Enhancement of Human Epidermal Cell Defense against UVB Damage by Fermentation of Passiflora edulis Sims Peel with Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Preparation of PW and PF
2.3. Determination of PW and PF Composition
2.4. In Vitro Antioxidant Activity Assay
2.5. Cell Culture and Viability Experiment
2.6. Measurement of Intracellular ROS Levels
2.7. ELISA
2.8. qRT-PCR
2.9. Safety Determination of PF
2.9.1. Red Blood Cell Hemolysis Test
2.9.2. Chicken Embryo Chorioallantoic Membrane Test (HET-CAM)
2.10. Statistical Analysis
3. Results
3.1. Active Substance Content of PF and PW
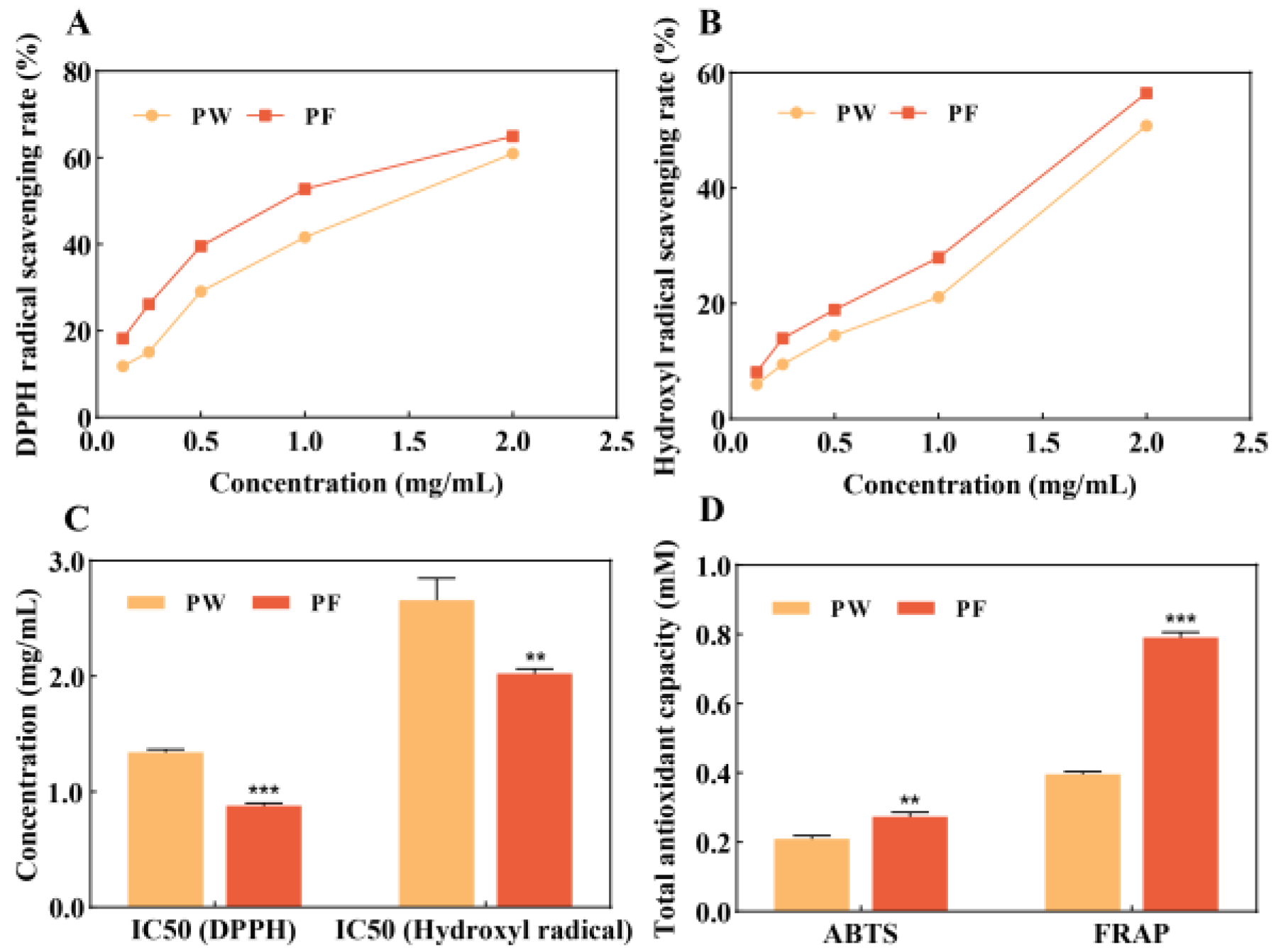
3.2. In Vitro Antioxidant Activity of PF and PW
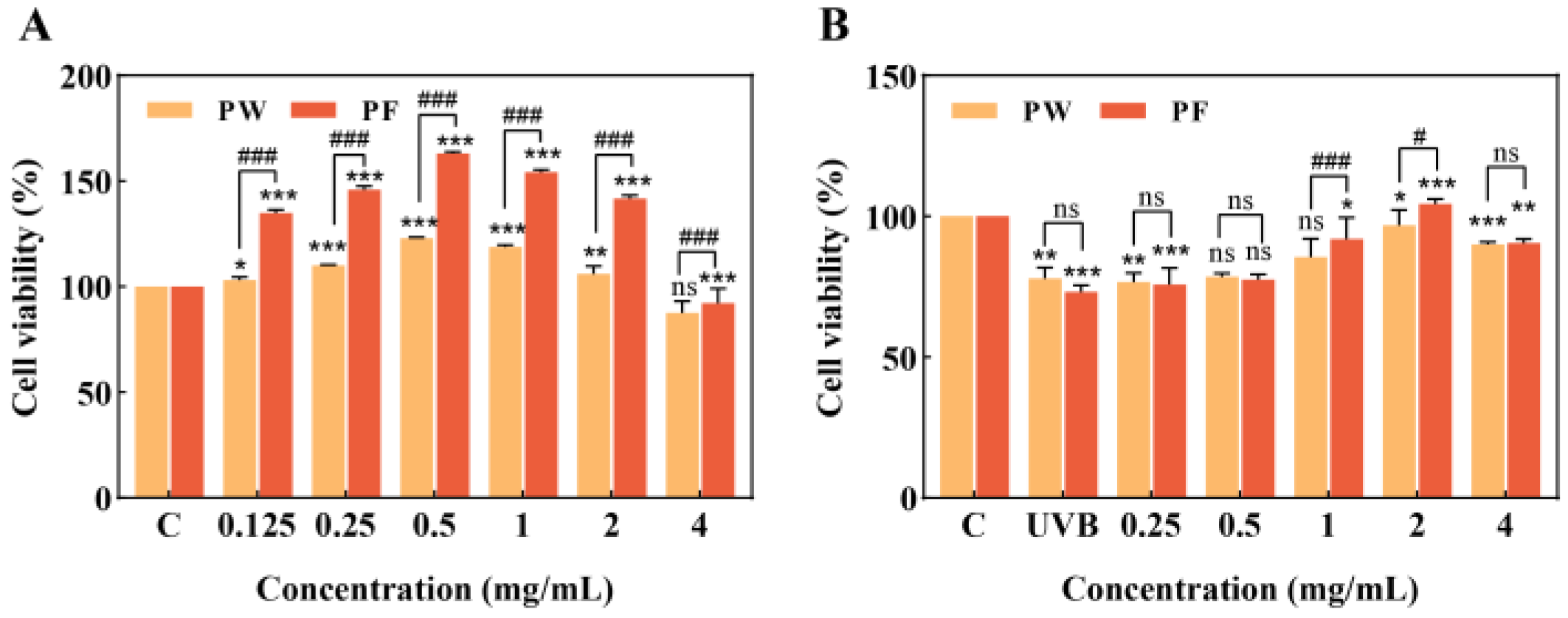
3.3. Effects of PF and PW on HaCaT Cell Activity
3.4. ROS Inhibition and Antioxidant Capacity of PF and PW
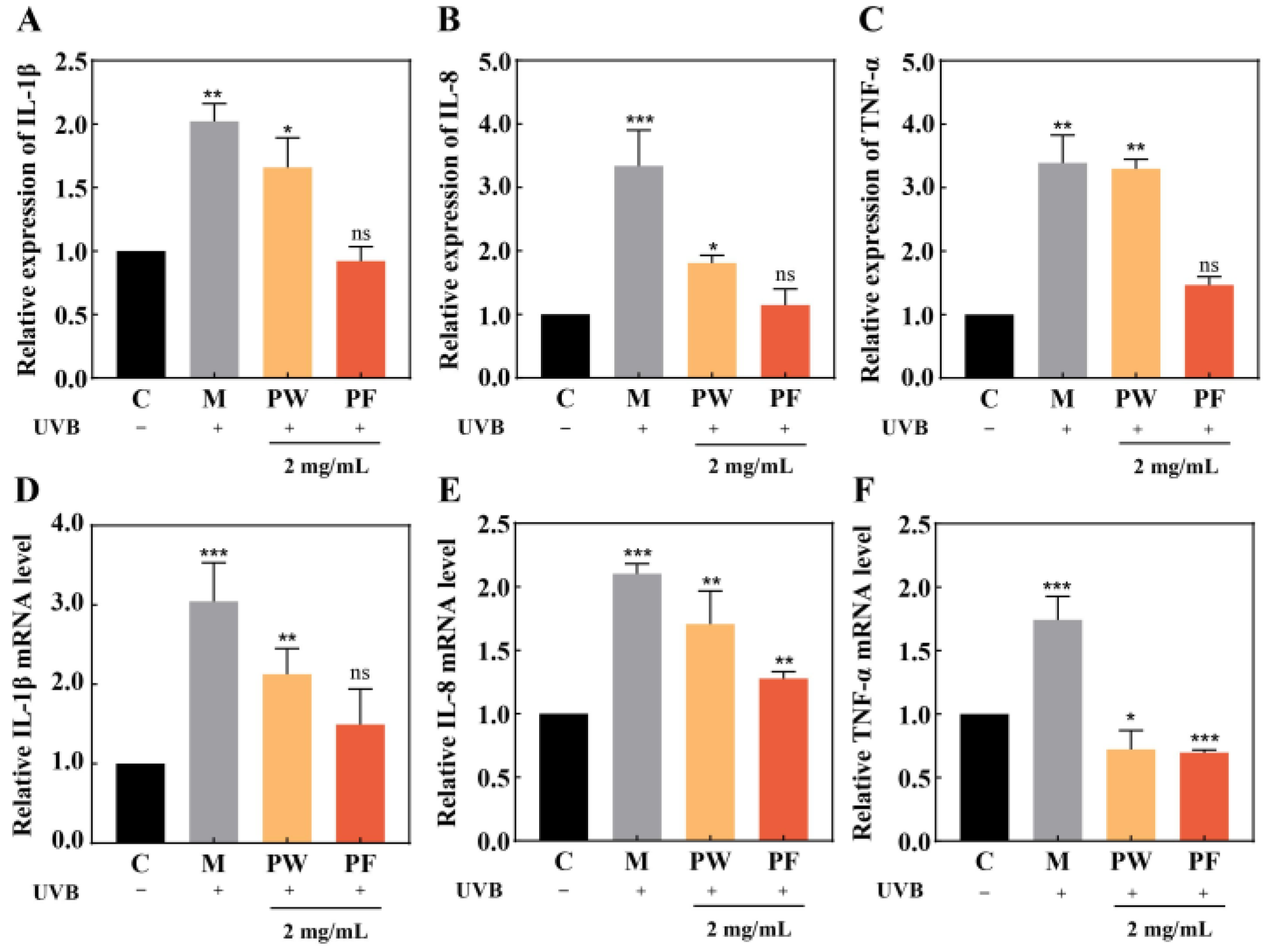
3.5. Effects of PF and PW on Cellular Inflammatory Factors and Gene Expression
3.6. Effects of PF and PW on Skin Barrier-Related Factors and Gene Expression
3.7. Regulation of PI3K/AKT/mTOR Signalling Pathway
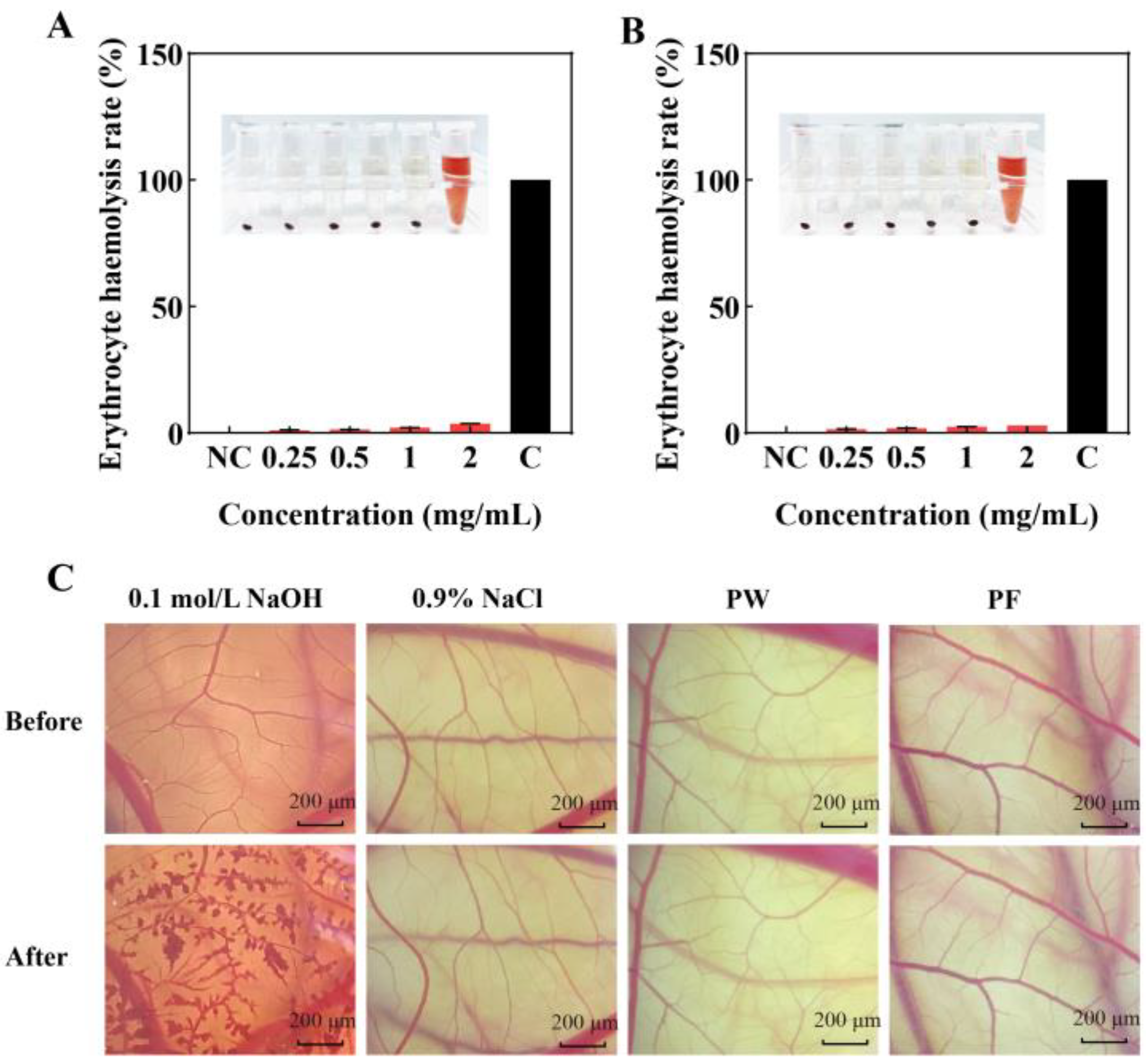
3.8. Safety of PF and PW
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Q.; Teng, J.; Wei, B.; Huang, L.; Xia, N. Phenolic Compounds, Bioactivity, and Bioaccessibility of Ethanol Extracts from Passion Fruit Peel Based on Simulated Gastrointestinal Digestion. Food Chem. 2021, 356, 129682. [Google Scholar] [CrossRef] [PubMed]
- dos Reis, L.C.R.; Facco, E.M.P.; Salvador, M.; Flôres, S.H.; de Oliveira Rios, A. Antioxidant Potential and Physicochemical Characterization of Yellow, Purple and Orange Passion Fruit. J. Food Sci. Technol. 2018, 55, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Cazarin, C.B.B.; Rodriguez-Nogales, A.; Algieri, F.; Utrilla, M.P.; Rodríguez-Cabezas, M.E.; Garrido-Mesa, J.; Guerra-Hernández, E.; de Campos Braga, P.A.; Reyes, F.G.R.; Maróstica, M.R.; et al. Intestinal Anti-Inflammatory Effects of Passiflora Edulis Peel in the Dextran Sodium Sulphate Model of Mouse Colitis. J. Funct. Foods 2016, 26, 565–576. [Google Scholar] [CrossRef]
- He, X.; Luan, F.; Yang, Y.; Wang, Z.; Zhao, Z.; Fang, J.; Wang, M.; Zuo, M.; Li, Y. Passiflora Edulis: An Insight Into Current Researches on Phytochemistry and Pharmacology. Front. Pharmacol. 2020, 11, 617. [Google Scholar] [CrossRef]
- Teng, H.; He, Z.; Li, X.; Shen, W.; Wang, J.; Zhao, D.; Sun, H.; Xu, X.; Li, C.; Zha, X. Chemical Structure, Antioxidant and Anti-Inflammatory Activities of Two Novel Pectin Polysaccharides from Purple Passion Fruit (Passiflora Edulia Sims) Peel. J. Mol. Struct. 2022, 1264, 133309. [Google Scholar] [CrossRef]
- Liu, M.; Su, Y.J.; Lin, Y.L.; Wang, Z.W.; Gao, H.M.; Li, F.; Wei, X.Y.; Jiang, H.L. Optimization of Green Extraction of Anthocyanins from Purple Passion Fruit Peels by Response Surface Methodology. J. Food Process Preserv. 2018, 42, e13756. [Google Scholar] [CrossRef]
- Kulkarni, S.G.; Vijayanand, P. Effect of Extraction Conditions on the Quality Characteristics of Pectin from Passion Fruit Peel (Passiflora Edulis f. Flavicarpa L.). LWT 2010, 43, 1026–1031. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Sousa, R.C.S.; Dias, M.M.S.; Coimbra, J.S.R. Extraction of Pectin from Passion Fruit Peel. Food Eng. Rev. 2020, 12, 460–472. [Google Scholar] [CrossRef]
- Maalej, H.; Boisset, C.; Hmidet, N.; Colin-Morel, P.; Buon, L.; Nasri, M. Depolymerization of Pseudomonas Stutzeri Exopolysaccharide upon Fermentation as a Promising Production Process of Antibacterial Compounds. Food Chem. 2017, 227, 22–32. [Google Scholar] [CrossRef]
- Huynh, N.T.; van Camp, J.; Smagghe, G.; Raes, K. Improved Release and Metabolism of Flavonoids by Steered Fermentation Processes: A Review. Int. J. Mol. Sci. 2014, 15, 19369–19388. [Google Scholar] [CrossRef]
- Lim, S.-J.; Lee, K.-J. A Microbial Fermentation of Soybean and Cottonseed Meal Increases Antioxidant Activity and Gossypol Detoxification in Diets for Nile Tilapia, Oreochromis Niloticus. J. World Aquac. Soc. 2011, 42, 494–503. [Google Scholar] [CrossRef]
- Xu, J.; Hussain, M.; Su, W.; Yao, Q.; Yang, G.; Zhong, Y.; Zhou, L.; Huang, X.; Wang, Z.; Gu, Q.; et al. Effects of Novel Cellulase (Cel 906) and Probiotic Yeast Fermentation on Antioxidant and Anti-Inflammatory Activities of Vine Tea (Ampelopsis Grossedentata). Front. Bioeng. Biotechnol. 2022, 10, 1006316. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, R.; Wang, Y.; An, X.; Liu, N.; Song, M.; Yang, Y.; Yin, N.; Qi, J. Characterization and Antioxidant Activity of Wheat Bran Polysaccharides Modified by Saccharomyces Cerevisiae and Bacillus Subtilis Fermentation. J. Cereal. Sci. 2021, 97, 103157. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. Ros Homeostasis and Metabolism: A Dangerous Liason in Cancer Cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Takagi, Y.; Nishizaka, T.; Baek, J.H.; Kim, H.J.; Lee, S.H. Subclinical Cutaneous Inflammation Remained after Permeability Barrier Disruption Enhances UV Sensitivity by Altering ER Stress Responses and Topical Pseudoceramide Prevents Them. Arch Dermatol. Res. 2017, 309, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Park, N.J.; Bong, S.K.; Lee, S.; Jung, Y.; Jegal, H.; Kim, J.; Kim, S.K.; Kim, Y.K.; Kim, S.N. Compound K Improves Skin Barrier Function by Increasing SPINK5 Expression. J. Ginseng. Res. 2020, 44, 799–807. [Google Scholar] [CrossRef]
- Takagi, Y.; Nakagawa, H.; Kondo, H.; Takemaw, Y.; Imokawaw, G. Decreased Levels of Covalently Bound Ceramide Are Associated with Ultraviolet B-Induced Perturbation of the Skin Barrier. J. Investig. Dermatol. 2004, 123, 1102–1109. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, Y.; An, Q.; Wang, D.; You, S.; Zhao, D.; Zhang, J.; Wang, C.; Li, M. Anti-Photoaging Effect of Rhodiola Rosea Fermented by Lactobacillus Plantarum on UVA-Damaged Fibroblasts. Nutrients 2022, 14, 2324. [Google Scholar] [CrossRef]
- Wagener, F.A.D.T.G.; Carels, C.E.; Lundvig, D.M.S. Targeting the Redox Balance in Inflammatory Skin Conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef]
- Zhang, X.L.; Zhang, Y.D.; Wang, T.; Guo, H.Y.; Liu, Q.M.; Su, H.X. Evaluation on Antioxidant Effect of Xanthohumol by Different Antioxidant Capacity Analytical Methods. J. Chem. 2014, 2014, 249485. [Google Scholar] [CrossRef]
- Wojas-Pelc, A.; Marcinkiewicz, J. What Is a Role of Haeme Oxygenase-1 in Psoriasis? Current Concepts of Pathogenesis. Int. J. Exp. Pathol. 2007, 88, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Numata, I.; Okuyama, R.; Memezawa, A.; Ito, Y.; Takeda, K.; Furuyama, K.; Shibahara, S.; Aiba, S. Functional Expression of Heme Oxygenase-1 in Human Differentiated Epidermis and Its Regulation by Cytokines. J. Investig. Dermatol. 2009, 129, 2594–2603. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Fahey, J.W.; Wade, K.L.; Jenkins, S.N.; Shapiro, T.A.; Fuchs, E.J.; Kerns, M.L.; Talalay, P. Induction of the Phase 2 Response in Mouse and Human Skin by Sulforaphane-Containing Broccoli Sprout Extracts. Cancer Epidemiol. Biomark. Prev. 2007, 16, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.S.; Choi, J.Y.; Lee, D.H.; Yun, S.J.; Lee, J.B.; Lee, S.C. Differentiation-Dependent Expression of NADP(H):Quinone Oxidoreductase-1 via NF-E2 Related Factor-2 Activation in Human Epidermal Keratinocytes. J. Dermatol. Sci. 2011, 62, 147–153. [Google Scholar] [CrossRef]
- Kim, H.; Cho, H.; Seo, Y.K.; Kim, S.; Yoon, M.Y.; Kang, H.; Park, C.S.; Park, J.K. Inhibitory Effects of Sea Buckthorn (Hippophae Rhamnoides L.) Seed on UVB-Induced Photoaging in Human Dermal Fibroblasts. Biotechnol. Bioprocess Eng. 2012, 17, 465–474. [Google Scholar] [CrossRef]
- Hasham, R.; Choi, H.K.; Sarmidi, M.R.; Park, C.S. Protective Effects of a Ficus Deltoidea (Mas Cotek) Extract against UVB-Induced Photoageing in Skin Cells. Biotechnol. Bioprocess Eng. 2013, 18, 185–193. [Google Scholar] [CrossRef]
- Endoh, I.; di Girolamo, N.; Hampartzoumian, T.; Cameron, B.; Geczy, C.L.; Tedla, N. Ultraviolet B Irradiation Selectively Increases the Production of Interleukin-8 in Human Cord Blood-Derived Mast Cells. Clin. Exp. Immunol. 2007, 148, 161–167. [Google Scholar] [CrossRef]
- Kishibe, M. Physiological and Pathological Roles of Kallikrein-Related Peptidases in the Epidermis. J. Dermatol. Sci. 2019, 95, 50–55. [Google Scholar] [CrossRef]
- McGovern, J.A.; Meinert, C.; de Veer, S.J.; Hollier, B.G.; Parker, T.J.; Upton, Z. Attenuated Kallikrein-Related Peptidase Activity Disrupts Desquamation and Leads to Stratum Corneum Thickening in Human Skin Equivalent Models. Br. J. Dermatol. 2017, 176, 145–158. [Google Scholar] [CrossRef]
- Fischer, J.; Wu, Z.; Kantyka, T.; Sperrhacke, M.; Dimitrieva, O.; Koblyakova, Y.; Ahrens, K.; Graumann, N.; Baurecht, H.; Reiss, K.; et al. Characterization of Spink6 in Mouse Skin: The Conserved Inhibitor of Kallikrein-Related Peptidases Is Reduced by Barrier Injury. J. Investig. Dermatol. 2014, 134, 1305–1312. [Google Scholar] [CrossRef]
- Boury-Jamot, M.; Sougrat, R.; Tailhardat, M.; Varlet, B.L.; Bonté, F.; Dumas, M.; Verbavatz, J.M. Expression and Function of Aquaporins in Human Skin: Is Aquaporin-3 Just a Glycerol Transporter? Biochim. Biophys. Acta Biomembr. 2006, 1758, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, S.; Hattori, K.; Date, A.; Tamura, H. Human Keratinocyte Caspase-14 Expression Is Altered in Human Epidermal 3D Models by Dexamethasone and by Natural Products Used in Cosmetics. Arch Dermatol. Res. 2013, 305, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt Signaling and Redox Metabolism in Cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef] [PubMed]
- Ying, Y.; Xingfen, Y.; Wengai, Z.; Jinheng, C.; Jinyu, X.; Guangyu, Y.; Xiaohua, T.; Xiaoping, X.; Xikun, X.; Junming, H.; et al. Combined In Vitro Tests as an Alternative to In Vivo Eye irritation Tests. Altern. Lab Anim. 2010, 38, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Proksch, E.; Brandner, J.M.; Jensen, J.M. The Skin: An Indispensable Barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.J.; Lee, Y.H.; Yeh, Y.L.; Wang, Y.J.; Wang, B.J. The Roles of Autophagy and the Inflammasome during Environmental Stress-Triggered Skin Inflammation. Int. J. Mol. Sci. 2016, 17, 2063. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Woo, Y.K.; Cho, H.J. Regulation of Anti-Oxidative, Anti-Inflammatory, and Anti-Apoptotic Activity of Advanced Cooling Composition (ACC) in UVB-Irradiated Human Hacat Keratinocytes. Int. J. Mol. Sci. 2020, 21, 6527. [Google Scholar] [CrossRef] [PubMed]
- Chittock, J.; Cooke, A.; Lavender, T.; Brown, K.; Wigley, A.; Victor, S.; Cork, M.J.; Danby, S.G. Development of Stratum Corneum Chymotrypsin-like Protease Activity and Natural Moisturizing Factors from Birth to 4 Weeks of Age Compared with Adults. Br. J. Dermatol. 2016, 175, 713–720. [Google Scholar] [CrossRef]
- Oh, Y.; Lim, H.W.; Kim, K.; Lim, C.J. Ginsenoside Re Improves Skin Barrier Function in HaCaT Keratinocytes under Normal Growth Conditions. Biosci. Biotechnol. Biochem. 2016, 80, 2165–2167. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Tang, B.; Yu, J.; Lu, Y.; Zhang, J.; Yan, Y.; Deng, H.; Han, L.; Li, S.; et al. P.Granatum Peel Polysaccharides Ameliorate Imiquimod-Induced Psoriasis-Like Dermatitis in Mice via Suppression of NF-ΚB and STAT3 Pathways. Front. Pharmacol. 2022, 12, 806844. [Google Scholar] [CrossRef]
- Nemoto-Hasebe, I.; Akiyama, M.; Nomura, T.; Sandilands, A.; McLean, W.H.I.; Shimizu, H. Clinical Severity Correlates with Impaired Barrier in Filaggrin-Related Eczema. J. Investig. Dermatol. 2009, 129, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Hoste, E.; Kemperman, P.; Devos, M.; Denecker, G.; Kezic, S.; Yau, N.; Gilbert, B.; Lippens, S.; de Groote, P.; Roelandt, R.; et al. Caspase-14 Is Required for Filaggrin Degradation to Natural Moisturizing Factors in the Skin. J. Investig. Dermatol. 2011, 131, 2233–2241. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Je, Y.J.; Lee, S.S.; Li, Z.J.; Choi, D.K.; Kwon, Y.B.; Sohn, K.C.; Im, M.; Seo, Y.J.; Lee, J.H. Changes in Transepidermal Water Loss and Skin Hydration According to Expression of Aquaporin-3 in Psoriasis. Ann. Dermatol. 2012, 24, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Zheng, J.; Xu, X.N.; Gu, C.; Li, W. Uranium Induces Kidney Cells Apoptosis via Reactive Oxygen Species Generation, Endoplasmic Reticulum Stress and Inhibition of PI3K/AKT/MTOR Signaling in Culture. Environ. Toxicol. 2022, 37, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Fan, D. Ginsenoside Rk1 Induces Cell Death through ROS-Mediated PTEN/PI3K/Akt/MTOR Signaling Pathway in MCF-7 Cells. J. Funct. Foods 2019, 57, 255–265. [Google Scholar] [CrossRef]
- Li, B.; Xi, P.; Wang, Z.; Han, X.; Xu, Y.; Zhang, Y.; Miao, J. PI3K/Akt/MTOR Signaling Pathway Participates in Streptococcus Uberis-Induced Inflammation in Mammary Epithelial Cells in Concert with the Classical TLRs/NF-ĸB Pathway. Vet. Microbiol. 2018, 227, 103–111. [Google Scholar] [CrossRef]
- Yang, Z.; Pu, M.; Dong, X.; Ji, F.; Veeraraghavan, V.P.; Yang, H. Piperine Loaded Zinc Oxide Nanocomposite Inhibits the PI3K/AKT/MTOR Signaling Pathway via Attenuating the Development of Gastric Carcinoma: In Vitro and in Vivo Studies. Arab. J. Chem. 2020, 13, 5501–5516. [Google Scholar] [CrossRef]
- Shen, M.; Guo, M.; Wang, Z.; Li, Y.; Kong, D.; Shao, J.; Tan, S.; Chen, A.; Zhang, F.; Zhang, Z.; et al. ROS-Dependent Inhibition of the PI3K/Akt/MTOR Signaling Is Required for Oroxylin A to Exert Anti-Inflammatory Activity in Liver Fibrosis. Int. Immunopharmacol. 2020, 85, 106637. [Google Scholar] [CrossRef]
- Feng, J.; Liao, Y.; Xu, X.; Yi, Q.; He, L.; Tang, L. HnRNP A1 Promotes Keratinocyte Cell Survival Post UVB Radiation through PI3K/Akt/MTOR Pathway. Exp. Cell Res. 2018, 362, 394–399. [Google Scholar] [CrossRef]
- Bridgeman, B.B.; Wang, P.; Ye, B.; Pelling, J.C.; Volpert, O.V.; Tong, X. Inhibition of MTOR by Apigenin in UVB-Irradiated Keratinocytes: A New Implication of Skin Cancer Prevention. Cell Signal. 2016, 28, 460–468. [Google Scholar] [CrossRef]
- Buerger, C.; Shirsath, N.; Lang, V.; Diehl, S.; Kaufmann, R.; Weigert, A.; Han, Y.Y.; Ringel, C.; Wolf, P. Blocking MTOR Signalling with Rapamycin Ameliorates Imiquimod-Induced Psoriasis in Mice. Acta Derm. Venereol. 2017, 97, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.L.; Lu, J.H.; Noda, M.; Wu, C.Y.; Liu, J.D.; Sakakibara, M.; Tsai, M.H.; Yu, H.S.; Lin, M.W.; Huang, Y.B.; et al. Derinat Protects Skin against Ultraviolet-B (UVB)-Induced Cellular Damage. Molecules 2015, 20, 20297–20311. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Guo, K.; Chen, Y.; Zhao, J.; Jing, R.; Wang, L.; Li, X.; Hu, Z.; Xu, N.; Li, X. Keratinocyte Growth Factor 2 Ameliorates UVB-Induced Skin Damage via Activating the AhR/Nrf2 Signaling Pathway. Front. Pharmacol. 2021, 12, 655281. [Google Scholar] [CrossRef] [PubMed]





| Primers | Direction | Primer Sequence (5′–3′) |
|---|---|---|
| β-actin | F | TGGCACCCAGCACAATGAA |
| R | CTAAGTCATAGTCCGCCTAGAAGCA | |
| IL-1β | F | AACCTCTTCGAGGCACAAGG |
| R | GTCCTGGAAGGAGCACTTCAT | |
| IL-8 | F | GGAGAAGTTTTTGAAGAGGGCTG |
| R | ACAGACCCACACAATACATGAAG | |
| TNF-α | F | TCTCCTTCCTGATCGTGGCA |
| R | CAGCTTGAGGGTTTGCTACAAC | |
| KLK-7 | F | TCAGATCCTCTCGAGCCCAG |
| R | CAGGTGCACGGTGTACTCAT | |
| FLG | F | TGAGGCATACCCAGAGGACT |
| R | CTGTATCGCGGTGAGAGGAT | |
| AQP3 | F | CTTCTTTGACCAGGACCGGC |
| R | GGGCCAGCTTCACATTCTCT | |
| Caspase 14 | F | ATCCAGACCCTGGTGGATGT |
| R | GATACAGCCGTTTCCGGAGG | |
| PI3K | F | TCCCTTCGATAAGAGTCGAGG |
| R | GCAGTCTTGTCGCAAAGTCC | |
| AKT | F | AAGTCATCGTGGCCAAGGAC |
| R | ACAGGTGGAAGAACAGCTCG | |
| mTOR | F | CTTAGAGGACAGCGGGGAAG |
| R | TCCTTTAATATTCGCGCGGC |
| Stimulus Score | Classification |
|---|---|
| IS < 1 | No irritation |
| 1 ≤ IS < 5 | Mid irritation |
| 5 ≤ IS < 9 | Moderate irritation |
| IS ≥ 9 | Strong irritation |
| Sample | Total Flavonoids (mg/mL) | Total Phenols (mg/mL) | Total Proteins (mg/mL) | Total Sugars (mg/mL) |
|---|---|---|---|---|
| PW | 0.080 ± 0.001 | 0.052 ± 0.003 | 0.086 ± 0.004 | 0.109 ± 0.003 |
| PF | 0.084 ± 0.002 | 0.088 ± 0.007 | 0.122 ± 0.012 | 0.160 ± 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, J.; Sun, Q.; Wang, Z.; Song, Z.; Geng, J.; Wang, C.; Li, M.; Wang, D. Enhancement of Human Epidermal Cell Defense against UVB Damage by Fermentation of Passiflora edulis Sims Peel with Saccharomyces cerevisiae. Nutrients 2023, 15, 501. https://doi.org/10.3390/nu15030501
Fang J, Sun Q, Wang Z, Song Z, Geng J, Wang C, Li M, Wang D. Enhancement of Human Epidermal Cell Defense against UVB Damage by Fermentation of Passiflora edulis Sims Peel with Saccharomyces cerevisiae. Nutrients. 2023; 15(3):501. https://doi.org/10.3390/nu15030501
Chicago/Turabian StyleFang, Jiaxuan, Qianru Sun, Ziwen Wang, Zixin Song, Jiman Geng, Changtao Wang, Meng Li, and Dongdong Wang. 2023. "Enhancement of Human Epidermal Cell Defense against UVB Damage by Fermentation of Passiflora edulis Sims Peel with Saccharomyces cerevisiae" Nutrients 15, no. 3: 501. https://doi.org/10.3390/nu15030501
APA StyleFang, J., Sun, Q., Wang, Z., Song, Z., Geng, J., Wang, C., Li, M., & Wang, D. (2023). Enhancement of Human Epidermal Cell Defense against UVB Damage by Fermentation of Passiflora edulis Sims Peel with Saccharomyces cerevisiae. Nutrients, 15(3), 501. https://doi.org/10.3390/nu15030501






