |
| SAPS II | r = −0.02 (p > 0.05) | r = −0.01 (p > 0.05) | r = −0.03 (p > 0.05) | r = 0.004 (p > 0.05) | |
| SOFA | r = −0.09 (p > 0.05) | r = −0.09 (p > 0.05) | r = −0.085 (p > 0.05) | r = −0.14 (p > 0.05) | |
| Day 7 | APACHE II | r = 0.14 (p > 0.05) | r = 0.14 (p > 0.05) | r = −0.11 (p > 0.05) | r = 0.21 (p > 0.05) |
| SAPS II | r = 0.02 (p > 0.05) | r = 0.16 (p > 0.05) | r = −0.24 (p < 0.05) | r = 0.28 (p < 0.05) | |
| SOFA | r = 0.2 (p > 0.05) | r = 0.19. (p > 0.05) | r = −0.13 (p > 0.05) | r = 0.21 (p > 0.05) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzych, Ł.J.; Taborek, M.; Winiarska, K.; Danel, J.; Nowotarska, A.; Jaworski, T. Medical Nutrition Therapy in Critically Ill Patients with COVID-19—A Single-Center Observational Study. Nutrients 2023, 15, 1086. https://doi.org/10.3390/nu15051086
Krzych ŁJ, Taborek M, Winiarska K, Danel J, Nowotarska A, Jaworski T. Medical Nutrition Therapy in Critically Ill Patients with COVID-19—A Single-Center Observational Study. Nutrients. 2023; 15(5):1086. https://doi.org/10.3390/nu15051086
Chicago/Turabian StyleKrzych, Łukasz J., Maria Taborek, Katarzyna Winiarska, Justyna Danel, Agnieszka Nowotarska, and Tomasz Jaworski. 2023. "Medical Nutrition Therapy in Critically Ill Patients with COVID-19—A Single-Center Observational Study" Nutrients 15, no. 5: 1086. https://doi.org/10.3390/nu15051086
APA StyleKrzych, Ł. J., Taborek, M., Winiarska, K., Danel, J., Nowotarska, A., & Jaworski, T. (2023). Medical Nutrition Therapy in Critically Ill Patients with COVID-19—A Single-Center Observational Study. Nutrients, 15(5), 1086. https://doi.org/10.3390/nu15051086






