Treatment of HMG-CoA Lyase Deficiency—Longitudinal Data on Clinical and Nutritional Management of 10 Australian Cases
Abstract
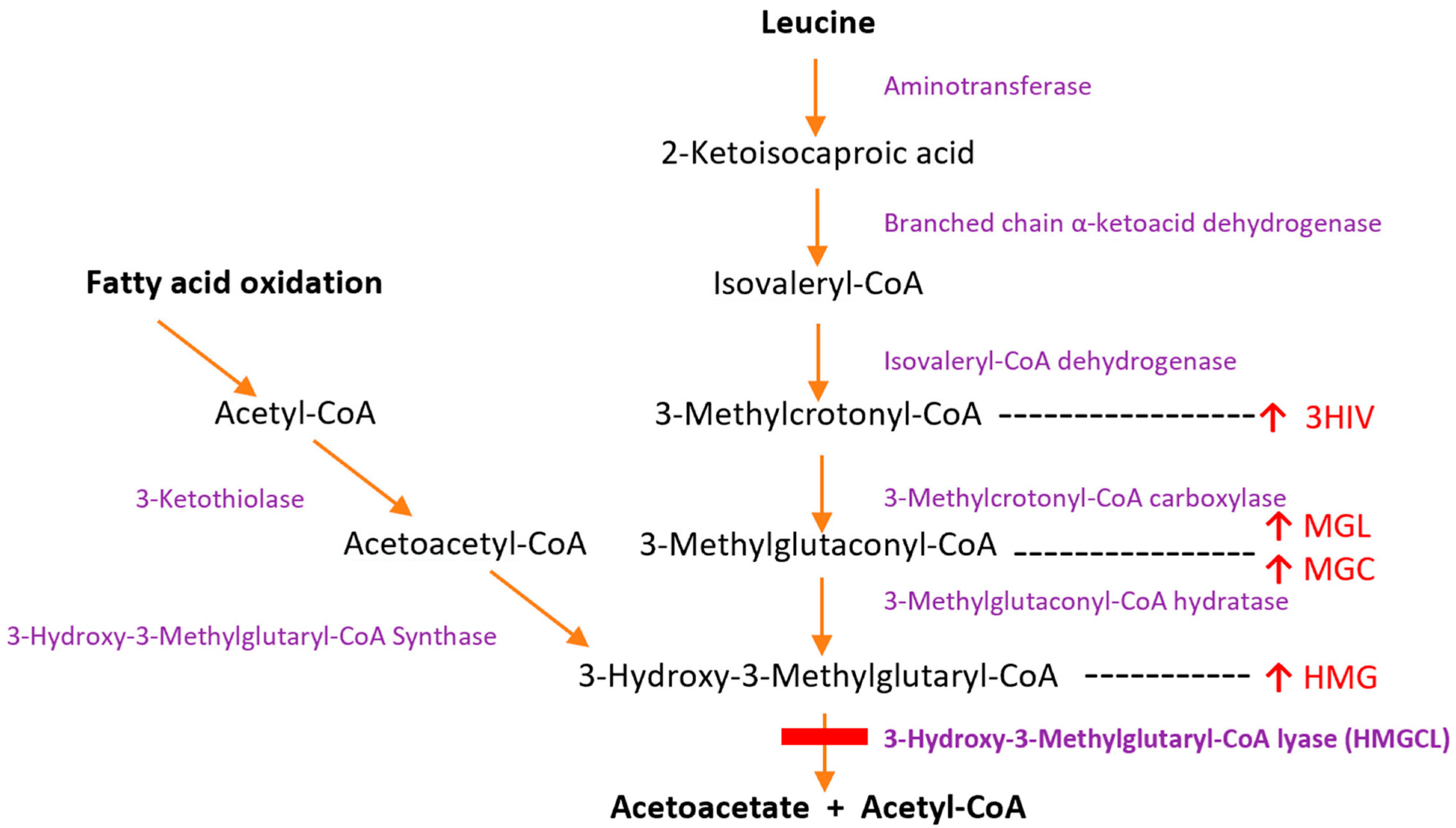
1. Introduction
1.1. History
1.2. Review of Treatment
1.3. This Study
2. Materials and Methods
3. Results
3.1. The Impact of Newborn Screening
3.2. Treatment
4. Discussion
4.1. Data on Illness and Fasting
4.2. Clinical Presentation in This Cohort
4.3. Pathophysiology
4.4. Utilization of Ketone Bodies
Stereoisomer Utilization
4.5. Development of Practice Guidelines
4.5.1. Glucose and Complex Carbohydrates
4.5.2. Fat- and Protein-Restricted Diet
4.5.3. Ketones
4.5.4. L-Carnitine
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pié, J.; López-Viñas, E.; Puisac, B.; Menao, S.; Pié, A.; Casale, C.; Ramos, F.J.; Hegardt, F.G.; Gómez-Puertas, P.; Casals, N. Molecular genetics of HMG-CoA lyase deficiency. Mol. Genet. Metab. 2007, 92, 198–209. [Google Scholar] [CrossRef]
- Faull, K.; Bolton, P.; Halpern, B.; Hammond, J.; Danks, D.M.; Hähnel, R.; Wilkinson, S.P.; Wysocki, S.J.; Masters, P.L. Letter: Patient with defect in leucine metabolism. N. Engl. J. Med. 1976, 294, 1013. [Google Scholar] [CrossRef]
- Wysocki, S.J.; Hähnel, R. 3-hydroxy-3-methylglutaric aciduria: 3-Hydroxy-3-methylglutaryl-coenzyme a lyase levels in leucocytes. Clin. Chim. Acta 1976, 73, 373–375. [Google Scholar] [CrossRef]
- Mitchell, G.A.; Robert, M.F.; Hruz, P.W.; Wang, S.; Fontaine, G.; Behnke, C.E.; Mende-Mueller, L.M.; Schappert, K.; Lee, C.; Gibson, K.M.; et al. 3-Hydroxy-3-methylglutaryl coenzyme A lyase (HL). Cloning of human and chicken liver HL cDNAs and characterization of a mutation causing human HL deficiency. J. Biol. Chem. 1993, 268, 4376–4381. [Google Scholar] [CrossRef]
- Grünert, S.C.; Schlatter, S.M.; Schmitt, R.N.; Gemperle-Britschgi, C.; Mrázová, L.; Balcı, M.C.; Bischof, F.; Çoker, M.; Das, A.M.; Demirkol, M.; et al. 3-Hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: Clinical presentation and outcome in a series of 37 patients. Mol. Genet. Metab. 2017, 121, 206–215. [Google Scholar] [CrossRef]
- Holdar, S.; Rahbeeni, Z.; Ramzan, K.; Imtiaz, F. Hepatic Manifestations of 3-Hydroxy-3-Methylglutaryl-Coenzyme-A Lyase Deficiency in Saudi Patients: Experience of a Tertiary Care Center. J. Pediatr. Genet. 2021, 10, 105–110. [Google Scholar] [CrossRef]
- Grünert, S.C.; Sass, J.O. 3-hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: One disease—Many faces. Orphanet. J. Rare Dis. 2020, 15, 48. [Google Scholar] [CrossRef]
- Wysocki, S.J.; Hähnel, R.; Truscott, R.J.; Halpern, B.; Wilcken, B. Hyperammonaemia and urinary organic acids. Lancet 1979, 2, 371–372. [Google Scholar] [CrossRef]
- Wysocki, S.J.; Hähnel, R. 3-Hydroxy-3-methylglutaryl-coenzyme a lyase deficiency: A review. J. Inherit. Metab. Dis. 1986, 9, 225–233. [Google Scholar] [CrossRef]
- Shilkin, R.; Wilson, G.; Owles, E. 3-Hydroxy-3-methylglutaryl coenzyme A lyase deficiency. Follow-up of first described case. Acta Paediatr. Scand. 1981, 70, 265–268. [Google Scholar] [CrossRef]
- Hammond, J.; Wilcken, B. 3-hydroxy-3-methylglutaric, 3-methylglutaconic and 3-methylglutaric acids can be non-specific indicators of metabolic disease. J. Inherit. Metab. Dis. 1984, 7 (Suppl. 2), 117–118. [Google Scholar] [CrossRef] [PubMed]
- Ozand, P.T.; al Aqeel, A.; Gascon, G.; Brismar, J.; Thomas, E.; Gleispach, H. 3-Hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) lyase deficiency in Saudi Arabia. J. Inherit. Metab. Dis. 1991, 14, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.M.; Cassidy, S.B.; Seaver, L.H.; Wanders, R.J.; Kennaway, N.G.; Mitchell, G.A.; Spark, R.P. Fatal cardiomyopathy associated with 3-hydroxy-3-methylglutaryl-CoA lyase deficiency. J. Inherit. Metab. Dis. 1994, 17, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.M.; Breuer, J.; Kaiser, K.; Nyhan, W.L.; McCoy, E.E.; Ferreira, P.; Greene, C.L.; Blitzer, M.G.; Shapira, E.; Reverte, F.; et al. 3-Hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: Report of five new patients. J. Inherit. Metab. Dis. 1988, 11, 76–87. [Google Scholar] [CrossRef]
- Al-Sayed, M.; Imtiaz, F.; Alsmadi, O.A.; Rashed, M.S.; Meyer, B.F. Mutations underlying 3-hydroxy-3-methylglutaryl CoA lyase deficiency in the Saudi population. BMC Med. Genet. 2006, 7, 86. [Google Scholar] [CrossRef]
- Vargas, C.R.; Sitta, A.; Schmitt, G.; Ferreira, G.C.; Cardoso, M.L.; Coelho, D.; Gibson, K.M.; Wajner, M. Incidence of 3-hydroxy-3-methylglutaryl-coenzyme A lyase (HL) deficiency in Brazil, South America. J. Inherit. Metab. Dis. 2008, 31 (Suppl. 3), 511–515. [Google Scholar] [CrossRef]
- Cardoso, M.L.; Rodrigues, M.R.; Leão, E.; Martins, E.; Diogo, L.; Rodrigues, E.; Garcia, P.; Rolland, M.O.; Vilarinho, L. The E37X is a common HMGCL mutation in Portuguese patients with 3-hydroxy-3-methylglutaric CoA lyase deficiency. Mol. Genet. Metab. 2004, 82, 334–338. [Google Scholar] [CrossRef]
- Thompson, G.N.; Chalmers, R.A.; Halliday, D. The contribution of protein catabolism to metabolic decompensation in 3-hydroxy-3-methylglutaric aciduria. Eur. J. Pediatr. 1990, 149, 346–350. [Google Scholar] [CrossRef]
- Dalkeith, T.; Ellaway, C.J.; Thompson, S.; Dennison, B.; Matar, W.; Wilcken, B.; Bhattacharya, K. The use of 3-hydroxybutyrate in patients with fat oxidation disorders. In Proceedings of the International Congress of Inborn Errors of Metabolism, Barcelona, Spain, 3–6 September 2013. [Google Scholar]
- Bhattacharya, K.; Matar, W.; Tolun, A.A.; Devanapalli, B.; Thompson, S.; Dalkeith, T.; Lichkus, K.; Tchan, M. The use of sodium DL-3-Hydroxybutyrate in severe acute neuro-metabolic compromise in patients with inherited ketone body synthetic disorders. Orphanet. J. Rare Dis. 2020, 15, 53. [Google Scholar] [CrossRef]
- Gibson, K.M.; Breuer, J.; Nyhan, W.L. 3-Hydroxy-3-methylglutaryl-coenzyme A lyase deficiency: Review of 18 reported patients. Eur. J. Pediatr. 1988, 148, 180–186. [Google Scholar] [CrossRef]
- Jones, K.J.; Wilcken, B.; Kilham, H. The long-term evolution of a case of 3-hydroxy-3-methylglutaryl-coenzyme A lyase deficiency associated with deafness and retinitis pigmentosa. J. Inherit. Metab. Dis. 1997, 20, 833–834. [Google Scholar] [CrossRef] [PubMed]
- Stacey, T.E.; de Sousa, C.; Tracey, B.M.; Whitelaw, A.; Mistry, J.; Timbrell, P.; Chalmers, R.A. Dizygotic twins with 3-hydroxy-3-methylglutaric aciduria; unusual presentation, family studies and dietary management. Eur. J. Pediatr. 1985, 144, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Langendonk, J.G.; Roos, J.C.; Angus, L.; Williams, M.; Karstens, F.P.; de Klerk, J.B.; Maritz, C.; Ben-Omran, T.; Williamson, C.; Lachmann, R.H.; et al. A series of pregnancies in women with inherited metabolic disease. J. Inherit. Metab. Dis. 2012, 35, 419–424. [Google Scholar] [CrossRef]
- Pipitone, A.; Raval, D.B.; Duis, J.; Vernon, H.; Martin, R.; Hamosh, A.; Valle, D.; Gunay-Aygun, M. The management of pregnancy and delivery in 3-hydroxy-3-methylglutaryl-CoA lyase deficiency. Am. J. Med. Genet. A 2016, 170, 1600–1602. [Google Scholar] [CrossRef]
- Puisac, B.; Teresa-Rodrigo, M.E.; Arnedo, M.; Gil-Rodríguez, M.C.; Pérez-Cerdá, C.; Ribes, A.; Pié, A.; Bueno, G.; Gómez-Puertas, P.; Pié, J. Analysis of aberrant splicing and nonsense-mediated decay of the stop codon mutations c.109G>T and c.504_505delCT in 7 patients with HMG-CoA lyase deficiency. Mol. Genet. Metab. 2013, 108, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Puisac, B.; Ramos, M.; Arnedo, M.; Menao, S.; Gil-Rodríguez, M.C.; Teresa-Rodrigo, M.E.; Pié, A.; de Karam, J.C.; Wesselink, J.J.; Giménez, I.; et al. Characterization of splice variants of the genes encoding human mitochondrial HMG-CoA lyase and HMG-CoA synthase, the main enzymes of the ketogenesis pathway. Mol. Biol. Rep. 2012, 39, 4777–4785. [Google Scholar] [CrossRef]
- Muroi, J.; Yorifuji, T.; Uematsu, A.; Nakahata, T. Cerebral infarction and pancreatitis: Possible complications of patients with 3-hydroxy-3-methylglutaryl-CoA lyase deficiency. J. Inherit. Metab. Dis. 2000, 23, 636–637. [Google Scholar] [CrossRef]
- Wortmann, S.B.; Kluijtmans, L.A.; Engelke, U.F.H.; Wevers, R.A.; Morava, E. The 3-methylglutaconic acidurias: What’s new? J. Inherit. Metab. Dis. 2012, 35, 13–22. [Google Scholar] [CrossRef]
- Morris, A.A. Cerebral ketone body metabolism. J. Inherit. Metab. Dis. 2005, 28, 109–121. [Google Scholar] [CrossRef]
- Jones, D.E.; Perez, L.; Ryan, R.O. 3-Methylglutaric acid in energy metabolism. Clin. Chim. Acta 2020, 502, 233–239. [Google Scholar] [CrossRef]
- Roland, D.; Jissendi-Tchofo, P.; Briand, G.; Vamecq, J.; Fontaine, M.; Ultré, V.; Acquaviva-Bourdain, C.; Mention, K.; Dobbelaere, D. Coupled brain and urine spectroscopy—In vivo metabolomic characterization of HMG-CoA lyase deficiency in 5 patients. Mol. Genet. Metab. 2017, 121, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.A.; Gauthier, N.; Lesimple, A.; Wang, S.P.; Mamer, O.; Qureshi, I. Hereditary and acquired diseases of acyl-coenzyme A metabolism. Mol. Genet. Metab. 2008, 94, 4–15. [Google Scholar] [CrossRef]
- Fukao, T.; Mitchell, G.; Sass, J.O.; Hori, T.; Orii, K.; Aoyama, Y. Ketone body metabolism and its defects. J. Inherit. Metab. Dis. 2014, 37, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Hertzog, A.; Selvanathan, A.; Pandithan, D.; Kim, W.T.; Kava, M.P.; Boneh, A.; Coman, D.; Tolun, A.A.; Bhattacharya, K. 3-Methylglutaconyl-CoA hydratase deficiency: When ascertainment bias confounds a biochemical diagnosis. JIMD Rep. 2022, 63, 568–574. [Google Scholar] [CrossRef]
- Stanley, C.A. Hyperinsulinism/hyperammonemia syndrome: Insights into the regulatory role of glutamate dehydrogenase in ammonia metabolism. Mol. Genet. Metab. 2004, 81, S45–S51. [Google Scholar] [CrossRef]
- Häberle, J.; Chakrapani, A.; Ah Mew, N.; Longo, N. Hyperammonaemia in classic organic acidaemias: A review of the literature and two case histories. Orphanet. J. Rare Dis. 2018, 13, 219. [Google Scholar] [CrossRef]
- Ryder, B.; Inbar-Feigenberg, M.; Glamuzina, E.; Halligan, R.; Vara, R.; Elliot, A.; Coman, D.; Minto, T.; Lewis, K.; Schiff, M.; et al. New insights into carnitine-acylcarnitine translocase deficiency from 23 cases: Management challenges and potential therapeutic approaches. J. Inherit. Metab. Dis. 2021, 44, 903–915. [Google Scholar] [CrossRef]
- Sklirou, E.; Alodaib, A.N.; Dobrowolski, S.F.; Mohsen, A.A.; Vockley, J. Physiological Perspectives on the Use of Triheptanoin as Anaplerotic Therapy for Long Chain Fatty Acid Oxidation Disorders. Front. Genet. 2020, 11, 598760. [Google Scholar] [CrossRef]
- Plecko, B.; Stoeckler-Ipsiroglu, S.; Schober, E.; Harrer, G.; Mlynarik, V.; Gruber, S.; Moser, E.; Moeslinger, D.; Silgoner, H.; Ipsiroglu, O. Oral β-Hydroxybutyrate Supplementation in Two Patients with Hyperinsulinemic Hypoglycemia: Monitoring of β-Hydroxybutyrate Levels in Blood and Cerebrospinal Fluid, and in the Brain by In Vivo Magnetic Resonance Spectroscopy. Pediatr. Res. 2002, 52, 301–306. [Google Scholar] [CrossRef][Green Version]
- Van Hove, J.L.; Grünewald, S.; Jaeken, J.; Demaerel, P.; Declercq, P.E.; Bourdoux, P.; Niezen-Koning, K.; Deanfeld, J.E.; Leonard, J.V. D,L-3-hydroxybutyrate treatment of multiple acyl-CoA dehydrogenase deficiency (MADD). Lancet 2003, 361, 1433–1435. [Google Scholar] [CrossRef]
- van Rijt, W.J.; Jager, E.A.; Allersma, D.P.; Aktuğlu Zeybek, A.; Bhattacharya, K.; Debray, F.G.; Ellaway, C.J.; Gautschi, M.; Geraghty, M.T.; Gil-Ortega, D.; et al. Efficacy and safety of D,L-3-hydroxybutyrate (D,L-3-HB) treatment in multiple acyl-CoA dehydrogenase deficiency. Genet. Med. 2020, 22, 908–916. [Google Scholar] [CrossRef]
- Edmond, J. Ketone bodies as precursors of sterols and fatty acids in the developing rat. J. Biol. Chem. 1974, 249, 72–80. [Google Scholar] [CrossRef]
- van der Knaap, M.S.; Bakker, H.D.; Valk, J. MR imaging and proton spectroscopy in 3-hydroxy-3-methylglutaryl coenzyme A lyase deficiency. AJNR Am. J. Neuroradiol. 1998, 19, 378–382. [Google Scholar]
- Bougneres, P.F.; Lemmel, C.; Ferré, P.; Bier, D.M. Ketone body transport in the human neonate and infant. J. Clin. Investig. 1986, 77, 42–48. [Google Scholar] [CrossRef]
- Bier, D.M.; Leake, R.D.; Haymond, M.W.; Arnold, K.J.; Gruenke, L.D.; Sperling, M.A.; Kipnis, D.M. Measurement of “true” glucose production rates in infancy and childhood with 6,6-dideuteroglucose. Diabetes 1977, 26, 1016–1023. [Google Scholar] [CrossRef]
- Zafeiriou, D.I.; Vargiami, E.; Mayapetek, E.; Augoustidou-Savvopoulou, P.; Mitchell, G.A. 3-Hydroxy-3-methylglutaryl coenzyme a lyase deficiency with reversible white matter changes after treatment. Pediatr. Neurol. 2007, 37, 47–50. [Google Scholar] [CrossRef]
- Baumgartner, M.R.; Hörster, F.; Dionisi-Vici, C.; Haliloglu, G.; Karall, D.; Chapman, K.A.; Huemer, M.; Hochuli, M.; Assoun, M.; Ballhausen, D.; et al. Proposed guidelines for the diagnosis and management of methylmalonic and propionic acidemia. Orphanet. J. Rare Dis. 2014, 9, 130. [Google Scholar] [CrossRef]
- Boy, N.; Mühlhausen, C.; Maier, E.M.; Heringer, J.; Assmann, B.; Burgard, P.; Dixon, M.; Fleissner, S.; Greenberg, C.R.; Harting, I.; et al. Proposed recommendations for diagnosing and managing individuals with glutaric aciduria type I: Second revision. J. Inherit. Metab. Dis. 2017, 40, 75–101. [Google Scholar] [CrossRef]
| ID | Age (Years) | Age at Diagnosis | Presentation | Ethnicity | Positive NBS | Initial Urinary Organic Acid Elevations | Initial Plasma Acylcarnitine Elevations | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3HMG | 3MGC | 3MGL | 3HIVA | DCA | 3OHB | C5OH (µmol/L) | C6DCA (µmol/L) | ||||||
| 1 | NA | 7 months | Hypoglycaemia, acidosis | Caucasian | NA | + + + | + + + | + + + | + + + | Not done | Not done | ||
| 2 | 39 | 3 months | Hypoglycaemia, acidosis | Chilean | NA | ++ | + + + | + + + | + + + | - | - | Not done | Not done |
| 3 | 33 | 4 days | Hypoglycaemia, acidosis | Caucasian | NA | ++ | + + + | + | ++ | - | - | Not done | Not done |
| 4 | 27 | 10 months | Hypoglycaemia, acidosis | Slovakian | NA | + + + | + + + | + | + | - | - | Not done | Not done |
| 5 | 13 | 3 days | Acidosis, hyperammonaemia Hypoglycaemia | Israeli | Y | + + + | + + + | + + + | + + + | - | - | 2.2 (<0.2) | 1.8 |
| 6# | 6.5 | 4 days | Weight loss > 10% | Iraqi | Y | ++ | + + + | + | - | - | - | 0.36 (0.01–0.15) | 0.18 (0.03–0.11) |
| 7 | 3 | 2.5 y | Developmental delay | Lebanese | N | + | + + + | + | - | - | - | 0.02 (0–0.1) | 0.12 (0–0.08) |
| 8 | 2 | 6 days | Weight loss > 10%, vomiting | Pakistan | N | + + + | + + + | ++ | + | ||||
| 9 | 1.8 | 9 months | Liver failure | Caucasian | N | ++ | + + + | + | ++ | + + + | + + + | 0 (0–0.1) * | 0.03 (0–0.08) * |
| 10 | 0.8 | D7 (NBS) | Mild vomiting | Lebanese | Y | + + + | + + + | ++ | + | - | - | 1.26 (0–0.1) | 1.2 (0–0.08) |
| ID | Macronutrient/Diet Rx on Diagnosis | Current Age | Current Protein Intake | Current Fat Intake (% Total Energy) | Ketones/Day when Well | Unwell Ketones | Night Rx | Current Carnitine Supplement | Admissions since Diagnosis | Intellectual Outcomes # |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Leucine restriction | NA | - | - | - | - | - | - | - | - |
| 2 | Leucine restriction | 39 | 0.5 g/kg/day | 18% | None | None | None | None | 5 | Moderate delay |
| 3 | - | 33 | 0.8 g/kg/day | - | None | None | None | 100 mg/kg/day to start | 9 | Normal |
| 4 | Fat and protein restriction | 27 | 1 g/kg/day | ~10% | None | None | None | 1.5 g per day | 1 | Normal (blind) * |
| 5 | Fat and protein restriction | 13 | 1.4 g/kg/day | 8% | Nocte | 4 hrly | None | 100 mg/kg/day | 5 | Normal |
| 6 | Fat and protein restriction | 7 | 3.4 g/kg/day | 8–20% | Nocte | 4 hrly | UCCS 1.5 g/kg/day | 100 mg/kg/day | 2 | Normal |
| 7 | None | 3.5 | Normal | Normal | Nocte | 4 hrly | None | 100 mg/kg/day | 0 | Moderate delay |
| 8 | None | 2.1 | Normal | Normal | Twice daily | 4 hrly | UCCS 1 g/kg/day | 40 mg/kg/day | 12 | Mild DD |
| 9 | Fat and protein restriction | 1.5 | 1.7 g/kg/day | 30% | Twice daily | 4 hrly | UCCS introduced | 100 mg/kg/day | 1 | Normal |
| 10 | Fat and protein restriction | 0.9 | 1.8 g/kg/day | 30% | Twice daily | 4 hrly | Frequent feeds (4 hrly) | 100 mg/kg/day | 0 | Normal |
| Age | When Well | When Unwell (All Ages) |
|---|---|---|
| At all ages |
|
|
| Neonatal |
| |
| Infant |
| |
| From one year of age |
| |
| Older children |
| |
| Teenagers/adults |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, S.; Hertzog, A.; Selvanathan, A.; Batten, K.; Lewis, K.; Nisbet, J.; Mitchell, A.; Dalkeith, T.; Billmore, K.; Moore, F.; et al. Treatment of HMG-CoA Lyase Deficiency—Longitudinal Data on Clinical and Nutritional Management of 10 Australian Cases. Nutrients 2023, 15, 531. https://doi.org/10.3390/nu15030531
Thompson S, Hertzog A, Selvanathan A, Batten K, Lewis K, Nisbet J, Mitchell A, Dalkeith T, Billmore K, Moore F, et al. Treatment of HMG-CoA Lyase Deficiency—Longitudinal Data on Clinical and Nutritional Management of 10 Australian Cases. Nutrients. 2023; 15(3):531. https://doi.org/10.3390/nu15030531
Chicago/Turabian StyleThompson, Susan, Ashley Hertzog, Arthavan Selvanathan, Kiera Batten, Katherine Lewis, Janelle Nisbet, Ashleigh Mitchell, Troy Dalkeith, Kate Billmore, Francesca Moore, and et al. 2023. "Treatment of HMG-CoA Lyase Deficiency—Longitudinal Data on Clinical and Nutritional Management of 10 Australian Cases" Nutrients 15, no. 3: 531. https://doi.org/10.3390/nu15030531
APA StyleThompson, S., Hertzog, A., Selvanathan, A., Batten, K., Lewis, K., Nisbet, J., Mitchell, A., Dalkeith, T., Billmore, K., Moore, F., Tolun, A. A., Devanapalli, B., Bratkovic, D., Hilditch, C., Rahman, Y., Tchan, M., & Bhattacharya, K. (2023). Treatment of HMG-CoA Lyase Deficiency—Longitudinal Data on Clinical and Nutritional Management of 10 Australian Cases. Nutrients, 15(3), 531. https://doi.org/10.3390/nu15030531








