Narrow Leafed Lupin (Lupinus angustifolius L.) β-Conglutin Seed Proteins as a New Natural Cytotoxic Agents against Breast Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Overexpression and Purification of β-Conglutins
2.2. Cell Lines and Culture Conditions
2.3. MTT Assay
2.4. Trypan Blue Assay
2.5. Apoptotic and Ferroptotic Cell Identification
2.6. ROS Measurement
2.7. DNA Damage Assay
2.8. Quantification and Characterization of CSCs
2.9. Sphere Formation Assay
2.10. Western Blot
2.11. Autophagy Detection
2.12. Statistical Analysis
3. Results
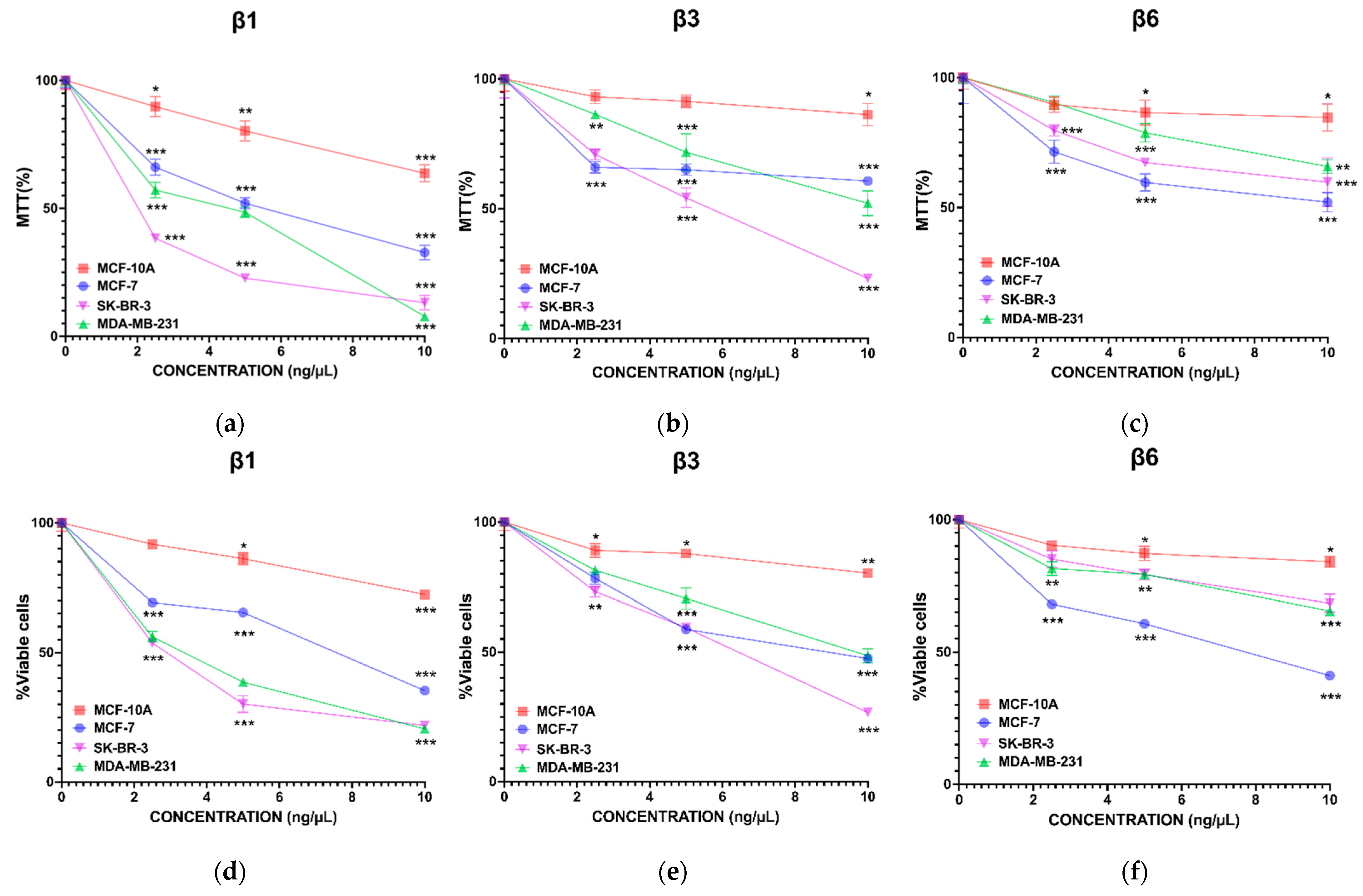
3.1. β-Conglutins Inhibit Breast Cancer Cell Lines Growth in a 2D In Vitro Model
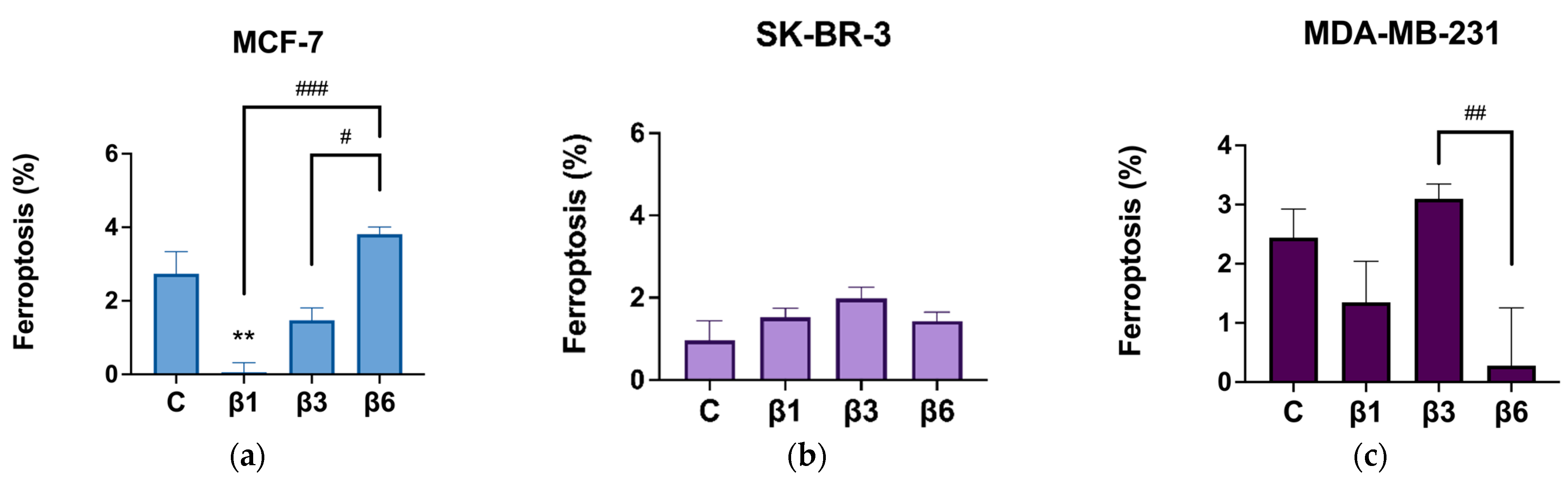
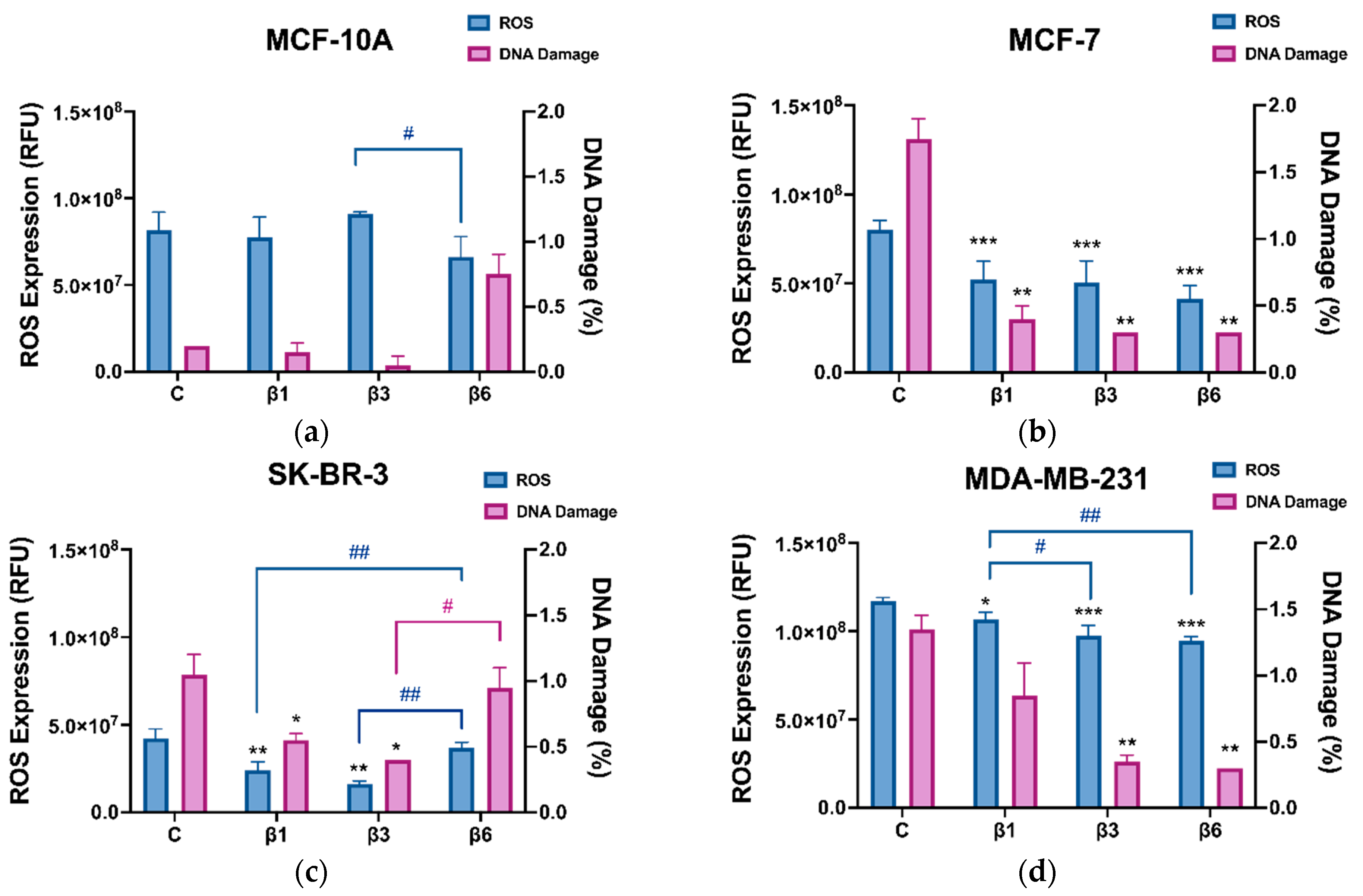
3.2. β-Conglutins Induce Caspase-Independent Apoptosis in Breast Cancer Cell Lines
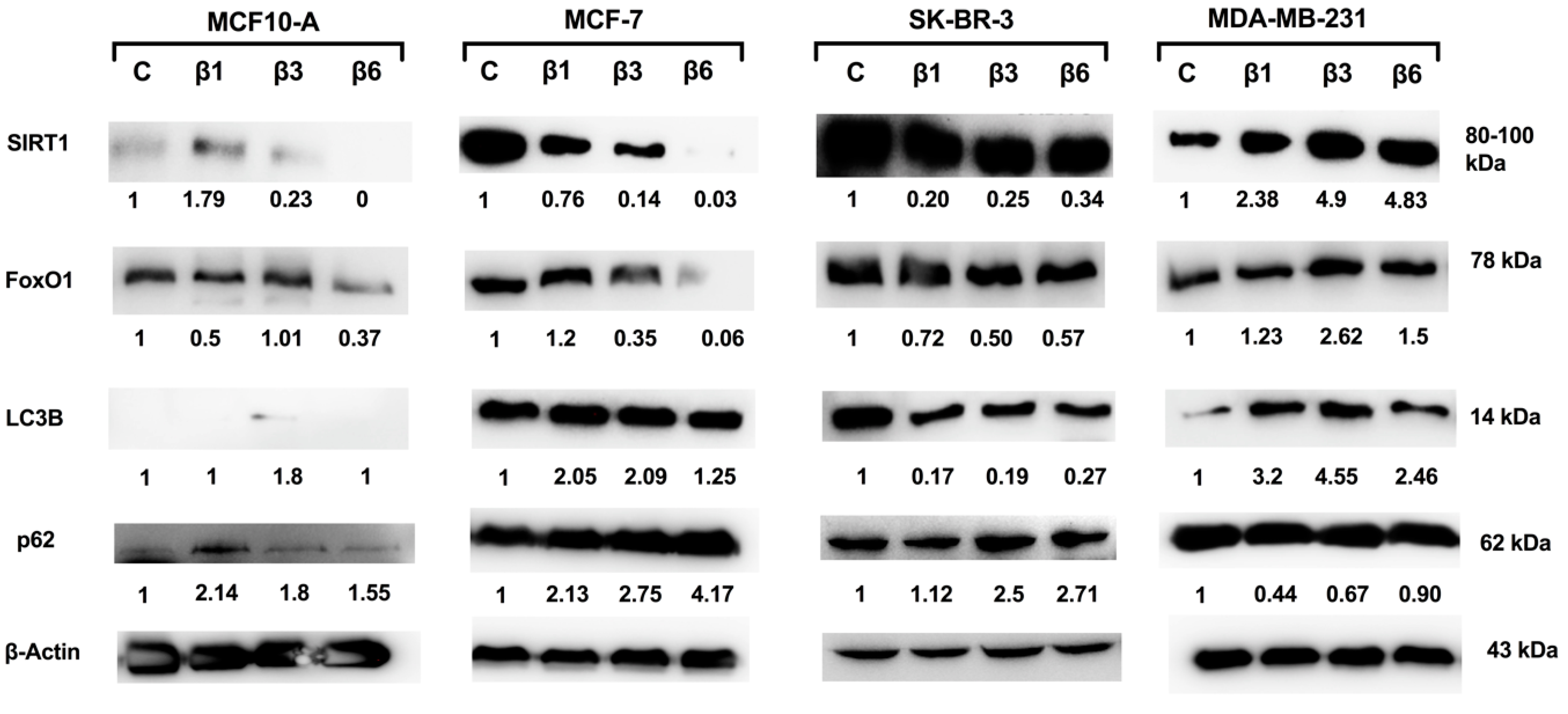
3.3. Implication of SIRT1/FoxO1 Pathway in β-Conglutins Effect on In Vitro Cultured Breast Cancer Cells
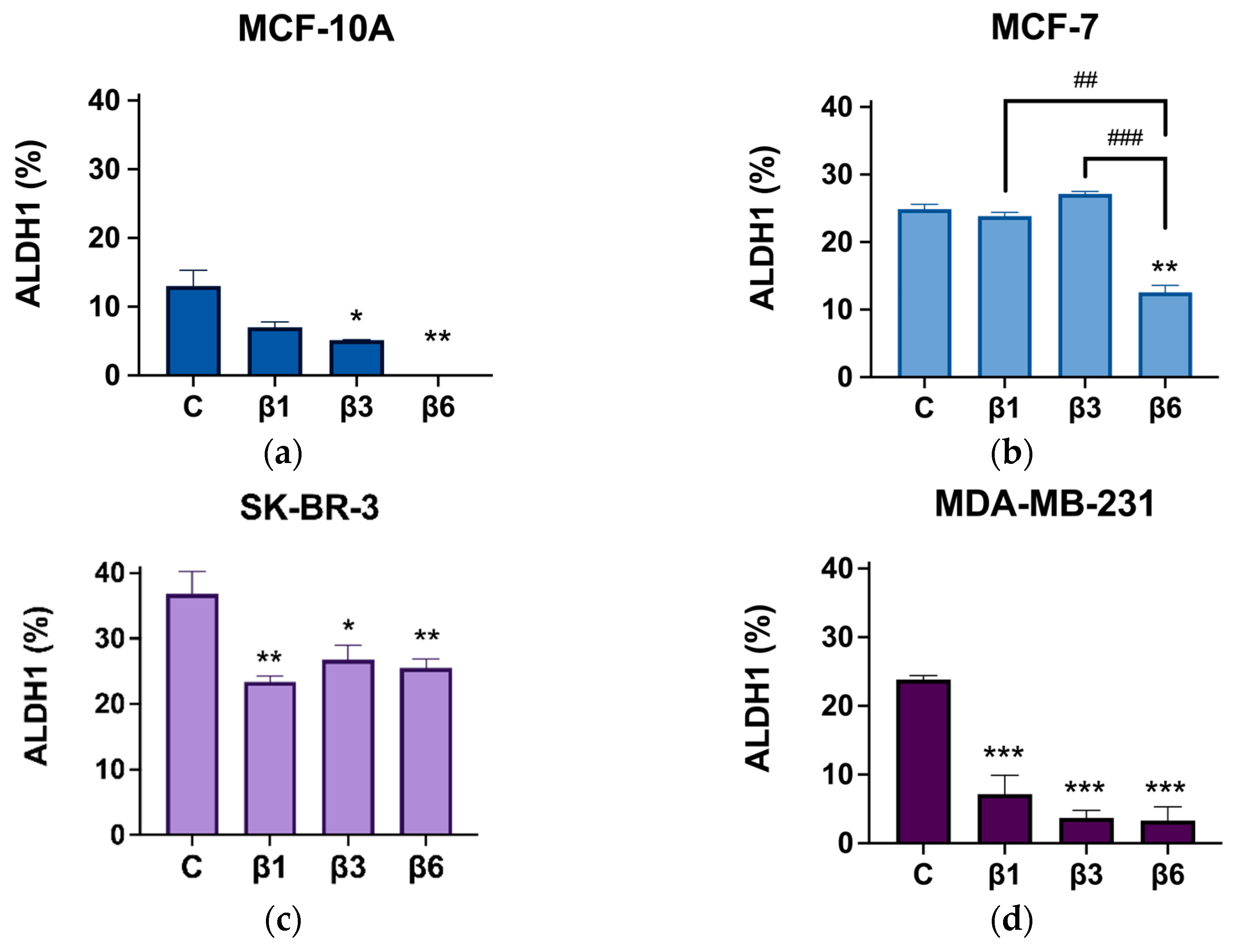
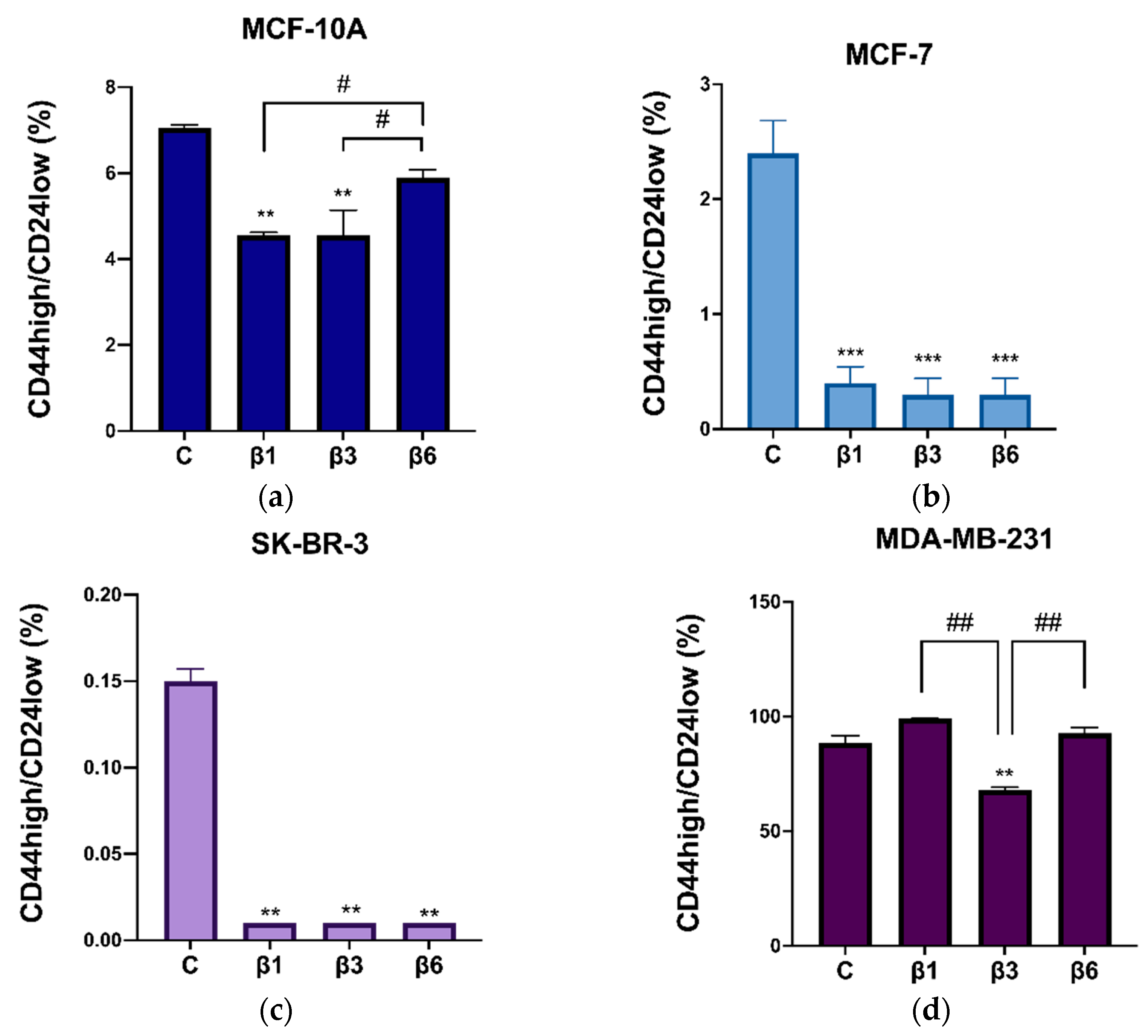
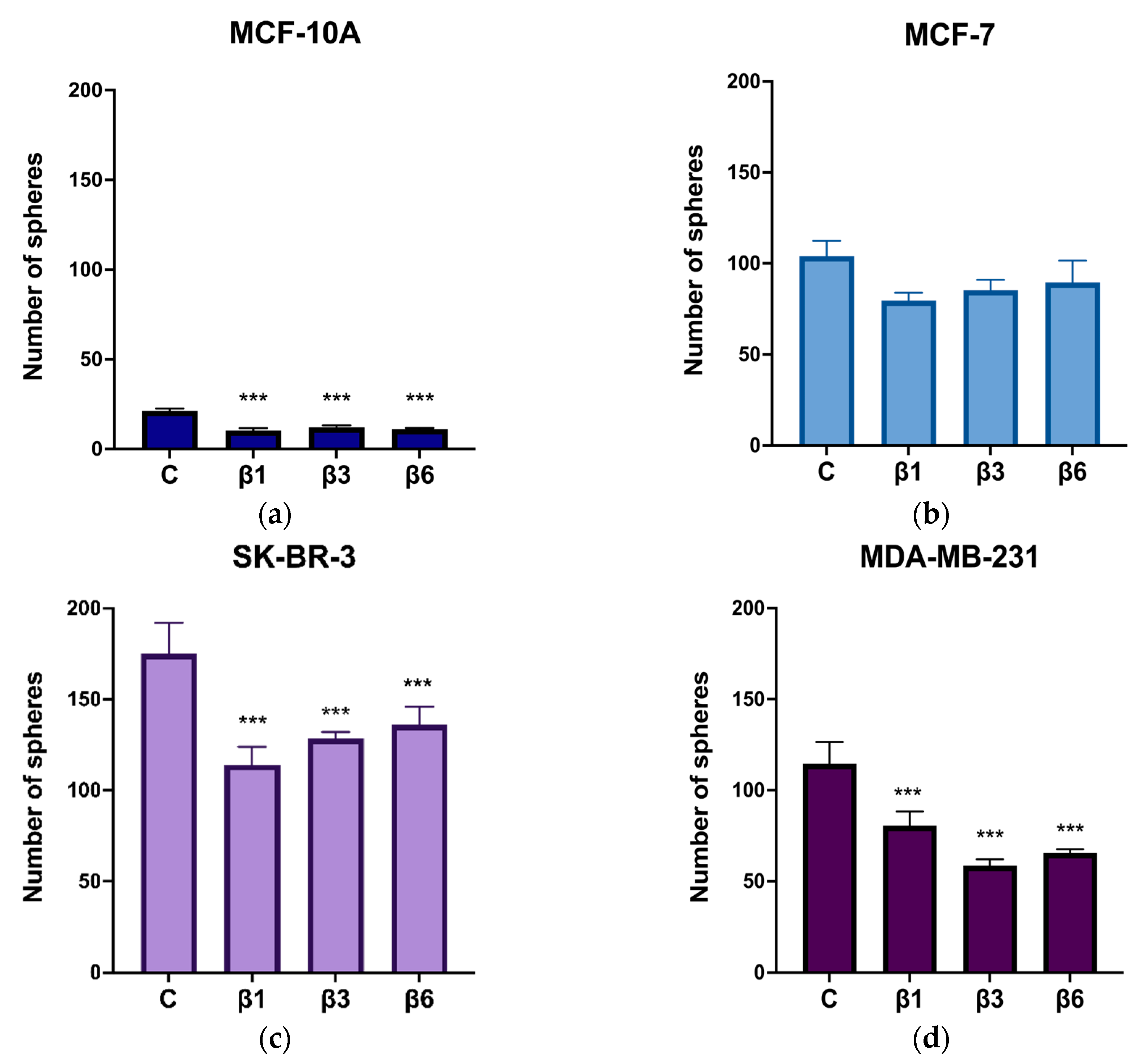
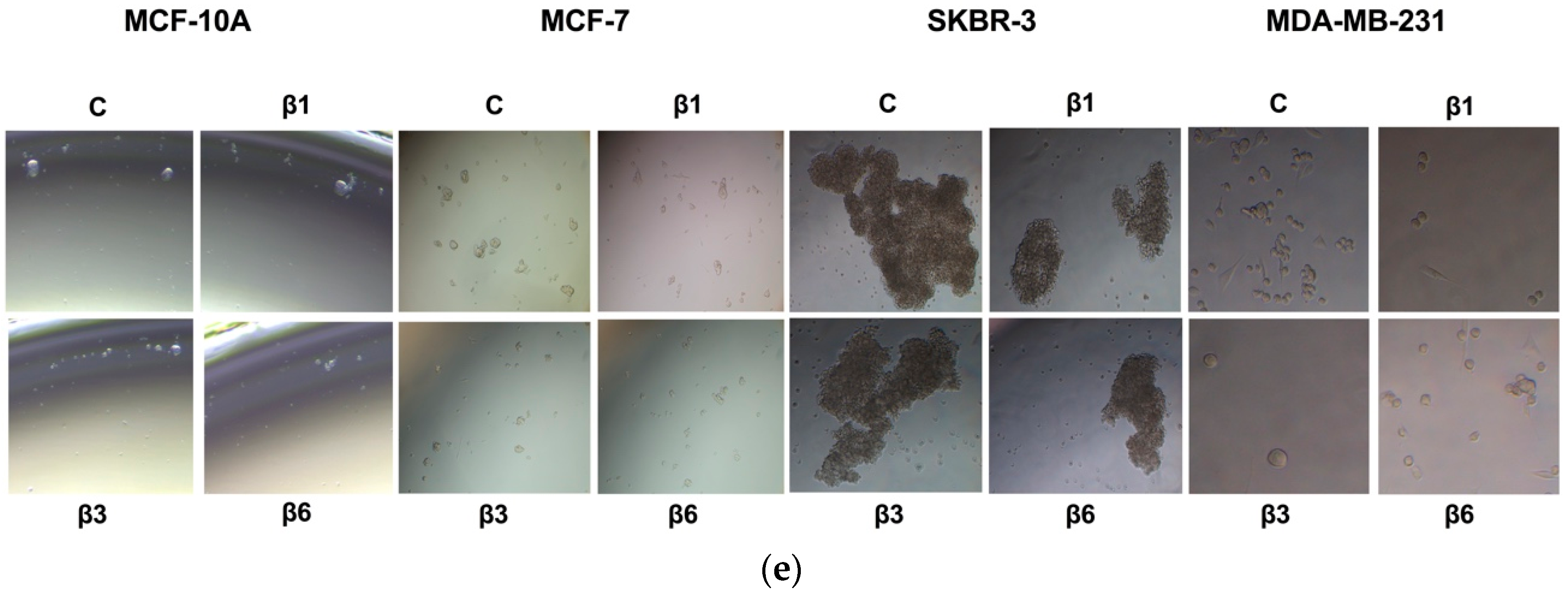
3.4. β-Conglutins Regulate Stemness Phenotype in Breast Cancer
4. Discussion
5. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Ríos-Arrabal, S.; Artacho-Cordón, F.; Torralbo, A.I.; López-Ruiz, E.; Marchal, J.A.; Núñez, M.I. Radiation and Stemness Phenotype May Influence Individual Breast Cancer Outcomes: The Crucial Role of MMPs and Microenvironment. Cancers 2019, 11, 1781. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Tomao, S.; Tomao, F.; Rossi, L.; Zaccarelli, E.; Caruso, D.; Minozzi, M.; Frati, L.; Papa, A. Vici Triple-negative breast cancer: New perspectives for targeted therapies. OncoTargets Ther. 2015, 177, 67673. [Google Scholar] [CrossRef]
- Stages of Breast Cancer | Understand Breast Cancer Staging. Available online: https://www.cancer.org/cancer/breast-cancer/understanding-a-breast-cancer-diagnosis/stages-of-breast-cancer.html (accessed on 26 September 2022).
- Olivares-Urbano, M.A.; Griñán-Lisón, C.; Zurita, M.; Moral, R.; Ríos-Arrabal, S.; Artacho-Cordón, F.; Arrebola, J.P.; González, A.R.; León, J.; Antonio Marchal, J.; et al. Matrix metalloproteases and TIMPs as prognostic biomarkers in breast cancer patients treated with radiotherapy: A pilot study. J. Cell. Mol. Med. 2020, 24, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Fatehi, D.; Soltani, A.; Ghatrehsamani, M. SRT1720, a potential sensitizer for radiotherapy and cytotoxicity effects of NVB-BEZ235 in metastatic breast cancer cells. Pathol. Res. Pract. 2018, 214, 889–895. [Google Scholar] [CrossRef] [PubMed]
- American Joint Committee on Cancer. AJCC Cancer Staging Manual; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Maughan, K.L.; Lutterbie, M.A.; Ham, P.S. Treatment of breast cancer. Am. Fam. Physician 2010, 81, 1339–1346. [Google Scholar] [PubMed]
- Griñán-Lisón, C.; Olivares-Urbano, M.A.; Jiménez, G.; López-Ruiz, E.; Val, C.; Morata-Tarifa, C.; Entrena, J.M.; González-Ramírez, A.R.; Boulaiz, H.; Zurita Herrera, M.; et al. miRNAs as radio-response biomarkers for breast cancer stem cells. Mol. Oncol. 2020, 14, 556–570. [Google Scholar] [CrossRef]
- Morrison, R.; Schleicher, S.M.; Sun, Y.; Niermann, K.J.; Kim, S.; Spratt, D.E.; Chung, C.H.; Lu, B. Targeting the Mechanisms of Resistance to Chemotherapy and Radiotherapy with the Cancer Stem Cell Hypothesis. J. Oncol. 2011, 2011, 941876. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Meng, X.; Gan, R.-Y.; Zhang, J.-J.; Li, H.-B. Dietary Natural Products for Prevention and Treatment of Breast Cancer. Nutrients 2017, 9, 728. [Google Scholar] [CrossRef]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.-P.; Li, S.; Li, H.-B. Spices for Prevention and Treatment of Cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Y.; Zhou, T.; Zheng, J.; Li, S.; Li, H.-B. Dietary Natural Products for Prevention and Treatment of Liver Cancer. Nutrients 2016, 8, 156. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Chen, W.Y.; Michels, K.B.; Cho, E.; Willett, W.C.; Eliassen, A.H. Fruit and vegetable consumption in adolescence and early adulthood and risk of breast cancer: Population based cohort study. BMJ 2016, 352, i2343. [Google Scholar] [CrossRef] [PubMed]
- Lucas, M.M.; Stoddard, F.L.; Annicchiarico, P.; Frías, J.; Martínez-Villaluenga, C.; Sussmann, D.; Duranti, M.; Seger, A.; Zander, P.M.; Pueyo, J.J. The future of lupin as a protein crop in Europe. Front. Plant Sci. 2015, 6, 705. [Google Scholar] [CrossRef] [PubMed]
- Kouris-Blazos, A.; Belski, R. Health benefits of legumes and pulses with a focus on Australian sweet lupins. Asia Pac. J. Clin. Nutr. 2016, 25, 1–17. [Google Scholar] [CrossRef]
- Belski, R.; Mori, T.A.; Puddey, I.B.; Sipsas, S.; Woodman, R.J.; Ackland, T.R.; Beilin, L.J.; Dove, E.R.; Carlyon, N.B.; Jayaseena, V.; et al. Effects of lupin-enriched foods on body composition and cardiovascular disease risk factors: A 12-month randomized controlled weight loss trial. Int. J. Obes. 2011, 35, 810–819. [Google Scholar] [CrossRef]
- Dove, E.R.; Mori, T.A.; Chew, G.T.; Barden, A.E.; Woodman, R.J.; Puddey, I.B.; Sipsas, S.; Hodgson, J.M. Lupin and soya reduce glycaemia acutely in type 2 diabetes. Br. J. Nutr. 2011, 106, 1045–1051. [Google Scholar] [CrossRef]
- Foley, R.C.; Gao, L.-L.; Spriggs, A.; Soo, L.Y.C.; Goggin, D.E.; Smith, P.M.C.; Atkins, C.A.; Singh, K.B. Identification and characterisation of seed storage protein transcripts from Lupinus angustifolius. BMC Plant Biol. 2011, 11, 59. [Google Scholar] [CrossRef]
- Foley, R.C.; Jimenez-Lopez, J.C.; Kamphuis, L.G.; Hane, J.K.; Melser, S.; Singh, K.B. Analysis of conglutin seed storage proteins across lupin species using transcriptomic, protein and comparative genomic approaches. BMC Plant Biol. 2015, 15, 106. [Google Scholar] [CrossRef]
- Lima-Cabello, E.; Alche, V.; Foley, R.C.; Andrikopoulos, S.; Morahan, G.; Singh, K.B.; Alche, J.D.; Jimenez-Lopez, J.C. Narrow-leafed lupin (Lupinus angustifolius L.) β-conglutin proteins modulate the insulin signaling pathway as potential type 2 diabetes treatment and inflammatory-related disease amelioration. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Jimenez-Lopez, J.C. Narrow-leafed lupin (Lupinus angustifolius L.) β-conglutin: A multifunctional family of proteins with roles in plant defence, human health benefits, and potential uses as functional food. Legume Sci. 2020, 2, e33. [Google Scholar] [CrossRef]
- Singh, N.; Baby, D.; Rajguru, J.P.; Patil, P.B.; Thakkannavar, S.S.; Pujari, V.B. Inflammation and Cancer. Ann. Afr. Med. 2019, 18, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Yao, H.; Caito, S.; Sundar, I.K.; Rahman, I. Redox regulation of SIRT1 in inflammation and cellular senescence. Free Radic. Biol. Med. 2013, 61, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Rahman, I. Perspectives on translational and therapeutic aspects of SIRT1 in inflammaging and senescence. Biochem. Pharmacol. 2012, 84, 1332–1339. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Tang, X.; Qian, M.; Liu, Z.; Meng, F.; Fu, L.; Wang, Z.; Zhu, W.-G.; Huang, J.-D.; Zhou, Z.; et al. A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene 2018, 37, 6299–6315. [Google Scholar] [CrossRef] [PubMed]
- Fukui, M.; Choi, H.J.; Wang, P.; Zhu, B.T. Mechanism underlying resveratrol’s attenuation of paclitaxel cytotoxicity in human breast cancer cells: Role of the SIRT1-FOXO1-HER3 signaling pathway. Cancer Treat. Res. Commun. 2021, 28, 100386. [Google Scholar] [CrossRef]
- Yang, Z.J.; Chee, C.E.; Huang, S.; Sinicrope, F.A. The role of autophagy in cancer: Therapeutic implications. Mol. Cancer Ther. 2011, 10, 1533–1541. [Google Scholar] [CrossRef]
- Marsh, T.; Debnath, J. Autophagy suppresses breast cancer metastasis by degrading NBR1. Autophagy 2020, 16, 1164–1165. [Google Scholar] [CrossRef]
- Wu, S.; Chu, Y.; Yang, Y.; Li, Y.; He, P.; Zheng, Y.; Zhang, C.; Liu, Q.; Han, L.; Huang, R. Inhibition of macrophage autophagy induced by Salmonella enterica serovar typhi plasmid. Front. Biosci. Landmark Ed. 2014, 19, 490–503. [Google Scholar] [CrossRef]
- Dhingra, A.; Alexander, D.; Reyes-Reveles, J.; Sharp, R.; Boesze-Battaglia, K. Microtubule-Associated Protein 1 Light Chain 3 (LC3) Isoforms in RPE and Retina. Adv. Exp. Med. Biol. 2018, 1074, 609–616. [Google Scholar] [CrossRef]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef]
- Kabeya, Y.; Mizushima, N.; Ueno, T.; Yamamoto, A.; Kirisako, T.; Noda, T.; Kominami, E.; Ohsumi, Y.; Yoshimori, T. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000, 19, 5720–5728. [Google Scholar] [CrossRef] [PubMed]
- Ríos-Arrabal, S.; Puentes-Pardo, J.D.; Moreno-SanJuan, S.; Szuba, Á.; Casado, J.; García-Costela, M.; Escudero-Feliu, J.; Verbeni, M.; Cano, C.; González-Puga, C.; et al. Endothelin-1 as a Mediator of Heme Oxygenase-1-Induced Stemness in Colorectal Cancer: Influence of p53. J. Pers. Med. 2021, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Lumivero, 2023 XLSTAT | Software estadístico Excel. Available online: https://www.xlstat.com/es/ (accessed on 9 January 2023).
- Suzuki, M.; Bandoski, C.; Bartlett, J.D. Fluoride induces oxidative damage and SIRT1/autophagy through ROS-mediated JNK signaling. Free Radic. Biol. Med. 2015, 89, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Nasrin, N.; Kaushik, V.K.; Fortier, E.; Wall, D.; Pearson, K.J.; De Cabo, R.; Bordone, L. JNK1 phosphorylates SIRT1 and promotes its enzymatic activity. PLoS ONE 2009, 4, e8414. [Google Scholar] [CrossRef]
- Salminen, A.; Kaarniranta, K.; Kauppinen, A. Crosstalk between Oxidative Stress and SIRT1: Impact on the Aging Process. Int. J. Mol. Sci. 2013, 14, 3834–3859. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, A.; Molkentin, J.D.; Paik, J.-H.; DePinho, R.A.; Yutzey, K.E. FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J. Biol. Chem. 2011, 286, 7468–7478. [Google Scholar] [CrossRef]
- Simmons, G.E.; Pruitt, W.M.; Pruitt, K. Diverse roles of SIRT1 in cancer biology and lipid metabolism. Int. J. Mol. Sci. 2015, 16, 950–965. [Google Scholar] [CrossRef]
- Boudreau, H.E.; Casterline, B.W.; Burke, D.J.; Leto, T.L. Wild-type and mutant p53 differentially regulate NADPH oxidase 4 in TGF-β-mediated migration of human lung and breast epithelial cells. Br. J. Cancer 2014, 110, 2569–2582. [Google Scholar] [CrossRef]
- Motadi, L.R.; Lekganyane, M.M.; Moela, P. RBBP6 expressional effects on cell proliferation and apoptosis in breast cancer cell lines with distinct p53 statuses. Cancer Manag. Res. 2018, 10, 3357–3369. [Google Scholar] [CrossRef]
- Ghaleb, A.; Padellan, M.; Marchenko, N. Mutant p53 drives the loss of heterozygosity by the upregulation of Nek2 in breast cancer cells. Breast Cancer Res. 2020, 22, 133. [Google Scholar] [CrossRef]
- Jordan, J.J.; Inga, A.; Conway, K.; Edmiston, S.; Carey, L.A.; Wu, L.; Resnick, M.A. Altered-Function p53 Missense Mutations Identified in Breast Cancers Can Have Subtle Effects on Transactivation. Mol. Cancer Res. 2010, 8, 701–716. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, O.S.; Brown, B.L.; Dobson, P.R.M. AMPK Activation of Apoptotic Markers in Human Breast Cancer Cell Lines with Different p53 Backgrounds: MCF-7, MDA-MB-231 and T47D Cells. Asian Pac. J. Cancer Prev. APJCP 2019, 20, 3763–3770. [Google Scholar] [CrossRef] [PubMed]
- Amaro, F.; Silva, D.; Reguengo, H.; Oliveira, J.C.; Quintas, C.; Vale, N.; Gonçalves, J.; Fresco, P. β-Adrenoceptor Activation in Breast MCF-10A Cells Induces a Pattern of Catecholamine Production Similar to that of Tumorigenic MCF-7 Cells. Int. J. Mol. Sci. 2020, 21, 7968. [Google Scholar] [CrossRef]
- Schratter, G.; Scheruebel, S.; Langthaler, S.; Ester, K.; Pelzmann, B.; Ghaffari-Tabrizi-Wizsy, N.; Rezania, S.; Gorischek, A.; Platzer, D.; Zorn-Pauly, K.; et al. GIRK1 triggers multiple cancer-related pathways in the benign mammary epithelial cell line MCF10A. Sci. Rep. 2019, 9, 19277. [Google Scholar] [CrossRef] [PubMed]
- Won, Y.-S.; Seo, K.-I. Lupiwighteone induces caspase-dependent and -independent apoptosis on human breast cancer cells via inhibiting PI3K/Akt/mTOR pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 135, 110863. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-A.; Jang, J.-H.; Lee, Y.-H.; Sung, E.-G.; Song, I.-H.; Kim, J.-Y.; Kim, S.; Sohn, H.-Y.; Lee, T.-J. Dioscin induces caspase-independent apoptosis through activation of apoptosis-inducing factor in breast cancer cells. Apoptosis Int. J. Program. Cell Death 2014, 19, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, E.; Sahin, A.A.; Chen, J.S.; Krishnamurthy, R.R.; Aggarwal, N.; Brun, A.-M.; Sapino, A.; Zhang, F.; Sharma, D.; Yang, X.-H.; et al. Down-regulation of caspase 3 in breast cancer: A possible mechanism for chemoresistance. Oncogene 2002, 21, 8843–8851. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Guo, P.; Xie, X.; Wang, Y.; Chen, G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 2017, 21, 648–657. [Google Scholar] [CrossRef] [PubMed]
- Sui, S.; Xu, S.; Pang, D. Emerging role of ferroptosis in breast cancer: New dawn for overcoming tumor progression. Pharmacol. Ther. 2022, 232, 107992. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Wang, J.-Q.; Assaraf, Y.G.; Ren, L.; Gupta, P.; Wei, L.; Ashby, C.R.; Yang, D.-H.; Chen, Z.-S. Modulating ROS to overcome multidrug resistance in cancer. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2018, 41, 1–25. [Google Scholar] [CrossRef]
- Dayem, A.A.; Choi, H.-Y.; Kim, J.-H.; Cho, S.-G. Role of oxidative stress in stem, cancer, and cancer stem cells. Cancers 2010, 2, 859–884. [Google Scholar] [CrossRef] [PubMed]
- Hecht, F.; Cazarin, J.M.; Lima, C.E.; Faria, C.C.; Leitão, A.A.D.C.; Ferreira, A.C.F.; Carvalho, D.P.; Fortunato, R.S. Redox homeostasis of breast cancer lineages contributes to differential cell death response to exogenous hydrogen peroxide. Life Sci. 2016, 158, 7–13. [Google Scholar] [CrossRef]
- Lee, K.-M.; Giltnane, J.M.; Balko, J.M.; Schwarz, L.J.; Guerrero-Zotano, A.L.; Hutchinson, K.E.; Nixon, M.J.; Estrada, M.V.; Sánchez, V.; Sanders, M.E.; et al. MYC and MCL1 Cooperatively Promote Chemotherapy-Resistant Breast Cancer Stem Cells via Regulation of Mitochondrial Oxidative Phosphorylation. Cell Metab. 2017, 26, 633–647.e7. [Google Scholar] [CrossRef] [PubMed]
- Isoalantolactone induces apoptosis in human breast cancer cells via ROS-mediated mitochondrial pathway and downregulation of SIRT1—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27600429/ (accessed on 9 January 2023).
- Dilmac, S.; Kuscu, N.; Caner, A.; Yildirim, S.; Yoldas, B.; Farooqi, A.A.; Tanriover, G. SIRT1/FOXO Signaling Pathway in Breast Cancer Progression and Metastasis. Int. J. Mol. Sci. 2022, 23, 10227. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Luo, J. The role of SIRT1 in tumorigenesis. N. Am. J. Med. Sci. 2011, 4, 104–106. [Google Scholar] [CrossRef]
- Yang, H.; Chen, Y.; Jiang, Y.; Wang, D.; Yan, J.; Zhou, Z. TP53 mutation influences the efficacy of treatment of colorectal cancer cell lines with a combination of sirtuin inhibitors and chemotherapeutic agents. Exp. Ther. Med. 2020, 20, 1415–1422. [Google Scholar] [CrossRef]
- Xu, Y.; Qin, Q.; Chen, R.; Wei, C.; Mo, Q. SIRT1 promotes proliferation, migration, and invasion of breast cancer cell line MCF-7 by upregulating DNA polymerase delta1 (POLD1). Biochem. Biophys. Res. Commun. 2018, 502, 351–357. [Google Scholar] [CrossRef]
- Wang, R.-H.; Zheng, Y.; Kim, H.-S.; Xu, X.; Cao, L.; Luhasen, T.; Lee, M.-H.; Xiao, C.; Vassilopoulos, A.; Chen, W.; et al. Interplay among BRCA1, SIRT1, and Survivin during BRCA1-associated tumorigenesis. Mol. Cell 2008, 32, 11–20. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, W.; Linke, S.P.; Cao, L.; Li, W.M.; Furth, P.A.; Harris, C.C.; Deng, C.X. Genetic interactions between tumor suppressors Brca1 and p53 in apoptosis, cell cycle and tumorigenesis. Nat. Genet. 2001, 28, 266–271. [Google Scholar] [CrossRef]
- Wang, R.-H.; Sengupta, K.; Li, C.; Kim, H.-S.; Cao, L.; Xiao, C.; Kim, S.; Xu, X.; Zheng, Y.; Chilton, B.; et al. Impaired DNA damage response, genome instability, and tumorigenesis in SIRT1 mutant mice. Cancer Cell 2008, 14, 312–323. [Google Scholar] [CrossRef]
- Abdullah, M.L.; Al-Shabanah, O.; Hassan, Z.K.; Hafez, M.M. Eugenol-Induced Autophagy and Apoptosis in Breast Cancer Cells via PI3K/AKT/FOXO3a Pathway Inhibition. Int. J. Mol. Sci. 2021, 22, 9243. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yin, K.; Zhang, Y.; Lu, H.; Hou, L.; Zhao, H.; Xing, M. Novel pathways of fluoride-induced hepatotoxicity: P53-dependent ferroptosis induced by the SIRT1/FOXOs pathway and Nrf2/HO-1 pathway. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2022, 264, 109526. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Fan, S.; Qin, T.; Yang, J.; Sun, Y.; Lu, Y.; Mao, J.; Li, L. Role of autophagy in breast cancer and breast cancer stem cells (Review). Int. J. Oncol. 2018, 52, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Becker, A.; Zimmer, A.; Lu, J.; Buettner, R.; Kirfel, J. SNAI1-mediated epithelial-mesenchymal transition confers chemoresistance and cellular plasticity by regulating genes involved in cell death and stem cell maintenance. PLoS ONE 2013, 8, e66558. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Escudero-Feliu, J.; García-Costela, M.; Moreno-SanJuan, S.; Puentes-Pardo, J.D.; Arrabal, S.R.; González-Novoa, P.; Núñez, M.I.; Carazo, Á.; Jimenez-Lopez, J.C.; León, J. Narrow Leafed Lupin (Lupinus angustifolius L.) β-Conglutin Seed Proteins as a New Natural Cytotoxic Agents against Breast Cancer Cells. Nutrients 2023, 15, 523. https://doi.org/10.3390/nu15030523
Escudero-Feliu J, García-Costela M, Moreno-SanJuan S, Puentes-Pardo JD, Arrabal SR, González-Novoa P, Núñez MI, Carazo Á, Jimenez-Lopez JC, León J. Narrow Leafed Lupin (Lupinus angustifolius L.) β-Conglutin Seed Proteins as a New Natural Cytotoxic Agents against Breast Cancer Cells. Nutrients. 2023; 15(3):523. https://doi.org/10.3390/nu15030523
Chicago/Turabian StyleEscudero-Feliu, Julia, María García-Costela, Sara Moreno-SanJuan, Jose D. Puentes-Pardo, Sandra Ríos Arrabal, Paula González-Novoa, María Isabel Núñez, Ángel Carazo, Jose C. Jimenez-Lopez, and Josefa León. 2023. "Narrow Leafed Lupin (Lupinus angustifolius L.) β-Conglutin Seed Proteins as a New Natural Cytotoxic Agents against Breast Cancer Cells" Nutrients 15, no. 3: 523. https://doi.org/10.3390/nu15030523
APA StyleEscudero-Feliu, J., García-Costela, M., Moreno-SanJuan, S., Puentes-Pardo, J. D., Arrabal, S. R., González-Novoa, P., Núñez, M. I., Carazo, Á., Jimenez-Lopez, J. C., & León, J. (2023). Narrow Leafed Lupin (Lupinus angustifolius L.) β-Conglutin Seed Proteins as a New Natural Cytotoxic Agents against Breast Cancer Cells. Nutrients, 15(3), 523. https://doi.org/10.3390/nu15030523






