Assessment of Dietary Intake of Iodine and Risk of Iodine Deficiency in Children with Classical Galactosaemia on Dietary Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Study Design
2.2.1. Demographics
2.2.2. Assessment of Estimated Iodine Intake
2.2.3. Assessment of Estimated Iodine Intake versus Recommendations
2.2.4. Comparison of Iodine Intake in Classical Galactosaemia versus the General Population
2.2.5. Projected Impact of Iodine Fortification on Dairy Alternative Products
2.3. Statistical Analysis
3. Results
3.1. Demographics
3.2. Assessment of Estimated Iodine Intake in Age Groups
3.3. Assessment of Estimated Iodine Intake versus Recommendations
3.4. Comparison of Iodine Intake in CG versus the General Population
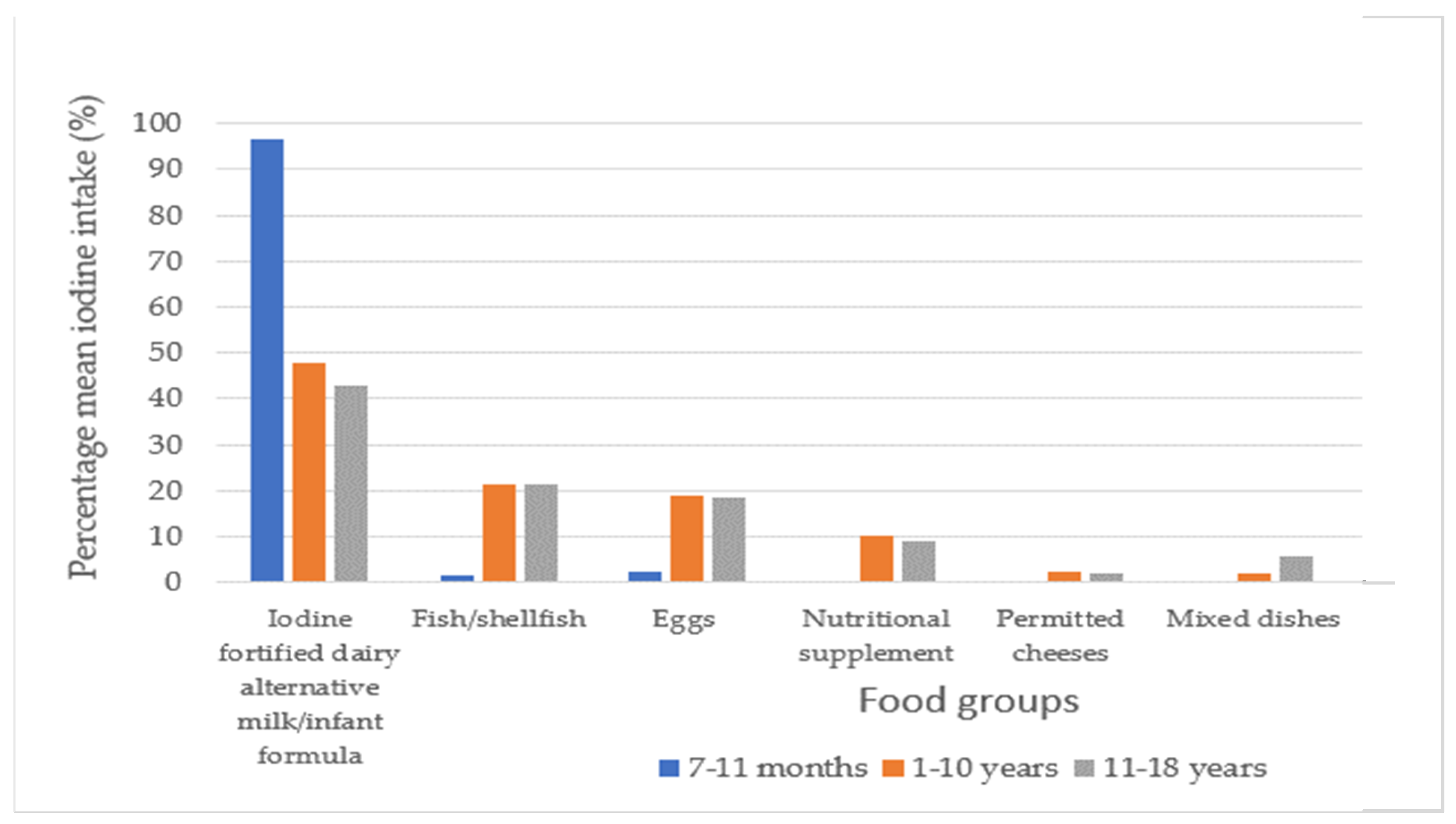
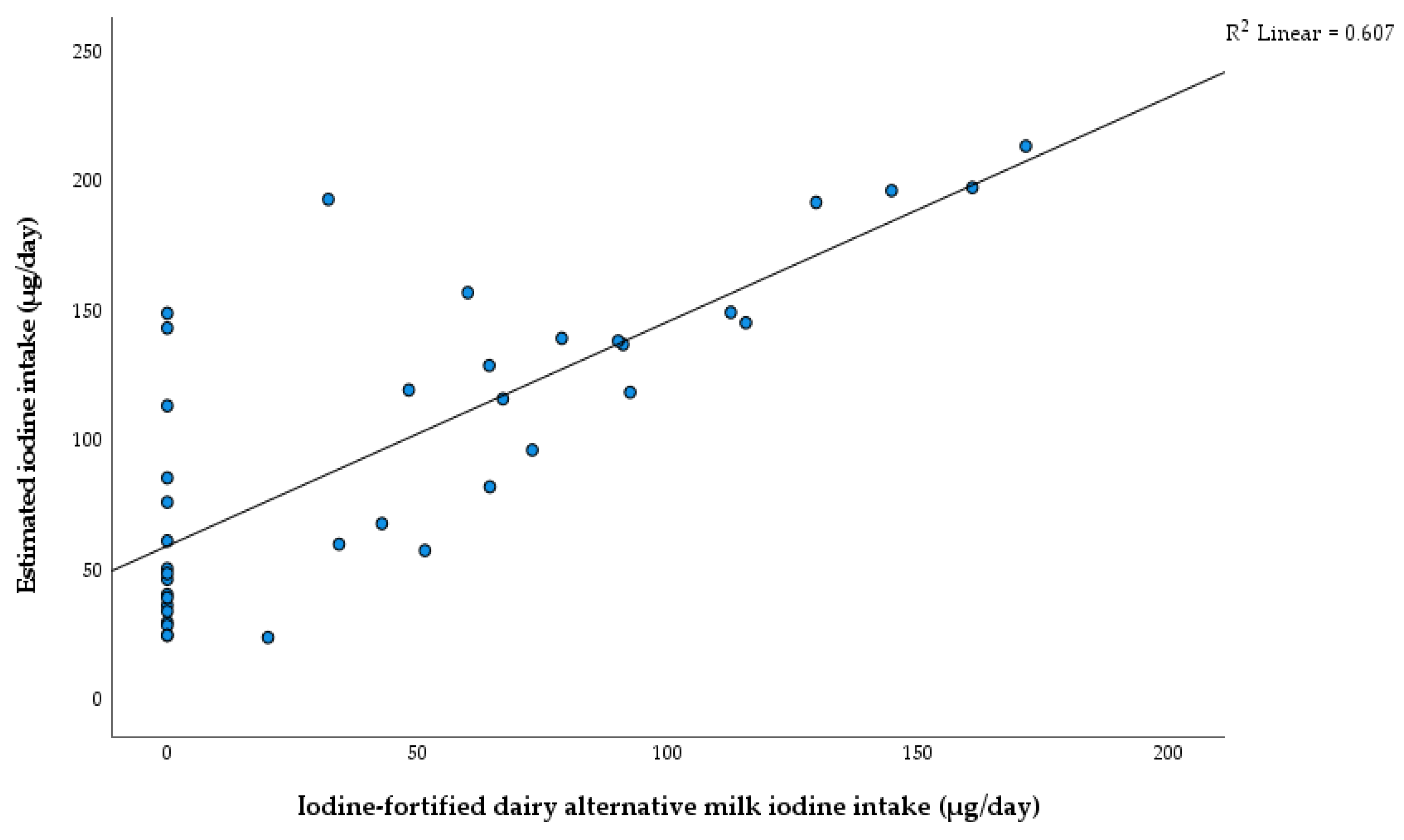
3.5. Dietary Sources of Iodine for Children with CG
3.6. Projected Impact of Iodine Fortification on Dairy Alternative Products in Ireland
4. Discussion
4.1. Demographics
4.2. Assessment of Estimated iodine Intake versus Recommendations
4.3. Comparison of Iodine Intake in CG versus the General Population
4.4. Dietary Sources of Iodine for Children with CG
4.5. Projected Impact of Iodine Fortification on Dairy Alternative Products in Ireland
4.6. Effect of Soya as a Goitrogen
4.7. Strengths and Limitations of the Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zimmermann, M.B. Iodine deficiency. Endocr. Rev. 2009, 30, 376–408. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Caballero, B.; Cousins, R.J.; Tucker, K.L.; Ziegler, T.R. Modern Nutrition in Health and Disease, 11th ed.; Wolters Kluwer Health Adis (ESP): Alphen aan den Rijn, The Netherlands, 2012; Available online: https://jhu.pure.elsevier.com/en/publications/modern-nutrition-in-health-and-disease-eleventh-edition (accessed on 18 April 2022).
- World Health Organization. Assessment of Iodine Deficiency Disorders and Monitoring Their Elimination: A Guide for Programme Managers; World Health Organization: Geneva, Switzerland, 2007. Available online: https://apps.who.int/iris/bitstream/handle/10665/43781/9789241595827_eng.pdf?sequence=1&isAllowed=y (accessed on 18 April 2022).
- Lightowler, H.J.; Davies, G.J. Iodine intake and iodine deficiency in vegans as assessed by the duplicate-portion technique and urinary iodine excretion. Br. J. Nutr. 1998, 80, 529–535. [Google Scholar] [CrossRef]
- Welling, L.; Bernstein, L.E.; Berry, G.T.; Burlina, A.B.; Eyskens, F.; Gautschi, M.; Grünewald, S.; Gubbels, C.S.; Knerr, I.; Labrune, P.; et al. International clinical guideline for the management of classical galactosemia: Diagnosis, treatment, and follow-up. J. Inherit. Metab. Dis. 2017, 40, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Ashino, J.; Okano, Y.; Suyama, I.; Yamazaki, T.; Yoshino, M.; Furuyama, J.-I.; Lin, H.-C.; Reichardt, J.K.V.; Isshiki, G. Molecular characterization of galactosemia (type 1) mutations in Japanese. Hum. Mutat. 1995, 6, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Coss, K.P.; Doran, P.P.; Owoeye, C.; Codd, M.B.; Hamid, N.; Mayne, P.D.; Crushell, E.; Knerr, I.; Monavari, A.A.; Treacy, E.P. Classical Galactosaemia in Ireland: Incidence, complications and outcomes of treatment. J. Inherit. Metab. Dis. 2013, 36, 21–27. [Google Scholar] [CrossRef]
- Murphy, M.; McHugh, B.; Tighe, O.; Mayne, P.; O’Neill, C.; Naughten, E.; Croke, D.T. Genetic basis of transferase-deficient galactosaemia in Ireland and the population history of the Irish Travellers. Eur. J. Hum. Genet. 1999, 7, 549–554. [Google Scholar] [CrossRef]
- Ficicioglu, C.; Yager, C.; Segal, S. Galactitol and galactonate in red blood cells of children with the Duarte/galactosemia genotype. Mol. Genet. Metab. 2005, 84, 152–159. [Google Scholar] [CrossRef]
- Oh, S.L.; Cheng, L.Y.; Zhou, J.F.J.; Henke, W.; Hagen, T. Galactose 1-phosphate accumulates to high levels in galactose-treated cells due to low GALT activity and absence of product inhibition of GALK. J. Inherit. Metab. Dis. 2020, 43, 529–539. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on Dietary Reference Values for iodine. EFSA J. 2014, 12, 3660. [Google Scholar] [CrossRef]
- Safefood. Iodine Status on the Island of Ireland. Available online: https://www.safefood.net/research-reports/iodine-status-ireland (accessed on 1 May 2022).
- Condo, D.; Makrides, M.; Skeaff, S.; Zhou, S.J. Development and validation of an iodine-specific FFQ to estimate iodine intake in Australian pregnant women. Br. J. Nutr. 2015, 113, 944–952. [Google Scholar] [CrossRef]
- Cheyette, C.; Balolia, Y. Carbs & Cals: Count Your Carbs & Calories with over 1,700 Food Photos, 6th revised ed.; Chello Publishing: London, UK, 2016; Available online: https://go.exlibris.link/4T47GwPg (accessed on 12 October 2021).
- Public Health England. McCance and Widdowson’s McCance and Widdowson’s Composition of Foods Integrated Dataset 2021; Published 19 March 2021. Available online: https://www.gov.uk/government/publications/composition-of-foods-integrated-dataset-cofid/ (accessed on 11 April 2022).
- EFSA. Scientific Panel on Dietetic Products, Nutrition and Allergies Tolerable Upper Intake Levels for Vitamins and Minerals; EFSA: Parma, Italy, 2006.
- Kane, E.; Buffini, M.; Nugent, A.; Kehoe, L.; Walton, J.; Kearney, J.; Flynn, A.; McNulty, B. Iodine intakes in Irish children aged 5–12 years. Proc. Nutr. Soc. 80 (OCE3) 2021, 80, E123. [Google Scholar] [CrossRef]
- Cohen, J. Set Correlation and Contingency Tables. Appl. Psychol. Meas. 1988, 12, 425–434. [Google Scholar] [CrossRef]
- Kurku, H.; Gencer, A.; Pirgon, O.; Buyukinan, M.; Aslan, N. Increased oxidative stress parameters in children with moderate iodine deficiency. J. Pediatr. Endocrinol. Metab. 2016, 29, 1159–1164. [Google Scholar] [CrossRef] [PubMed]
- Karbownik-Lewińska, M.; Stępniak, J.; Iwan, P.; Lewiński, A. Iodine as a potential endocrine disruptor-a role of oxidative stress. Endocrine 2022, 78, 219–240. [Google Scholar] [CrossRef]
- Haskovic, M.; Coelho, A.I.; Bierau, J.; Vanoevelen, J.M.; Steinbusch, L.K.M.; Zimmermann, L.J.I.; Villamor-Martinez, E.; Berry, G.T.; Rubio-Gozalbo, M.E. Pathophysiology and targets for treatment in hereditary galactosemia: A systematic review of animal and cellular models. J. Inherit. Metab. Dis. 2020, 43, 392–408. [Google Scholar] [CrossRef]
- Venkatesh, S.G.; Deshpande, V. A comparative review of the structure and biosynthesis of thyroglobulin. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 122, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Department of Health. All Ireland Traveller Health Study; Department of Health: Dublin, Ireland, 2010. [Google Scholar]
- Dewalt, D.A.; Berkman, N.D.; Sheridan, S.; Lohr, K.N.; Pignone, M.P. Literacy and health outcomes: A systematic review of the literature. J. Gen. Intern. Med. 2004, 19, 1228–1239. [Google Scholar] [CrossRef]
- Hohoff, E.; Perrar, I.; Jancovic, N.; Alexy, U. Age and time trends of dairy intake among children and adolescents of the DONALD study. Eur. J. Nutr. 2021, 60, 3861–3872. [Google Scholar] [CrossRef]
- Dror, D.K.; Allen, L.H. Dairy product intake in children and adolescents in developed countries: Trends, nutritional contribution, and a review of association with health outcomes. Nutr. Rev. 2014, 72, 68–81. [Google Scholar] [CrossRef]
- Vanderpump, M.P.; Lazarus, J.H.; Smyth, P.P.; Laurberg, P.; Holder, R.L.; Boelaert, K.; Franklyn, J.A.; British Thyroid Association UK Iodine Survey Group. Iodine status of UK schoolgirls: A cross-sectional survey. Lancet 2011, 377, 2007–2012. [Google Scholar] [CrossRef]
- Food Safety Authority of Ireland. FSAI Publishes Results of Total Diet Study. Available online: https://www.fsai.ie/news_centre/press_releases/total_diet_study_15032016.html (accessed on 18 April 2022).
- Bath, S.C.; Hill, S.; Infante, H.G.; Elghul, S.; Nezianya, C.J.; Rayman, M.P. Iodine concentration of milk-alternative drinks available in the UK in comparison to cows’ milk. Br. J. Nutr. 2017, 118, 525–532. [Google Scholar] [CrossRef]
- Combet, E.; Bouga, M.; Pan, B.; Lean, M.E.J.; Christopher, C.O. Iodine and pregnancy—A UK cross-sectional survey of dietary intake, knowledge and awareness. Br. J. Nutr. 2015, 114, 108–117. [Google Scholar] [CrossRef]
- O’Kane, S.M.; Pourshahidi, L.K.; Farren, K.M.; Mulhern, M.S.; Strain, J.J.; Yeates, A.J. Iodine knowledge is positively associated with dietary iodine intake among women of childbearing age in the UK and Ireland. Br. J. Nutr. 2016, 116, 1728–1735. [Google Scholar] [CrossRef]
- HSE Healthy Eating Guidelines: Milk, Yogurt, Cheese Fact Sheet. Available online: https://www.hse.ie/eng/about/who/healthwellbeing/our-priority-programmes/heal/food-pyramid-images/milk-yogurt-cheese-fact-sheet.pdf (accessed on 27 May 2022).
- BDA. Iodine: The Debate around Fortification. Available online: https://www.bda.uk.com/resource/iodine-the-debate-around-fortification.html (accessed on 17 May 2022).
- Doerge, D.R.; Sheehan, D.M. Goitrogenic and estrogenic activity of soy isoflavones. Environ. Health Perspect. 2002, 110 (Suppl. 3), 349–353. [Google Scholar] [CrossRef]
- Vance, K.; Makhmudov, A.; Jones, R.L.; Caldwell, K.L. Re: “Iodine Content in Milk Alternatives” by Ma et al. (Thyroid 2016;26:1308–1310). Thyroid 2017, 27, 748–749. [Google Scholar] [CrossRef]

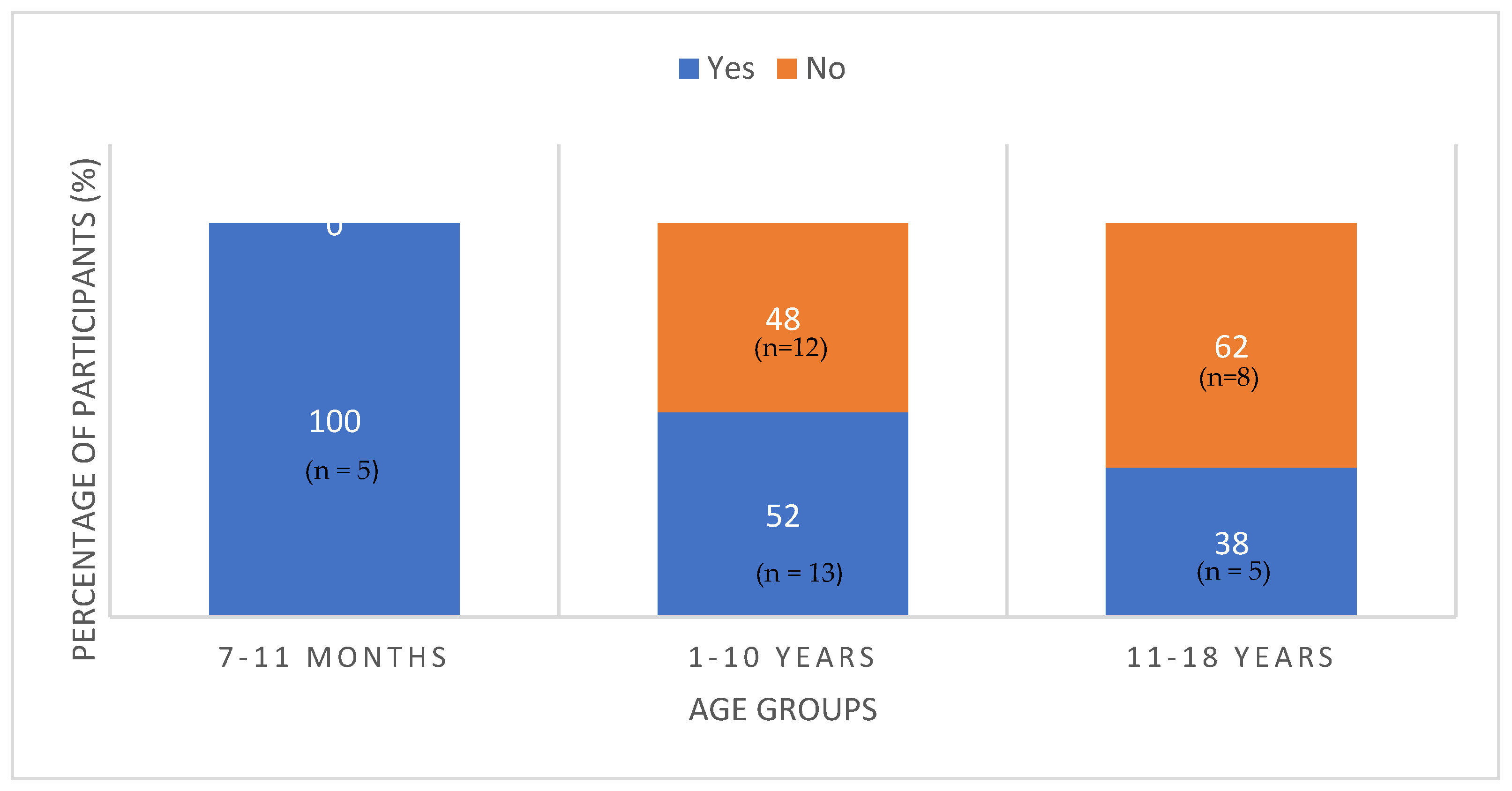
| Physiological Group | Health Consequences of Iodine Deficiency |
|---|---|
| All ages | Goitre Hypothyroidism |
| Foetus | Spontaneous abortion Stillbirth Congenital anomalies Perinatal mortality |
| Neonate | Endemic cretinism including mental deficiency with a combination of mutism, spastic diplegia, squint, hypothyroidism and short stature Infant mortality |
| Child and adolescent | Impaired cognitive function Delayed physical development Iodine-induced hyperthyroidism # |
| Adults | Impaired cognitive function Iodine-induced hyperthyroidism # |
| N = 43 | n | % | Meeting Recommendations (n) | p-Value | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Sex | 0.71 | ||||
| Male | 17 | 39.5 | 8 | 9 | |
| Female | 26 | 60.5 | 15 | 11 | |
| EFSA AI age groups | 0.06 | ||||
| 7–11 months | 5 | 11.7 | 5 | 0 | |
| 1–10 years | 25 | 58.1 | 13 | 12 | |
| 11–18 years | 13 | 30.2 | 5 | 8 | |
| Ethnicity/cultural group | 0.41 | ||||
| Irish | 20 | 46.5 | 12 | 8 | |
| Irish Traveller | 22 | 51.2 | 10 | 12 | |
| Any other white background | 1 | 2.3 | 1 | 0 | |
| Education level of parents | 0.11 | ||||
| Primary | 3 | 7.0 | 1 | 2 | |
| Secondary (lower and upper secondary) | 22 | 51.2 | 9 | 13 | |
| Third level and postgraduate | 18 | 41.9 | 13 | 5 | |
| Age Group | % Meeting EFSA AI Values | ||
|---|---|---|---|
| Current Intake | Fortification Strategy 1 | Fortification Strategy 2 | |
| 1–10 years | 52 | 72 | 100 |
| 11–18 years | 39 | 69 | 77 |
| 1–18 years | 47 | 71 | 92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milner, D.; Boyle, F.; McNulty, J.; Knerr, I. Assessment of Dietary Intake of Iodine and Risk of Iodine Deficiency in Children with Classical Galactosaemia on Dietary Treatment. Nutrients 2023, 15, 407. https://doi.org/10.3390/nu15020407
Milner D, Boyle F, McNulty J, Knerr I. Assessment of Dietary Intake of Iodine and Risk of Iodine Deficiency in Children with Classical Galactosaemia on Dietary Treatment. Nutrients. 2023; 15(2):407. https://doi.org/10.3390/nu15020407
Chicago/Turabian StyleMilner, Dearbhla, Fiona Boyle, Jenny McNulty, and Ina Knerr. 2023. "Assessment of Dietary Intake of Iodine and Risk of Iodine Deficiency in Children with Classical Galactosaemia on Dietary Treatment" Nutrients 15, no. 2: 407. https://doi.org/10.3390/nu15020407
APA StyleMilner, D., Boyle, F., McNulty, J., & Knerr, I. (2023). Assessment of Dietary Intake of Iodine and Risk of Iodine Deficiency in Children with Classical Galactosaemia on Dietary Treatment. Nutrients, 15(2), 407. https://doi.org/10.3390/nu15020407






