Vitamin D and Sjögren’s Disease: Revealing the Connections—A Systematic Review and Meta-Analysis
Abstract
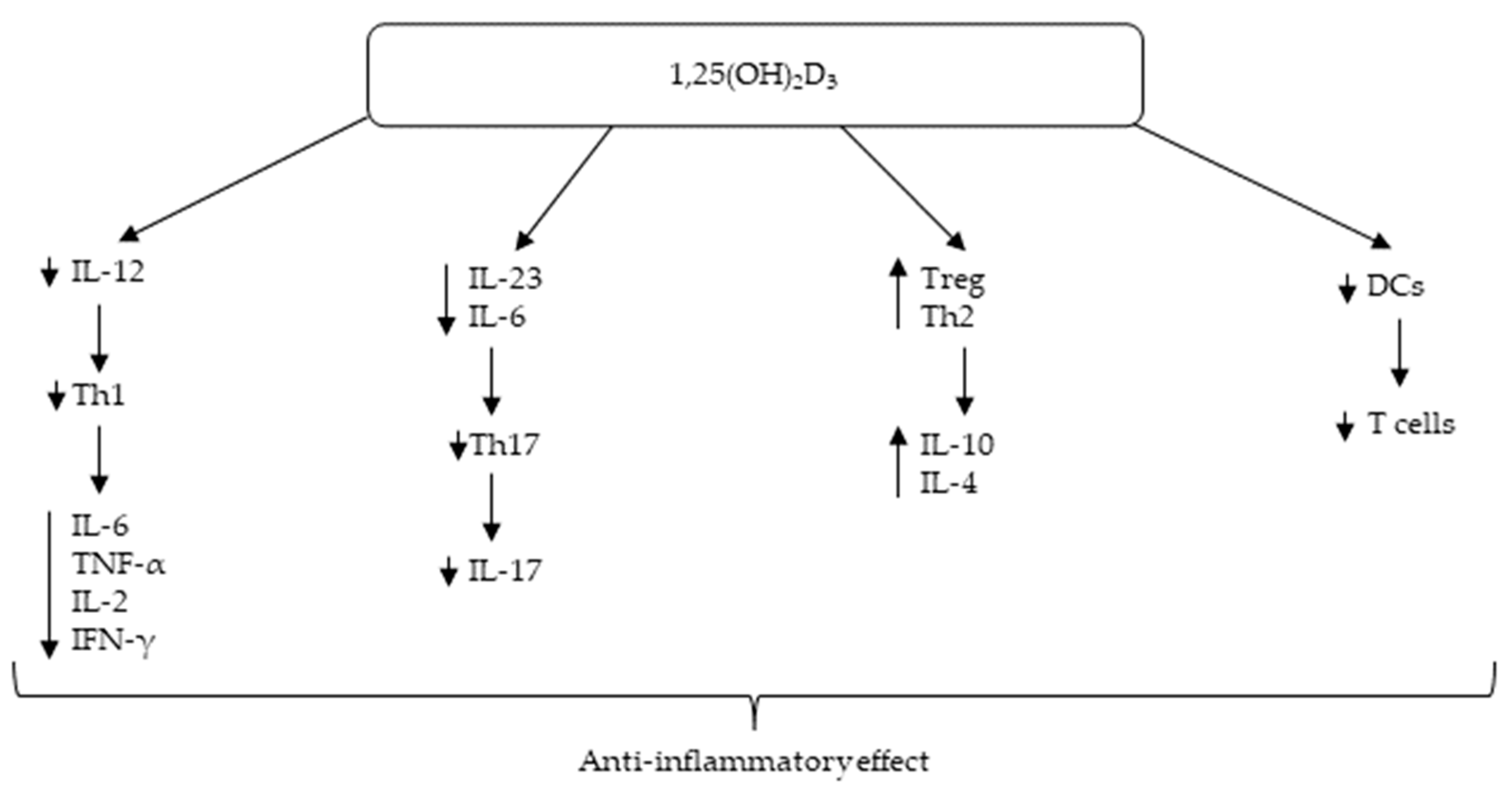
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Literature Selection
- study design: prospective or retrospective cohort study, randomized controlled trial, or observational studies;
- studies with a control group of either healthy or sicca syndrome subjects;
- studies assessing serum 25(OH)D3 levels in SD patients and controls;
- an available full version of the paper online;
- papers available in the English language.
- Therefore, the exclusion criteria were:
- abstracts and conference abstracts, letters, comments, case reports, reviews, or meta-analyses;
- studies not exploring patients with SD;
- studies with unavailable serum 25(OH)D3 levels for patients or controls;
- patients or healthy controls taking vitamin D supplements;
- studies with a control group consisting of patients with various comorbidities;
- unavailable serum 25(OH)D3 levels for patients or controls;
- papers not available online in full version or not available in the English language.
2.3. Data Extraction
2.4. Outcome Measures
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
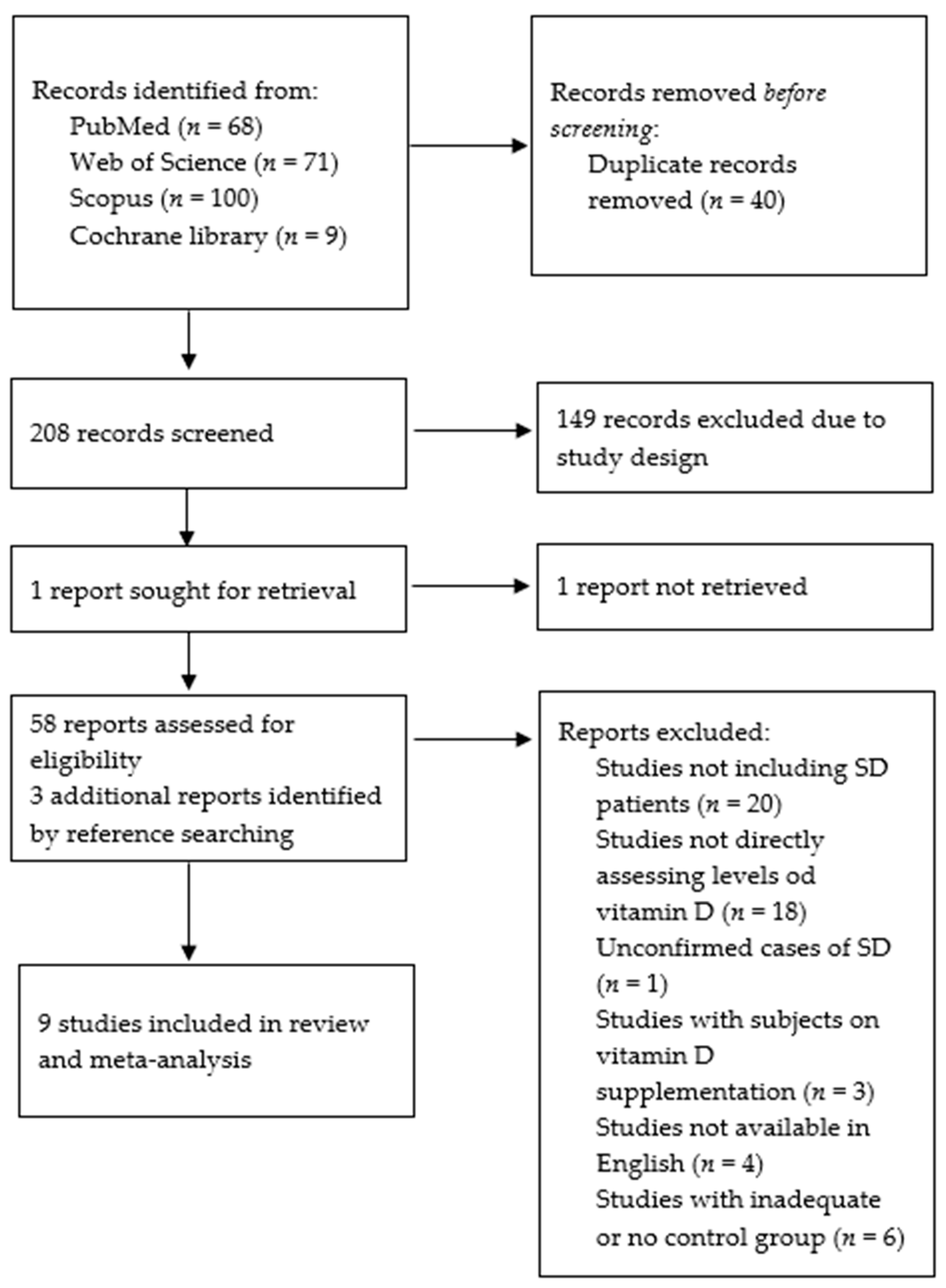
3.1. Literature Search Result
3.2. Characteristics of the Studies
3.3. Risk of Bias and Quality Assessment
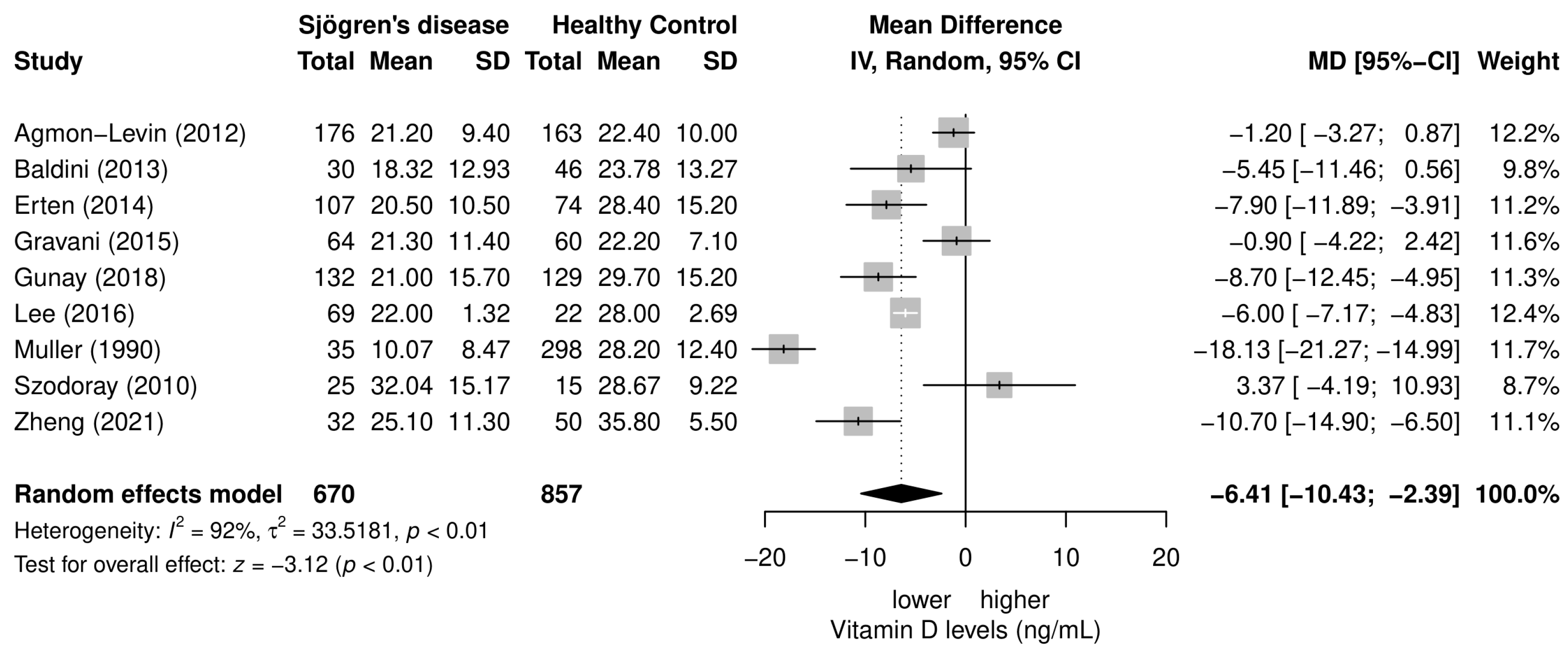
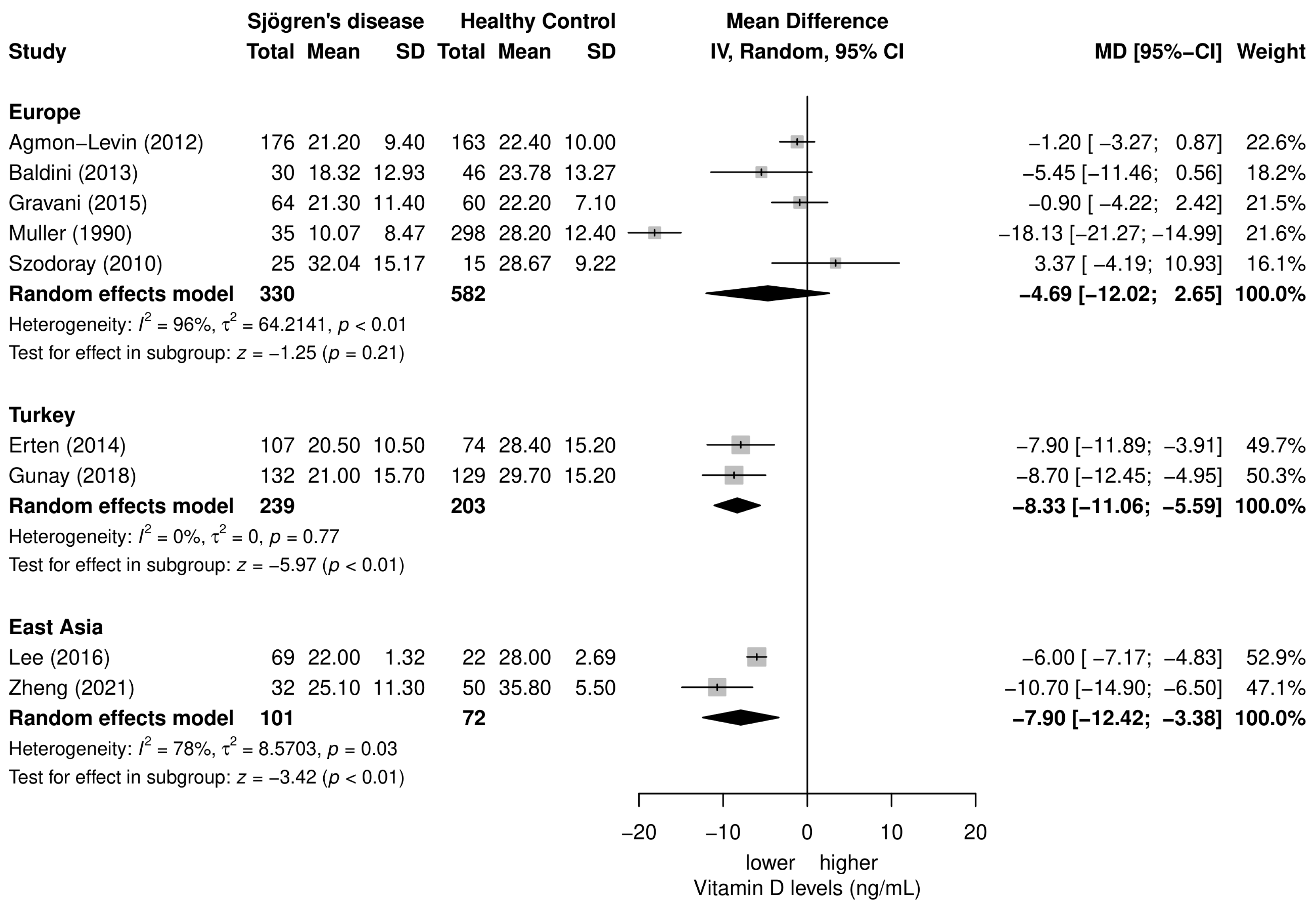
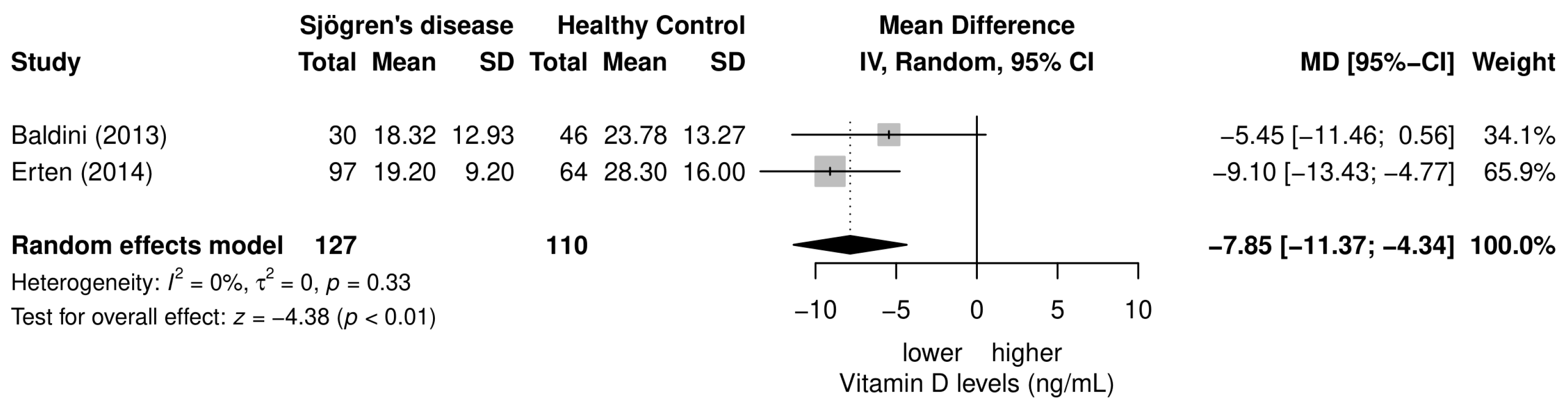
3.4. Meta-Analysis of the Differences in Serum 25(OH)D3 Levels between SD Patients and Healthy Controls
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, Molecular Mechanism of Action, and Pleiotropic Effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]
- Sassi, F.; Tamone, C.; D’Amelio, P. Vitamin D: Nutrient, Hormone, and Immunomodulator. Nutrients 2018, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004, 80, 1689S–1696S. [Google Scholar] [CrossRef] [PubMed]
- Chau, Y.-Y.; Kumar, J. Vitamin D in Chronic Kidney Disease. Indian J. Pediatr. 2012, 79, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D Metabolism, Mechanism of Action, and Clinical Applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Bartley, J. Vitamin D: Emerging roles in infection and immunity. Expert Rev. Anti. Infect. Ther. 2010, 8, 1359–1369. [Google Scholar] [CrossRef]
- Cantorna, M.; Snyder, L.; Lin, Y.-D.; Yang, L. Vitamin D and 1,25(OH)2D Regulation of T cells. Nutrients 2015, 7, 3011–3021. [Google Scholar] [CrossRef]
- Garcia-Carrasco, M.; Jiménez-Herrera, E.A.; Gálvez-Romero, J.L.; de Lara, L.V.; Mendoza-Pinto, C.; Etchegaray-Morales, I.; Munguía-Realpozo, P.; Ruíz-Argüelles, A.; Jose, R.; Vera-Recabarren, M.; et al. Vitamin D and Sjögren syndrome. Autoimmun. Rev. 2017, 16, 587–593. [Google Scholar] [CrossRef]
- Berardi, S.; Giardullo, L.; Corrado, A.; Cantatore, F.P. Vitamin D and connective tissue diseases. Inflamm. Res. 2020, 69, 453–462. [Google Scholar] [CrossRef]
- Bizzaro, G.; Antico, A.; Fortunato, A.; Bizzaro, N. Vitamin D and Autoimmune Diseases: Is Vitamin D Receptor (VDR) Polymorphism the Culprit? Isr. Med. Assoc. J. 2017, 19, 438–443. [Google Scholar]
- Marshall, L.L.; Stevens, G.A. Management of Primary Sjögren’s Syndrome. Consult Pharm. 2018, 33, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Bowman, S. Primary Sjögren’s syndrome. Lupus 2018, 27, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Tincani, A.; Andreoli, L.; Cavazzana, I.; Doria, A.; Favero, M.; Fenini, M.-G.; Franceschini, F.; Lojacono, A.; Nascimbeni, G.; Santoro, A.; et al. Novel aspects of Sjögren’s syndrome in 2012. BMC Med. 2013, 11, 93. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, J.; Jiang, Y. The association between vitamin D level and Sjögren’s syndrome: A meta-analysis. Int. J. Rheum. Dis. 2019, 22, 532–533. [Google Scholar] [CrossRef] [PubMed]
- Prisma Transparent Reporting of Systematic Reviews and Meta-Analyses. Available online: https://prisma-statement.org/prismastatement/Checklist.aspx (accessed on 5 September 2022).
- Ottawa Hospital Reasearch Institute. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 4 November 2022).
- Dubey, V.P.; Kievišienė, J.; Rauckiene-Michealsson, A.; Norkiene, S.; Razbadauskas, A.; Agostinis-Sobrinho, C. Bullying and Health Related Quality of Life among Adolescents—A Systematic Review. Children 2022, 9, 766. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-Project.Org/ (accessed on 24 April 2020).
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Muller, K.; Oxholm, P.; S0rensen, O.H.; Thymann, M.; Hoier-Madsen, M.; Bendtzen, K. Abnormal vitamin D3 metabolism in patients with primary Sjogren’s syndrome. Ann. Rheum. Dis. 1990, 49, 682–684. [Google Scholar] [CrossRef] [PubMed]
- Szodoray, P.; Horvath, I.F.; Papp, G.; Barath, S.; Gyimesi, E.; Csathy, L.; Kappelmayer, J.; Sipka, S.; Duttaroy, A.K.; Nakken, B.; et al. The immunoregulatory role of vitamins A, D and E in patients with primary Sjögren’s syndrome. Rheumatology 2010, 49, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Kivity, S.; Tzioufas, A.G.; López Hoyos, M.; Rozman, B.; Efes, I.; Shapira, Y.; Shamis, A.; Amital, H.; Youinou, P.; et al. Low levels of vitamin-D are associated with neuropathy and lymphoma among patients with Sjögren’s syndrome. J. Autoimmun. 2012, 39, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Baldini, C.; Delle Sedie, A.; Luciano, N.; Pepe, P.; Ferro, F.; Talarico, R.; Tani, C.; Mosca, M. Vitamin D in “early” primary Sjögren’s syndrome: Does it play a role in influencing disease phenotypes? Rheumatol. Int. 2014, 34, 1159–1164. [Google Scholar] [CrossRef]
- Erten, Ş.; Şahin, A.; Altunoğlu, A.; Gemcioğlu, E.; Koca, C. Comparison of plasma vitamin D levels in patients with Sjögren’s syndrome and healthy subjects. Int. J. Rheum. Dis. 2015, 18, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Gravani, F.; Papadaki, I.; Antypa, E.; Nezos, A.; Masselou, K.; Ioakeimidis, D.; Koutsilieris, M.; Moutsopoulos, H.M.; Mavragani, C.P. Subclinical atherosclerosis and impaired bone health in patients with primary Sjogren’s syndrome: Prevalence, clinical and laboratory associations. Arthritis Res. Ther. 2015, 17, 99. [Google Scholar] [CrossRef]
- Lee, S.J.; Oh, H.J.; Choi, B.Y.; Jang, Y.J.; Lee, J.Y.; Park, J.K.; Song, Y.W. Serum 25-Hydroxyvitamin D3 and BAFF Levels Are Associated with Disease Activity in Primary Sjogren’s Syndrome. J. Immunol. Res. 2016, 2016, 5781070. [Google Scholar] [CrossRef] [PubMed]
- Günay, N.E.; Buğday, İ.; Akalın, T. Relationships of the Vitamin D and Platelet Indices in Sjögren’s Syndrome. Korean J. Clin. Lab. Sci. 2018, 50, 484–491. [Google Scholar] [CrossRef]
- Zheng, M.; Zhu, S.; Li, Y.; Chen, J.; Lai, W. Interferon alpha serum level association with low vitamin D levels in Chinese patients with primary Sjögren’s syndrome. Eur. J. Inflamm. 2021, 19, 205873922110140. [Google Scholar] [CrossRef]
- Kuo, C.H.E.Y.; Huang, Y.C.; Lin, K.J.; Tsai, T.Y. Vitamin D deficiency is associated with severity of dry eye symptoms and primary Sjögren’s syndrome: A systematic review and meta-analysis. J. Nutr. Sci. Vitaminol. 2020, 66, 386–388. [Google Scholar] [CrossRef]
- Tsiaras, W.; Weinstock, M. Factors influencing vitamin D status. Acta Derm. Venereol. 2011, 91, 115–124. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Carpentier, Y.A.; Boelaert, M.; El Moumni, K.; Dufourny, G.; Bazelmans, C.; Levêque, A.; Gervy, C.; Goldman, S. Vitamin D deficiency and hyperparathyroidism in relation to ethnicity: A cross-sectional survey in healthy adults. Eur. J. Nutr. 2008, 48, 31–37. [Google Scholar] [CrossRef]
- Patel, R.; Shahane, A. The epidemiology of Sjögren’s syndrome. Clin. Epidemiol. 2014, 6, 247–255. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Forrest, K.Y.; Stuhldreher, W.L. Prevalence and correlates of vitamin D deficiency in US adults. Nutr. Res. 2011, 31, 48–54. [Google Scholar] [CrossRef]
- Muscogiuri, G.; Barrea, L.; Di Somma, C.; Laudisio, D.; Salzano, C.; Pugliese, G.; de Alteriis, G.; Colao, A.; Savastano, S. Sex Differences of Vitamin D Status across BMI Classes: An Observational Prospective Cohort Study. Nutrients 2019, 11, 3034. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.S.; Freitas, T.Q.; Bernardo, W.M.; Pereira, R.M.R. Vitamin D supplementation and disease activity in patients with immune-mediated rheumatic diseases. Medicine 2017, 96, e7024. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, T.; Prenzler, N.K.; Gingele, S.; Seeliger, B.; Körner, S.; Thiele, T.; Bönig, L.; Sühs, K.-W.; Witte, T.; Stangel, M.; et al. Neuro-Sjögren: Peripheral Neuropathy With Limb Weakness in Sjögren’s Syndrome. Front. Immunol. 2019, 10, 1600. [Google Scholar] [CrossRef] [PubMed]
- Kiraly, S.J.; Kiraly, M.A.; Hawe, R.D.; Makhani, N. Vitamin D as a Neuroactive Substance: Review. Sci. World J. 2006, 6, 125–139. [Google Scholar] [CrossRef]
- Cornet, A.; Baudet, C.; Neveu, I.; Baron-Van Evercooren, A.; Brachet, P.; Naveilhan, P. 1,25-Dihydroxyvitamin D3 regulates the expression of VDR and NGF gene in Schwann cells in vitro. J. Neurosci. Res. 1998, 53, 742–746. [Google Scholar] [CrossRef]
- Drake, M.T.; Maurer, M.J.; Link, B.K.; Habermann, T.M.; Ansell, S.M.; Micallef, I.N.; Kelly, J.L.; Macon, W.R.; Nowakowski, G.S.; Inwards, D.J.; et al. Vitamin D Insufficiency and Prognosis in Non-Hodgkin’s Lymphoma. J. Clin. Oncol. 2010, 28, 4191–4198. [Google Scholar] [CrossRef]
- Borchmann, S.; Cirillo, M.; Goergen, H.; Meder, L.; Sasse, S.; Kreissl, S.; Bröckelmann, P.J.; von Tresckow, B.; Fuchs, M.; Ullrich, R.T.; et al. Pretreatment Vitamin D Deficiency Is Associated With Impaired Progression-Free and Overall Survival in Hodgkin Lymphoma. J. Clin. Oncol. 2019, 37, 3528–3537. [Google Scholar] [CrossRef]
- Hickish, T.; Cunningham, D.; Colston, K.; Millar, B.; Sandle, J.; Mackay, A.; Soukop, M.; Sloane, J. The effect of 1,25-dihydroxyvitamin D3 on lymphoma cell lines and expression of vitamin D receptor in lymphoma. Br. J. Cancer 1993, 68, 668–672. [Google Scholar] [CrossRef]
- Park, H.Y.; Hong, Y.-C.; Lee, K.; Koh, J. Vitamin D status and risk of non-Hodgkin lymphoma: An updated meta-analysis. PLoS ONE 2019, 14, e0216284. [Google Scholar] [CrossRef]
- Seamans, K.M.; Cashman, K.D. Existing and potentially novel functional markers of vitamin D status: A systematic review. Am. J. Clin. Nutr. 2009, 89, 1997S–2008S. [Google Scholar] [CrossRef] [PubMed]
- Kim-Lee, C.; Suresh, L.; Ambrus, J.L. Gastrointestinal disease in Sjogren’s syndrome: Related to food hypersensitivities. Springerplus 2015, 4, 766. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | Study Design | Sample Size (SD/HC) | Age (Years; SD/HC) | Disease Duration (Years) | NOS |
|---|---|---|---|---|---|---|
| Muller [20], 1990 | Denmark | Cross-sectional | 35/298 | 51/NA | NA | 7/10 |
| Szodoray [21], 2010 | Hungary | Case-control | 25/15 | 56.4 | NA | 7/9 |
| Agmon-Levin [22], 2012 | Several European countries | Case-control | 176/163 | NA | NA | 7/9 |
| Baldini [23], 2013 | Italy | Prospective monocentric cross-sectional | 30/46 | NA | NA | 9/10 |
| Erten [24], 2014 | Turkey | Case-control | 107/74 | 45.24 ± 8/43.52 ± 8.89 | 44.1 ± 26.9 | 7/9 |
| Gravani [25], 2015 | Greece | Cohort study | 64/60 | 57.2 ± 12.4/56.4 ± 7.8 | 8.4 ± 7.0 | 6/9 |
| Lee [26], 2016 | Korea | Case-control | 69/22 | NA | 8.7 ± 0.78 | 6/9 |
| Günay [27], 2018 | Turkey | Analytical cross-sectional | 132/129 | 52/51.6 | NA | 10/10 |
| Zheng [28], 2021 | China | Retrospective cross-sectional | 32/50 | 43 ± 14/40 ± 12 | 3.3 | 9/10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radić, M.; Kolak, E.; Đogaš, H.; Gelemanović, A.; Bučan Nenadić, D.; Vučković, M.; Radić, J. Vitamin D and Sjögren’s Disease: Revealing the Connections—A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 497. https://doi.org/10.3390/nu15030497
Radić M, Kolak E, Đogaš H, Gelemanović A, Bučan Nenadić D, Vučković M, Radić J. Vitamin D and Sjögren’s Disease: Revealing the Connections—A Systematic Review and Meta-Analysis. Nutrients. 2023; 15(3):497. https://doi.org/10.3390/nu15030497
Chicago/Turabian StyleRadić, Mislav, Ela Kolak, Hana Đogaš, Andrea Gelemanović, Dora Bučan Nenadić, Marijana Vučković, and Josipa Radić. 2023. "Vitamin D and Sjögren’s Disease: Revealing the Connections—A Systematic Review and Meta-Analysis" Nutrients 15, no. 3: 497. https://doi.org/10.3390/nu15030497
APA StyleRadić, M., Kolak, E., Đogaš, H., Gelemanović, A., Bučan Nenadić, D., Vučković, M., & Radić, J. (2023). Vitamin D and Sjögren’s Disease: Revealing the Connections—A Systematic Review and Meta-Analysis. Nutrients, 15(3), 497. https://doi.org/10.3390/nu15030497








