Limosilactobacillus reuteri DSM 17938-Containing Infant Formulas and the Associations with Gastrointestinal Tolerance: A Cross-Sectional Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design and Participants
2.2. Outcome Measures
2.3. Statistical Analysis
2.4. Ethics Approval
3. Results
3.1. Infants’ Characteristics
3.2. Infant Gastrointestinal Symptom Questionnaire Composite Scores
3.3. Individual IGSQ Items and Additional GI-Tolerance Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weaver, L.T.; Ewing, G.; Taylor, L.C. The bowel habit of milk-fed infants. J. Pediatr. Gastroenterol. Nutr. 1988, 7, 568–571. [Google Scholar] [CrossRef] [PubMed]
- Forsyth, B.W.; McCarthy, P.L.; Leventhal, J.M. Problems of early infancy, formula changes, and mothers’ beliefs about their infants. J. Pediatr. 1985, 106, 1012–1017. [Google Scholar] [CrossRef] [PubMed]
- Quinlan, P.T.; Lockton, S.; Irwin, J.; Lucas, A.L. The Relationship between Stool Hardness and Stool Composition in Breast- and Formula-Fed Infants. J. Pediatr. Gastroenterol. Nutr. 1995, 20, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Iacono, G.; Merolla, R.; D’amico, D.; Bonci, E.; Cavataio, F.; Di Prima, L.; Scalici, C.; Indinnimeo, L.; Averna, M.R.; Carroccio, A.; et al. Gastrointestinal symptoms in infancy: A population-based prospective study. Dig. Liver Dis. 2005, 37, 432–438. [Google Scholar] [CrossRef]
- Liu, W.; Xiao, L.P.; Li, Y.; Wang, X.Q.; Xu, C.D. Epidemiology of mild gastrointestinal disorders among infants and young children in Shanghai area. Zhonghua Er Ke Za Zhi Chin. J. Pediatr. 2009, 47, 917–921. [Google Scholar]
- Iacovou, M.; Ralston, R.A.; Muir, J.; Walker, K.Z.; Truby, H. Dietary management of infantile colic: A systematic review. Matern. Child Health J. 2012, 16, 1319–1331. [Google Scholar] [CrossRef]
- Czinn, S.J.; Blanchard, S. Gastroesophageal reflux disease in neonates and infants: When and how to treat. Paediatr. Drugs 2013, 15, 19–27. [Google Scholar] [CrossRef]
- Nevo, N.; Rubin, L.; Tamir, A.; Levine, A.; Shaoul, R. Infant feeding patterns in the first 6 months: An assessment in full-term infants. J. Pediatr. Gastroenterol. Nutr. 2007, 45, 234–239. [Google Scholar] [CrossRef]
- Alarcon, P.A.; Tressler, R.L.; Mulvaney, A.; Lam, W.; Comer, G.M. Gastrointestinal tolerance of a new infant milk formula in healthy babies: An international study conducted in 17 countries. Nutrition 2002, 18, 484–489. [Google Scholar] [CrossRef]
- Kesavelu, D.; Sethi, G.; Bangale, N.; Anwar, F.; Rao, S. Common gastrointestinal distress among infants: Role of optimal nutritional interventions. Clin. Epidemiol. Glob. Health 2018, 6, 5–9. [Google Scholar] [CrossRef]
- Urbanska, M.; Szajewska, H. The efficacy of Lactobacillus reuteri DSM 17938 in infants and children: A review of the current evidence. Eur. J. Pediatr. 2014, 173, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Singal, A.G.; Higgins, P.D.; Waljee, A.K. A primer on effectiveness and efficacy trials. Clin. Transl. Gastroenterol. 2014, 5, e45. [Google Scholar] [CrossRef] [PubMed]
- Happy Tummy Consortium; Lavalle, L.; Sauvageot, N.; Cercamondi, C.I.; Egli, D.; Jankovic, I.; Vandenplas, Y. Infant feeding practice and gastrointestinal tolerance: A real-world, multi-country, cross-sectional observational study. BMC Pediatr. 2022, 22, 714. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.W.; Trabulsi, J.; Yao, M.; Bevans, K.B.; DeRusso, P.A. Validation of a Parent Report Questionnaire: The Infant Gastrointestinal Symptom Questionnaire. Clin. Pediatr. 2015, 54, 1167–1174. [Google Scholar] [CrossRef]
- Huysentruyt, K.; Koppen, I.; Benninga, M.; Cattaert, T.; Cheng, J.; De Geyter, C.; Faure, C.; Gottrand, F.; Hegar, B.; Hojsak, I.; et al. The Brussels Infant and Toddler Stool Scale: A Study on Interobserver Reliability. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 207–213. [Google Scholar] [CrossRef]
- Polack, F.P.; Khan, N.; Maisels, M.J. Changing partners: The dance of infant formula changes. Clin. Pediatr. 1999, 38, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, E.R.; Villares, J.M.M.; Ortega, F.D.; Martínez, A.C.; Sirvent, L.P.; Santana, L.; Rivero, J.C.; Alshweki, A.; Cercamondi, C.; Dahbane, S.; et al. Real-world study in infants fed with an infant formula with two human milk oligosaccharides. Nutr. Hosp. 2020, 37, 698–706. [Google Scholar] [CrossRef]
- Czerkies, L.; Finn, K.L.; Kineman, B.D.; Reichert, H.; Cohen, S.S.; Carvalho, R. Use of a partially hydrolyzed 100% whey-based infant formula with Lactobacillus reuteri in infants with caregiver-perceived intolerance. J. Pediatr. Health Nutr. 2019, 1, 19. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, L.; Ge, J.; Yan, J.; Northington, R.; Yao, M.; Nowacki, J.; Hays, N.P. Infant Feeding Regimens and Gastrointestinal Tolerance: A Multicenter, Prospective, Observational Cohort Study in China. Glob. Pediatr. Health 2018, 5, 2333794X17750271. [Google Scholar] [CrossRef]
- Storm, H.M.; Shepard, J.; Czerkies, L.M.; Kineman, B.; Cohen, S.S.; Reichert, H.; Carvalho, R. 2′-Fucosyllactose Is Well Tolerated in a 100% Whey, Partially Hydrolyzed Infant Formula With Bifidobacterium lactis: A Randomized Controlled Trial. Glob. Pediatr. Health 2019, 6, 2333794X19833995. [Google Scholar] [CrossRef] [PubMed]
- Simonson, J.; Haglund, K.; Weber, E.; Fial, A.; Hanson, L. Probiotics for the Management of Infantile Colic: A Systematic Review. MCN Am. J. Matern. Child Nurs. 2021, 46, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Dryl, R.; Szajewska, H. Probiotics for management of infantile colic: A systematic review of randomized controlled trials. Arch. Med. Sci. 2018, 14, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Castrellon, P.; Indrio, F.; Bolio-Galvis, A.; Jimenez-Gutierrez, C.; Jimenez-Escobar, I.; Lopez-Velazquez, G. Efficacy of Lactobacillus reuteri DSM 17938 for infantile colic: Systematic review with network meta-analysis. Medicine 2017, 96, e9375. [Google Scholar] [CrossRef]
- Schreck Bird, A.; Gregory, P.J.; Jalloh, M.A.; Risoldi Cochrane, Z.; Hein, D.J. Probiotics for the Treatment of Infantile Colic: A Systematic Review. J. Pharm. Pract. 2017, 30, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Sung, V.; D’Amico, F.; Cabana, M.D.; Chau, K.; Koren, G.; Savino, F.; Szajewska, H.; Deshpande, G.; Dupont, C.; Indrio, F.; et al. Lactobacillus reuteri to Treat Infant Colic: A Meta-analysis. Pediatrics 2018, 141, e20171811. [Google Scholar] [CrossRef]
- Xu, M.; Wang, J.; Wang, N.; Sun, F.; Wang, L.; Liu, X.H. The Efficacy and Safety of the Probiotic Bacterium Lactobacillus reuteri DSM 17938 for Infantile Colic: A Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2015, 10, e0141445. [Google Scholar] [CrossRef]
- Indrio, F.; Riezzo, G.; Raimondi, F.; Bisceglia, M.; Filannino, A.; Cavallo, L.; Francavilla, R. Lactobacillus reuteri accelerates gastric emptying and improves regurgitation in infants. Eur. J. Clin. Investig. 2011, 41, 417–422. [Google Scholar] [CrossRef]
- Garofoli, F.; Civardi, E.; Indrio, F.; Mazzucchelli, I.; Angelini, M.; Tinelli, C.; Stronati, M. The early administration of Lactobacillus reuteri DSM 17938 controls regurgitation episodes in full-term breastfed infants. Int. J. Food Sci. Nutr. 2014, 65, 646–648. [Google Scholar] [CrossRef]
- Savino, F.; Fornasero, S.; Ceratto, S.; De Marco, A.; Mandras, N.; Roana, J.; Tullio, V.; Amisano, G. Probiotics and gut health in infants: A preliminary case–control observational study about early treatment with Lactobacillus reuteri DSM 17938. Clin. Chim. Acta 2015, 451, 82–87. [Google Scholar] [CrossRef]
- Rosenfeldt, V.; Benfeldt, E.; Valerius, N.H.; Paerregaard, A.; Michaelsen, K.F. Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. J. Pediatr. 2004, 145, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.Y.; Pasyk, M.; Wang, B.; Forsythe, P.; Bienenstock, J.; Mao, Y.K.; Sharma, P.; Stanisz, A.M.; Kunze, W.A. Spatiotemporal maps reveal regional differences in the effects on gut motility for Lactobacillus reuteri and rhamnosus strains. Neurogastroenterol. Motil. 2013, 25, e205–e214. [Google Scholar] [CrossRef] [PubMed]
- Savino, F.; Garro, M.; Montanari, P.; Galliano, I.; Bergallo, M. Crying Time and RORgamma/FOXP3 Expression in Lactobacillus reuteri DSM17938-Treated Infants with Colic: A Randomized Trial. J. Pediatr. 2018, 192, 171–177.e171. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Islam, M.; Ziaul, M.; Tayab, M.; Alam, K.; Sahid, H.; Kamrul, M.; Mahmood, S.; Haque, A. Role of Probiotic Lactobacillus reuteri in Improving Gut Health and Immunity in Infants and Toddlers: A Review. Int. J. Nutr. Sci. 2022, 7, 75–80. [Google Scholar] [CrossRef]
- Chau, K.; Lau, E.; Greenberg, S.; Jacobson, S.; Yazdani-Brojeni, P.; Verma, N.; Koren, G. Probiotics for infantile colic: A randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. J. Pediatr. 2015, 166, 74–78. [Google Scholar] [CrossRef]
- Mi, G.L.; Zhao, L.; Qiao, D.D.; Kang, W.Q.; Tang, M.Q.; Xu, J.K. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: A prospective single blind randomized trial. Antonie Van Leeuwenhoek 2015, 107, 1547–1553. [Google Scholar] [CrossRef]
- Savino, F.; Cordisco, L.; Tarasco, V.; Palumeri, E.; Calabrese, R.; Oggero, R.; Roos, S.; Matteuzzi, D. Lactobacillus reuteri DSM 17938 in infantile colic: A randomized, double-blind, placebo-controlled trial. Pediatrics 2010, 126, e526–e533. [Google Scholar] [CrossRef]
- Vandenplas, Y.; Gerlier, L.; Caekelbergh, K.; Nan-Study-Group; Possner, M. An Observational Real-Life Study with a New Infant Formula in Infants with Functional Gastro-Intestinal Disorders. Nutrients 2021, 13, 3336. [Google Scholar] [CrossRef]

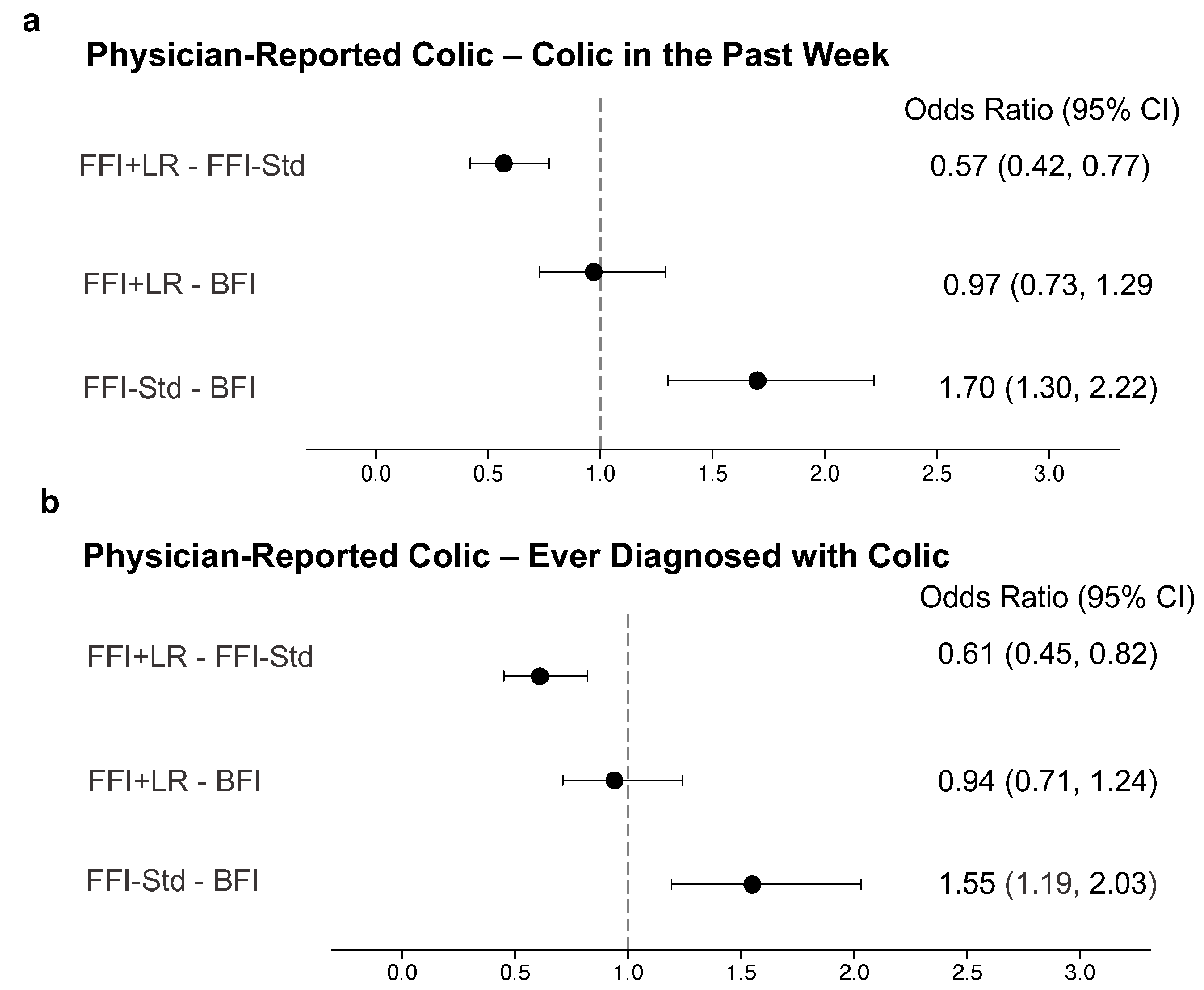
| Characteristic | BFI | FFI + LR | FFI-Std |
|---|---|---|---|
| n = 760 | n = 470 | n = 501 | |
| Age, days | 82.1 (0.9) | 80.2 (1.0) | 82.5 (1.1) |
| Sex | |||
| Female, % | 44.6 | 48.5 | 43.7 |
| Male, % | 55.4 | 51.5 | 56.3 |
| Delivery type * | |||
| Caesarean, % | 38.4 | 37.9 | 50.9 |
| Vaginal, % | 61.6 | 62.1 | 49.1 |
| Gestational age at birth, weeks | 38.7 (0.0) | 38.5 (0.1) | 38.5 (0.1) |
| Mother’s education * | |||
| Low (primary school or lower), % | 16.1 | 26.6 | 15.8 |
| Medium (high school or professional school), % | 42.0 | 46.8 | 45.5 |
| High (college or higher), % | 42.0 | 26.6 | 38.7 |
| History of gastrointestinal disease in parents | |||
| No, % | 74.5 | 77.2 | 76.8 |
| Yes, % | 25.5 | 22.8 | 23.2 |
| Birth weight *, g | 3068 (18) | 3001 (26) | 2972 (21) |
| Birth length *, cm | 49.3 (0.1) | 49.2 (0.2) | 48.6 (0.1) |
| Birth head circumference *, cm | 33.5 (0.1) | 33.6 (0.1) | 33.9 (0.1) |
| Weight at visit **, g | 5503 (35) | 5137 (48) | 5441 (42) |
| Length at visit **, cm | 58.6 (0.2) | 57.7 (0.2) | 58.9 (0.2) |
| Head circumference at visit ***, cm | 39.1 (0.1) | 38.8 (0.1) | 39.1 (0.1) |
| IGSQ Domain | IGSQ Measure | Comparison | Difference of Adjusted Mean | 95% CI | p-Value |
|---|---|---|---|---|---|
| Stool | 1. Hard stools: “How many times did your baby pass a hard stool?” | FFI + LR–FFI-Std | −0.12 | −0.21, −0.02 | 0.02 |
| FFI + LR–BFI | 0.06 | −0.02, 0.14 | 0.15 | ||
| FFI-Std–BFI | 0.17 | 0.09, 0.26 | <0.01 | ||
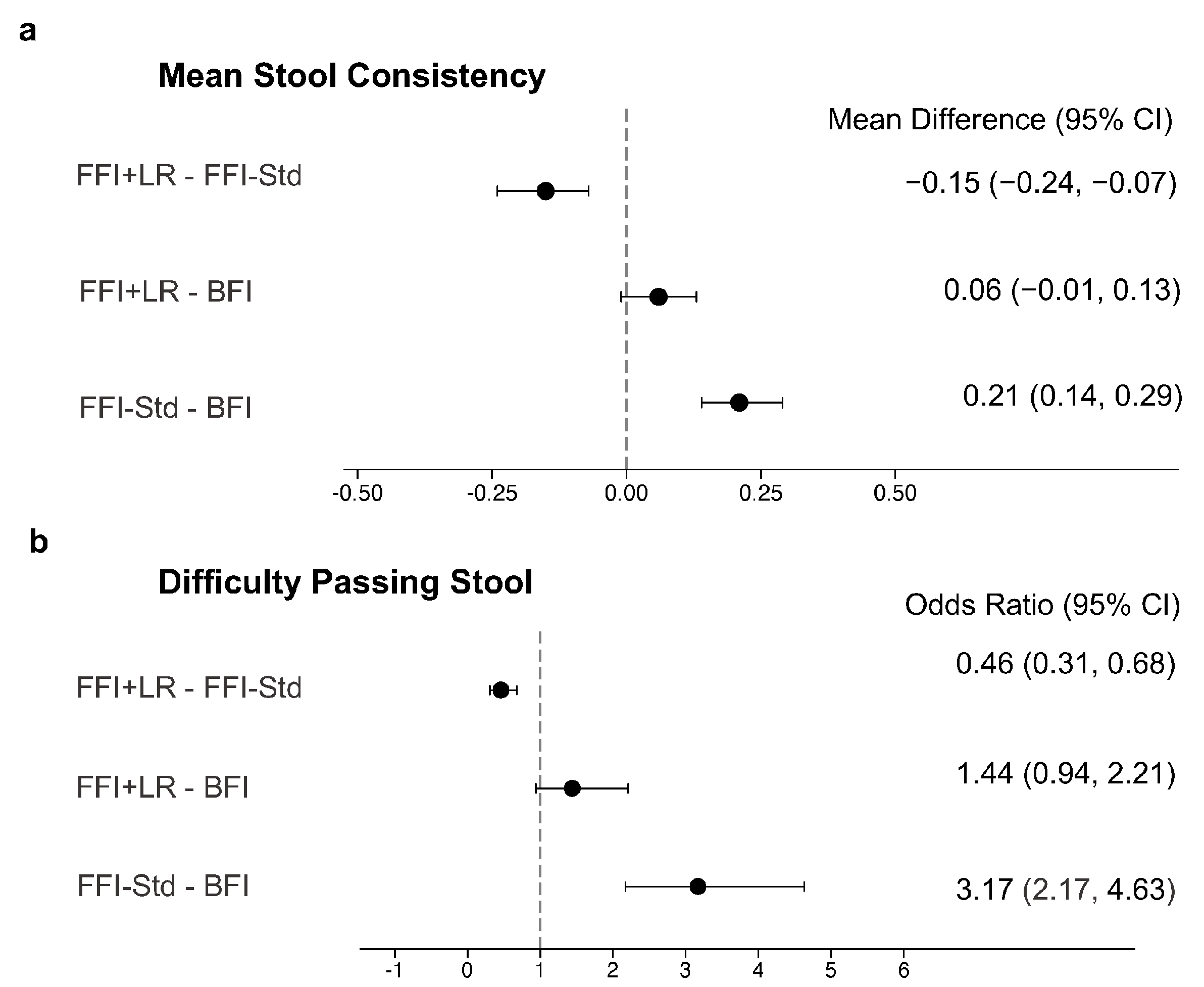
| 2. Difficulty in passing stool: “How many times did your baby have difficulty when passing a bowel movement?” | FFI + LR–FFI-Std | −0.05 | −0.18, 0.07 | 0.38 | |
| FFI + LR–BFI | 0.15 | 0.05, 0.26 | <0.01 | ||
| FFI-Std–BFI | 0.21 | 0.10, 0.31 | <0.01 | ||
| Spitting-up/Vomiting | 3. Frequency of spit up: “How many times did milk come out of your baby’s mouth?” | FFI + LR–FFI-Std | −0.18 | −0.34, −0.03 | 0.02 |
| FFI + LR–BFI | −0.09 | −0.22, 0.05 | 0.22 | ||
| FFI-Std–BFI | 0.10 | −0.03, 0.23 | 0.15 | ||
| 4. Volume of milk spit up: “How much milk usually came out each time?” | FFI + LR–FFI-Std | −0.20 | −0.32, −0.08 | <0.01 | |
| FFI + LR–BFI | −0.08 | −0.18, 0.03 | 0.14 | ||
| FFI-Std–BFI | 0.12 | 0.02, 0.22 | 0.02 | ||
| 5. Discomfort when spitting-up: “How often did your baby seem uncomfortable or fussy when milk came out of his or her mouth?” | FFI + LR–FFI-Std | −0.17 | −0.29, −0.05 | <0.01 | |
| FFI + LR–BFI | −0.05 | −0.15, 0.06 | 0.39 | ||
| FFI-Std–BFI | 0.12 | 0.02, 0.23 | 0.02 | ||
| 6. Frequency of arching back: “How many times did your baby arch his or her back as if in pain when milk came out of his or her mouth?” | FFI + LR–FFI-Std | −0.01 | −0.11, 0.09 | 0.91 | |
| FFI + LR–BFI | 0.01 | −0.08, 0.09 | 0.91 | ||
| FFI-Std–BFI | 0.01 | −0.07, 0.10 | 0.80 | ||
| Crying | 7. Total crying time: “How much total time did your baby usually cry in a day?” | FFI + LR–FFI-Std | −0.15 | −0.28, −0.01 | 0.03 |
| FFI + LR–BFI | 0.06 | −0.06, 0.17 | 0.32 | ||
| FFI-Std–BFI | 0.20 | 0.09, 0.32 | <0.01 | ||
| 8. Unable to soothe crying: “How many times were you unable to soothe your baby to stop his or her crying?” | FFI + LR–FFI-Std | −0.01 | −0.16, 0.13 | 0.85 | |
| FFI + LR–BFI | −0.10 | −0.22, 0.03 | 0.12 | ||
| FFI-Std–BFI | −0.08 | −0.20, 0.04 | 0.18 | ||
| 9. Crying after feeding: “How many times did your baby cry during or right after a feeding because the milk bothered your baby?” | FFI + LR–FFI-Std | −0.08 | −0.22, 0.06 | 0.26 | |
| FFI + LR–BFI | −0.09 | −0.21, 0.03 | 0.14 | ||
| FFI-Std–BFI | −0.01 | −0.13, 0.11 | 0.87 | ||
| Fussiness | 10. Frequency of fussiness: “On how many days was your baby fussy?” | FFI + LR–FFI-Std | 0.00 | −0.15, 0.15 | 0.99 |
| FFI + LR–BFI | −0.01 | −0.14, 0.12 | 0.89 | ||
| FFI-Std–BFI | −0.01 | −0.13, 0.11 | 0.88 | ||
| 11. Unable to soothe fussiness: “How many times were you unable to soothe your baby when he or she was fussy?” | FFI + LR–FFI-Std | −0.02 | −0.15, 0.10 | 0.70 | |
| FFI + LR–BFI | −0.03 | −0.14, 0.08 | 0.62 | ||
| FFI-Std–BFI | 0.00 | −0.11, 0.11 | 0.95 | ||
| Flatulence | 12. Frequency of gassiness: “How many times in a usual day was your baby gassy?” | FFI + LR–FFI-Std | −0.16 | −0.32, 0.00 | 0.05 |
| FFI + LR–BFI | −0.04 | −0.18, 0.10 | 0.61 | ||
| FFI-Std–BFI | 0.12 | −0.01, 0.26 | 0.07 | ||
| 13. Discomfort due to gas: “How often did gas seem to make your baby uncomfortable or fussy?” | FFI + LR–FFI-Std | −0.11 | −0.25, 0.03 | 0.11 | |
| FFI + LR–BFI | 0.01 | −0.11, 0.13 | 0.92 | ||
| FFI-Std–BFI | 0.12 | 0.002, 0.24 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Happy Tummy Consortium; Lavalle, L.; Sauvageot, N.; Cercamondi, C.I.; Jankovic, I.; Egli, D.; Vandenplas, Y. Limosilactobacillus reuteri DSM 17938-Containing Infant Formulas and the Associations with Gastrointestinal Tolerance: A Cross-Sectional Observational Study. Nutrients 2023, 15, 530. https://doi.org/10.3390/nu15030530
Happy Tummy Consortium, Lavalle L, Sauvageot N, Cercamondi CI, Jankovic I, Egli D, Vandenplas Y. Limosilactobacillus reuteri DSM 17938-Containing Infant Formulas and the Associations with Gastrointestinal Tolerance: A Cross-Sectional Observational Study. Nutrients. 2023; 15(3):530. https://doi.org/10.3390/nu15030530
Chicago/Turabian StyleHappy Tummy Consortium, Luca Lavalle, Nicolas Sauvageot, Colin Ivano Cercamondi, Ivana Jankovic, Delphine Egli, and Yvan Vandenplas. 2023. "Limosilactobacillus reuteri DSM 17938-Containing Infant Formulas and the Associations with Gastrointestinal Tolerance: A Cross-Sectional Observational Study" Nutrients 15, no. 3: 530. https://doi.org/10.3390/nu15030530
APA StyleHappy Tummy Consortium, Lavalle, L., Sauvageot, N., Cercamondi, C. I., Jankovic, I., Egli, D., & Vandenplas, Y. (2023). Limosilactobacillus reuteri DSM 17938-Containing Infant Formulas and the Associations with Gastrointestinal Tolerance: A Cross-Sectional Observational Study. Nutrients, 15(3), 530. https://doi.org/10.3390/nu15030530






