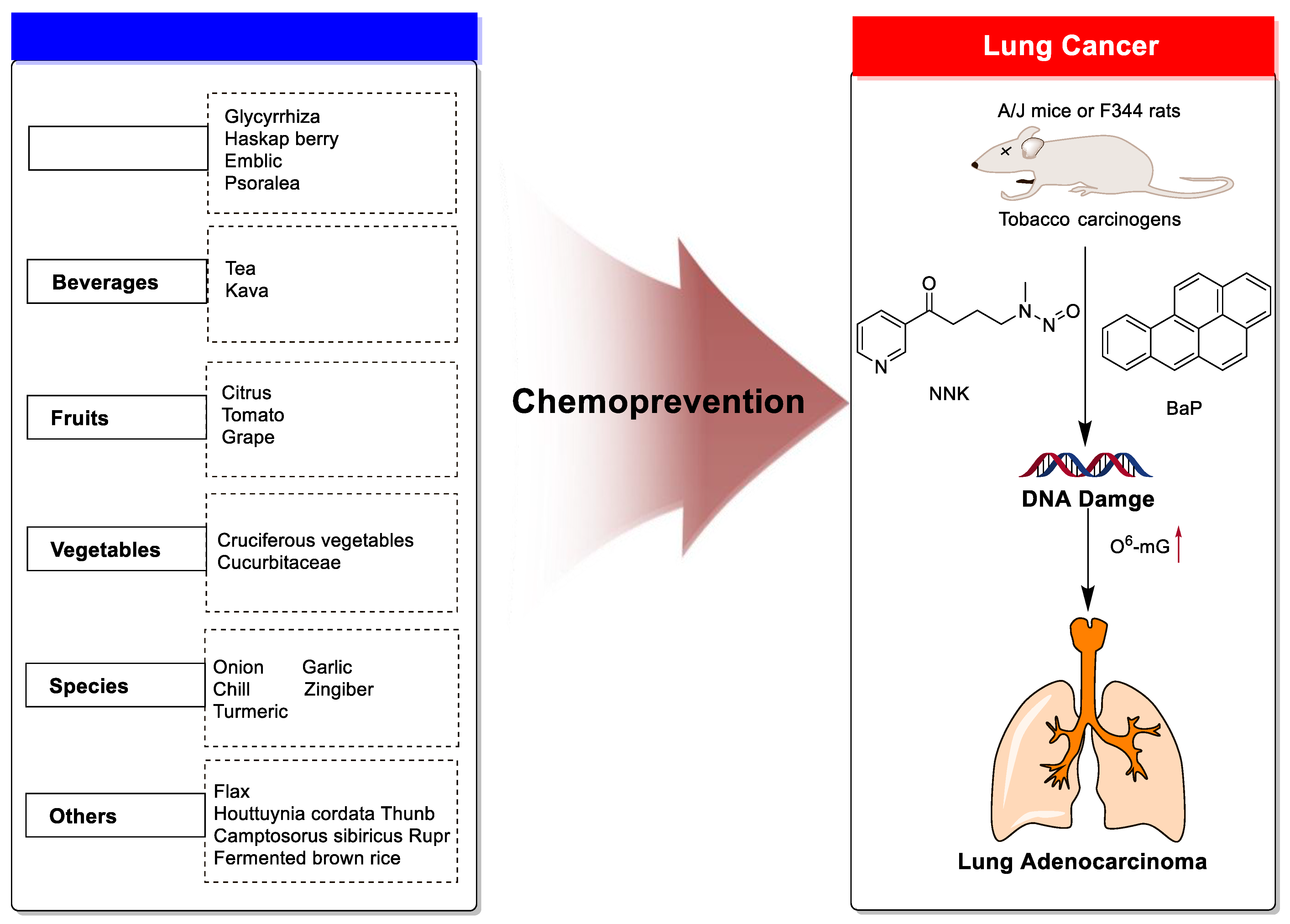
Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis
Abstract
1. Introduction
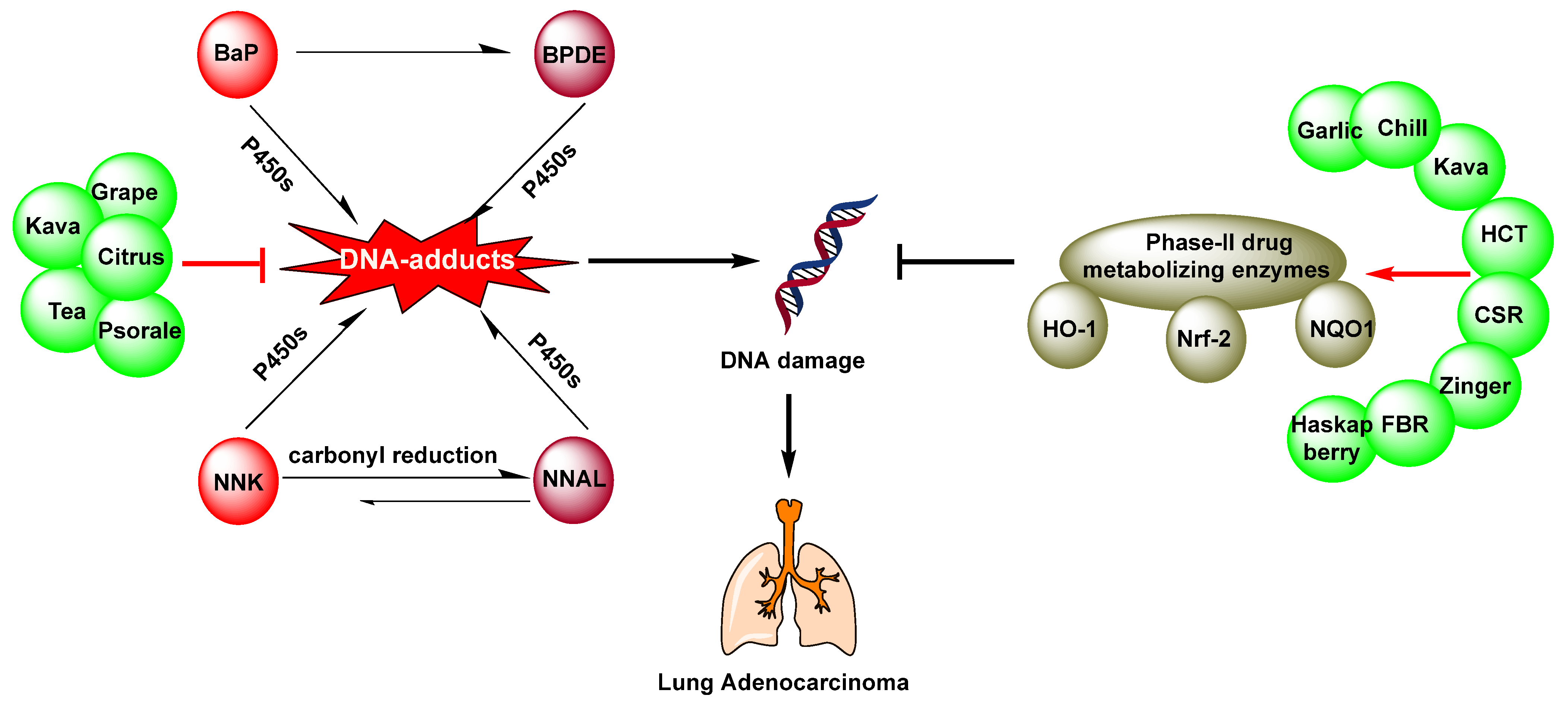
2. Chemoprevention of Lung Cancer by Dietary Phytochemicals
2.1. Medicine Plants
2.1.1. Glycyrrhiza
2.1.2. Haskap Berry
2.1.3. Emblic
2.1.4. Psoralea
2.2. Beverages
2.2.1. Tea
2.2.2. Kava
Kava
Dihydromethysticin
2.3. Fruits
2.3.1. Citrus
5-Demethylnobiletin
Nobiletin
Other Compounds
2.3.2. Tomatoes
2.3.3. Grape
2.4. Vegetables
2.4.1. Cruciferous Vegetables
Phenethyl Isothiocyanate
Indole-3-Carbinol
2.4.2. Cucurbitaceae
Cucurbitacin B
2.5. Spices
2.5.1. Garlic
2.5.2. Chilli
2.5.3. Zingiber
2.5.4. Turmeric
2.6. Other Plants
3. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AhR | Aryl hydrocarbon receptor |
| BaP | Benzo[a]pyrene |
| CS | Cigarette smoke |
| CuB | Cucurbitacin B |
| C3G | Cyanidin-3-O-glucoside |
| CYP450 | Cytochrome P450 |
| DADS | Diallyl disulfide |
| DAS | Diallyl sulfide |
| DATS | Diallyl trisulfide |
| DHM | Dihydromethysticin |
| DNMT1 | DNA methyltransferase 1 |
| EGFR | Epidermal growth factor receptor |
| EA | Ellagic acid |
| HMGB1 | High Mobility Group Box 1 |
| LicA | LicochalconeA |
| LPO | Lipid peroxidation |
| NSCLC | Non–small-cell lung cancer cells |
| O6-mG | O6-methylguanine |
| PTX | Paclitaxel |
| PEL | Phyllanthusemblica L. |
| PBPs | Polymeric black tea polyphenols |
| PD-L1 | Programmed cell death ligand 1 |
| PCNA | Proliferative cell nuclear antigen |
| RCT | Reverse cholesterol transport |
| DBQ | 2,6-dimethoxy-1,4-benzoquinone |
| PEITC | 2-Phenethyl isothiocyanate |
| EGCGD | 2R,3R6-methoxycarbonylgallocatechin-3-O-gallate |
| 3-mA | 3-methyladenine |
| NNK | 4-(N-methyl-N-nitrosamine)-1-(3-pyridyl)-butanone |
| NNAL | 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol |
| NNKOAc | 4-[(acetoxymethyl) nitrosamino]-1-(3-pyridyl)-1-butanone |
| 8-MOP | 8-methoxsalen |
| BCX | β-Cryptoxanthin |
| EGCG | (-)-epigallocatechin-3-gallate |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.X.; Zheng, R.S.; Zeng, H.M.; Zhang, S.W.; Zou, X.N.; Gu, X.Y.; Xia, C.F.; Yang, Z.X.; Li, H.; Chen, W.Q.; et al. [The incidence and mortality of lung cancer in China, 2014]. Zhonghua Zhong Liu Za Zhi [Chin. J. Oncol. ] 2018, 40, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Cooper, W.A.; Lam, D.C.; O’Toole, S.A.; Minna, J.D. Molecular biology of lung cancer. J. Thorac. Dis. 2013, 5 (Suppl. S5), S479–S490. [Google Scholar] [CrossRef] [PubMed]
- Pozza, D.H.; Andrade de Mello, R.B. Treatment Sequencing Strategies in Lung Cancer. Zhongguo Fei Ai Za Zhi = Chin. J. Lung Cancer 2022, 25, 323–336. [Google Scholar] [CrossRef]
- Zhang, L.; Qu, Z.; Song, A.; Yang, J.; Yu, J.; Zhang, W.; Zhuang, C. Garlic oil blocks tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis by inducing phase II drug-metabolizing enzymes. Food Chem. Toxicol. 2021, 157, 112581. [Google Scholar] [CrossRef]
- Wang, Y.; Bian, T.; Song, L.; Jiang, Y.; Huo, Z.; Salloum, R.G.; Warren, G.W.; Kaye, F.J.; Fujioka, N.; Jin, L.; et al. Reducing Chemotherapy-Induced DNA Damage via nAChR-Mediated Redox Reprograming-A New Mechanism for SCLC Chemoresistance Boosted by Nicotine. Cancers 2022, 14, 2272. [Google Scholar] [CrossRef]
- Bethune, G.; Bethune, D.; Ridgway, N.; Xu, Z. Epidermal growth factor receptor (EGFR) in lung cancer: An overview and update. J. Thorac. Dis. 2010, 2, 48–51. [Google Scholar]
- Cheng, W.L.; Chen, K.Y.; Lee, K.Y.; Feng, P.H.; Wu, S.M. Nicotinic-nAChR signaling mediates drug resistance in lung cancer. J. Cancer 2020, 11, 1125–1140. [Google Scholar] [CrossRef]
- Vu, T.; Jin, L.; Datta, P.K. Effect of Cigarette Smoking on Epithelial to Mesenchymal Transition (EMT) in Lung Cancer. J. Clin. Med. 2016, 5, 44. [Google Scholar] [CrossRef]
- Petrulis, J.R.; Perdew, G.H. The role of chaperone proteins in the aryl hydrocarbon receptor core complex. Chem.-Biol. Interact. 2002, 141, 25–40. [Google Scholar] [CrossRef]
- Tchou-Wong, K.-M.; Jiang, Y.; Yee, H.; LaRosa, J.; Lee, T.C.; Pellicer, A.; Jagirdar, J.; Gordon, T.; Goldberg, J.D.; Rom, W.N. Lung-Specific Expression of Dominant-Negative Mutant p53 in Transgenic Mice Increases Spontaneous and Benzo(a)pyrene-Induced Lung Cancer. Am. J. Respir. Cell Mol. Biol. 2002, 27, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Liskova, A.; Stefanicka, P.; Samec, M.; Smejkal, K.; Zubor, P.; Bielik, T.; Biskupska-Bodova, K.; Kwon, T.K.; Danko, J.; Büsselberg, D.; et al. Dietary phytochemicals as the potential protectors against carcinogenesis and their role in cancer chemoprevention. Clin. Exp. Med. 2020, 20, 173–190. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.; Blount, B.C.; Xia, B.; Wang, L. Assessing exposure to tobacco-specific carcinogen NNK using its urinary metabolite NNAL measured in US population: 2011–2012. J. Expo. Sci. Environ. Epidemiol. 2016, 26, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Ge, G.-Z.; Xu, T.-R.; Chen, C. Tobacco carcinogen NNK-induced lung cancer animal models and associated carcinogenic mechanisms. Acta Biochim. Biophys. Sin. 2015, 47, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Radziszewska, A.; Karczmarek-Borowska, B.; Gradalska-Lampart, M.; Filip, A.A. Epidemiology, prevention and risk morbidity factors for lung cancer. Pol. Merkur. Lek. Organ Pol. Tow. Lek. 2015, 38, 113–118. [Google Scholar]
- Polanski, J.; Jankowska-Polanska, B.; Rosinczuk, J.; Chabowski, M.; Szymanska-Chabowska, A. Quality of life of patients with lung cancer. OncoTargets Ther. 2016, 9, 1023–1028. [Google Scholar] [CrossRef]
- Kasala, E.R.; Bodduluru, L.N.; Barua, C.C.; Sriram, C.S.; Gogoi, R. Benzo(a)pyrene induced lung cancer: Role of dietary phytochemicals in chemoprevention. Pharmacol. Rep. 2015, 67, 996–1009. [Google Scholar] [CrossRef]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S.; et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef]
- Wang, C.; Yang, T.; Guo, X.F.; Li, D. The Associations of Fruit and Vegetable Intake with Lung Cancer Risk in Participants with Different Smoking Status: A Meta-Analysis of Prospective Cohort Studies. Nutrients 2019, 11, 1791. [Google Scholar] [CrossRef]
- Lam, T.K.; Gallicchio, L.; Lindsley, K.; Shiels, M.; Hammond, E.; Tao, X.; Chen, L.; Robinson, K.A.; Caulfield, L.E.; Herman, J.G.; et al. Cruciferous Vegetable Consumption and Lung Cancer Risk: A Systematic Review. Cancer Epidemiol. Biomark. Prev. 2009, 18, 184–195. [Google Scholar] [CrossRef] [PubMed]
- García-Lavandeira, J.A.; Ruano-Ravina, A.; Torres-Durán, M.; Parente-Lamelas, I.; Provencio, M.; Varela-Lema, L.; Fernández-Villar, A.; Piñeiro, M.; Barros-Dios, J.M.; Pérez-Ríos, M. Fruits and Vegetables and Lung Cancer Risk in Never Smokers. A Multicentric and Pooled Case-Control Study. Nutr. Cancer 2022, 74, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.R.; Abar, L.; Vingeliene, S.; Chan, D.S.M.; Aune, D.; Navarro-Rosenblatt, D.; Stevens, C.; Greenwood, D.; Norat, T. Fruits, vegetables and lung cancer risk: A systematic review and meta-analysis. Ann. Oncol. 2016, 27, 81–96. [Google Scholar] [CrossRef] [PubMed]
- de Seranno, S.; Meuwissen, R. Progress and applications of mouse models for human lung cancer. Eur. Respir. J. 2010, 35, 426. [Google Scholar] [CrossRef]
- Witschi, H. The complexities of an apparently simple lung tumor model: The A/J mouse. Exp. Toxicol. Pathol. Off. J. Ges. Fur Toxikol. Pathol. 2005, 57 (Suppl. S1), 171–181. [Google Scholar] [CrossRef] [PubMed]
- Witschi, H. A/J Mouse As A Model For Lung Tumorigenesis Caused By Tobacco Smoke: Strengths And Weaknesses. Exp. Lung Res. 2004, 31, 3–18. [Google Scholar] [CrossRef]
- Zheng, H.C.; Takano, Y. NNK-Induced Lung Tumors: A Review of Animal Model. J. Oncol. 2011, 2011, 635379. [Google Scholar] [CrossRef]
- Li, B.; Zhou, D.; Li, S.; Feng, Y.; Li, X.; Chang, W.; Zhang, J.; Sun, Y.; Qing, D.; Chen, G.; et al. Licochalcone A reverses NNK-induced ectopic miRNA expression to elicit in vitro and in vivo chemopreventive effects. Phytomedicine 2020, 76, 153245. [Google Scholar] [CrossRef]
- Wu, X.; Wang, W.; Chen, Y.; Liu, X.; Wang, J.; Qin, X.; Yuan, D.; Yu, T.; Chen, G.; Mi, Y.; et al. Glycyrrhizin Suppresses the Growth of Human NSCLC Cell Line HCC827 by Downregulating HMGB1 Level. Biomed. Res. Int. 2018, 2018, 6916797. [Google Scholar] [CrossRef]
- Deng, Q.P.; Wang, M.J.; Zeng, X.; Chen, G.G.; Huang, R.Y. Effects of Glycyrrhizin in a Mouse Model of Lung Adenocarcinoma. Cell. Physiol. Biochem. 2017, 41, 1383–1392. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.Y.; Chu, Y.L.; Jiang, Z.B.; Chen, X.M.; Zhang, X.; Zeng, X. Glycyrrhizin Suppresses Lung Adenocarcinoma Cell Growth Through Inhibition of Thromboxane Synthase. Cell. Physiol. Biochem. 2014, 33, 375–388. [Google Scholar] [CrossRef]
- Amararathna, M.; Hoskin, D.W.; Rupasinghe, H.P.V. Cyanidin-3-O-Glucoside-Rich Haskap Berry Administration Suppresses Carcinogen-Induced Lung Tumorigenesis in A/JCr Mice. Molecules 2020, 25, 3823. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-F.; Wu, C.-Y.; Lin, C.-F.; Liu, Y.-W.; Lin, T.-C.; Liao, H.-J.; Chang, G.-R. The anticancer effects of cyanidin 3-O-glucoside combined with 5-fluorouracil on lung large-cell carcinoma in nude mice. Biomed. Pharmacother. 2022, 151, 113128. [Google Scholar] [CrossRef]
- Wang, C.C.; Yuan, J.R.; Wang, C.F.; Yang, N.; Chen, J.; Liu, D.; Song, J.; Feng, L.; Tan, X.B.; Jia, X.B. Anti-inflammatory Effects of Phyllanthus emblica L. on Benzopyrene-Induced Precancerous Lung Lesion by Regulating the IL-1beta/miR-101/Lin28B Signaling Pathway. Integr. Cancer 2017, 16, 505–515. [Google Scholar] [CrossRef]
- Takeuchi, H.; Saoo, K.; Yokohira, M.; Ikeda, M.; Maeta, H.; Miyazaki, M.; Yamazaki, H.; Kamataki, T.; Imaida, K. Pretreatment with 8-methoxypsoralen, a potent human CYP2A6 inhibitor, strongly inhibits lung tumorigenesis induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in female A/J mice. Cancer Res. 2003, 63, 7581–7583. [Google Scholar] [PubMed]
- Takeuchi, H.; Saoo, K.; Matsuda, Y.; Yokohira, M.; Yamakawa, K.; Zeng, Y.; Kuno, T.; Kamataki, T.; Imaida, K. 8-Methoxypsoralen, a potent human CYP2A6 inhibitor, inhibits lung adenocarcinoma development induced by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in female A/J mice. Mol. Med. Rep. 2009, 2, 585–588. [Google Scholar] [CrossRef]
- Takeuchi, H.; Saoo, K.; Matsuda, Y.; Yokohira, M.; Yamakawa, K.; Zeng, Y.; Miyazaki, M.; Fujieda, M.; Kamataki, T.; Imaida, K. Dose dependent inhibitory effects of dietary 8-methoxypsoralen on NNK-induced lung tumorigenesis in female A/J mice. Cancer Lett. 2006, 234, 232–238. [Google Scholar] [CrossRef]
- Feng, M.; Zhang, H.; Cao, B.; Liu, S.; Mao, J.; Zhang, Q. Effects of 8-methoxypsoralen on the metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in mice. Drug Metab. Pharm. 2015, 30, 314–320. [Google Scholar] [CrossRef]
- Jin, H.; Chen, J.X.; Wang, H.; Lu, G.; Liu, A.; Li, G.; Tu, S.; Lin, Y.; Yang, C.S. NNK-induced DNA methyltransferase 1 in lung tumorigenesis in A/J mice and inhibitory effects of (-)-epigallocatechin-3-gallate. Nutr. Cancer 2015, 67, 167–176. [Google Scholar] [CrossRef]
- Lu, G.; Liao, J.; Yang, G.; Reuhl, K.R.; Hao, X.; Yang, C.S. Inhibition of adenoma progression to adenocarcinoma in a 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis model in A/J mice by tea polyphenols and caffeine. Cancer Res. 2006, 66, 11494–11501. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.-L.; Wang, M.; Rivenson, A.; Iatropoulos, M.J.; Reinhardt, J.C.; Pittman, B.; Ho, C.-T.; Amin, S.G. Inhibition of Lung Carcinogenesis by Black Tea in Fischer Rats Treated with a Tobacco-specific Carcinogen: Caffeine as an Important Constituent1. Cancer Res. 1998, 58, 4096–4101. [Google Scholar] [PubMed]
- Yang, G.-y.; Wang, Z.-Y.; Kim, S.; Liao, J.; Seril, D.N.; Chen, X.; Smith, T.J.; Yang, C.S. Characterization of Early Pulmonary Hyperproliferation and Tumor Progression and Their Inhibition by Black Tea in a 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced Lung Tumorigenesis Model with A/J Mice1. Cancer Res. 1997, 57, 1889–1894. [Google Scholar] [PubMed]
- Hudlikar, R.R.; Venkadakrishnan, V.B.; Kumar, R.; Thorat, R.A.; Kannan, S.; Ingle, A.D.; Desai, S.; Maru, G.B.; Mahimkar, M.B. Polymeric black tea polyphenols (PBPs) inhibit benzo(a)pyrene and 4-(methylnitrosamino)-1-(3-pyridyl)-1- butanone-induced lung carcinogenesis potentially through down-regulation of p38 and Akt phosphorylation in A/J mice. Mol. Carcinog. 2016, 56, 625–640. [Google Scholar] [CrossRef]
- Hudlikar, R.R.; Pai, V.; Kumar, R.; Thorat, R.A.; Kannan, S.; Ingle, A.D.; Maru, G.B.; Mahimkar, M.B. Dose-Related Modulatory Effects of Polymeric Black Tea Polyphenols (PBPs) on Initiation and Promotion Events in B(a)P and NNK-Induced Lung Carcinogenesis. Nutr. Cancer 2019, 71, 508–523. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Xiao, H.; You, H.; Lin, Y.; Jin, H.; Snagaski, B.; Yang, C.S. Synergistic Inhibition of Lung Tumorigenesis by a Combination of Green Tea Polyphenols and Atorvastatin. Clin. Cancer Res. 2008, 14, 4981–4988. [Google Scholar] [CrossRef]
- Khattab, S.A.; Hussien, W.F.; Raafat, N.; Ahmed Alaa El-Din, E. Effects of catechin hydrate in benzo[a]pyrene-induced lung toxicity: Roles of oxidative stress, apoptosis, and DNA damage. Toxicol. Mech. Methods 2021, 31, 467–475. [Google Scholar] [CrossRef]
- Liao, J.; Yang, G.Y.; Park, E.S.; Meng, X.; Sun, Y.; Jia, D.; Seril, D.N.; Yang, C.S. Inhibition of lung carcinogenesis and effects on angiogenesis and apoptosis in A/J mice by oral administration of green tea. Nutr. Cancer 2004, 48, 44–53. [Google Scholar] [CrossRef]
- Xu, Y.; Ho, C.-T.; Amin, S.G.; Han, C.; Chung, F.-L. Inhibition of Tobacco-specific Nitrosamine-induced Lung Tumorigenesis in A/J Mice by Green Tea and Its Major Polyphenol as Antioxidants1. Cancer Res. 1992, 52, 3875–3879. [Google Scholar] [PubMed]
- Rawangkan, A.; Wongsirisin, P.; Namiki, K.; Iida, K.; Kobayashi, Y.; Shimizu, Y.; Fujiki, H.; Suganuma, M. Green Tea Catechin Is an Alternative Immune Checkpoint Inhibitor that Inhibits PD-L1 Expression and Lung Tumor Growth. Molecules 2018, 23, 2071. [Google Scholar] [CrossRef] [PubMed]
- Dhatwalia, S.K.; Kumar, M.; Bhardwaj, P.; Dhawan, D.K. White tea—A cost effective alternative to EGCG in fight against benzo(a)pyrene (BaP) induced lung toxicity in SD rats. Food Chem. Toxicol. 2019, 131, 110551. [Google Scholar] [CrossRef] [PubMed]
- Leitzman, P.; Narayanapillai, S.C.; Balbo, S.; Zhou, B.; Upadhyaya, P.; Shaik, A.A.; O’Sullivan, M.G.; Hecht, S.S.; Lu, J.; Xing, C. Kava blocks 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in association with reducing O6-methylguanine DNA adduct in A/J mice. Cancer Prev. Res. 2014, 7, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.E.; Hermanson, D.; Wang, L.; Kassie, F.; Upadhyaya, P.; O’Sullivan, M.G.; Hecht, S.S.; Lu, J.; Xing, C. Lung tumorigenesis suppressing effects of a commercial kava extract and its selected compounds in A/J mice. Am. J. Chin. Med. 2011, 39, 727–742. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Corral, P.; Wang, Y.; Botello, J.; Kingston, R.; Daniels, T.; Salloum, R.G.; Johnston, E.; Huo, Z.; Lu, J.; et al. Kava as a Clinical Nutrient: Promises and Challenges. Nutrients 2020, 12, 3044. [Google Scholar] [CrossRef]
- Johnson, T.E.; Kassie, F.; O’Sullivan, M.G.; Negia, M.; Hanson, T.E.; Upadhyaya, P.; Ruvolo, P.P.; Hecht, S.S.; Xing, C. Chemopreventive Effect of Kava on 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone plus Benzo[a]pyrene–Induced Lung Tumorigenesis in A/J Mice. Cancer Prev. Res. 2008, 1, 430–438. [Google Scholar] [CrossRef]
- Shaik, A.A.; Hermanson, D.L.; Xing, C. Identification of methysticin as a potent and non-toxic NF-kappaB inhibitor from kava, potentially responsible for kava’s chemopreventive activity. Bioorg. Med. Chem Lett. 2009, 19, 5732–5736. [Google Scholar] [CrossRef]
- Narayanapillai, S.C.; Leitzman, P.; O’Sullivan, M.G.; Xing, C. Flavokawains A and B in Kava, Not Dihydromethysticin, Potentiate Acetaminophen-Induced Hepatotoxicity in C57BL/6 Mice. Chem. Res. Toxicol. 2014, 27, 1871–1876. [Google Scholar] [CrossRef]
- Hu, Q.; Corral, P.; Narayanapillai, S.C.; Leitzman, P.; Upadhyaya, P.; O’Sullivan, M.G.; Hecht, S.S.; Lu, J.; Xing, C. Oral Dosing of Dihydromethysticin Ahead of Tobacco Carcinogen NNK Effectively Prevents Lung Tumorigenesis in A/J Mice. Chem. Res. Toxicol. 2020, 33, 1980–1988. [Google Scholar] [CrossRef]
- Narayanapillai, S.C.; von Weymarn, L.B.; Carmella, S.G.; Leitzman, P.; Paladino, J.; Upadhyaya, P.; Hecht, S.S.; Murphy, S.E.; Xing, C. Dietary Dihydromethysticin Increases Glucuronidation of 4-(Methylnitrosamino)-1-(3-Pyridyl)-1-Butanol in A/J Mice, Potentially Enhancing Its Detoxification. Drug Metab. Dispos. 2016, 44, 422–427. [Google Scholar] [CrossRef]
- Narayanapillai, S.C.; Balbo, S.; Leitzman, P.; Grill, A.E.; Upadhyaya, P.; Shaik, A.A.; Zhou, B.; O’Sullivan, M.G.; Peterson, L.A.; Lu, J.; et al. Dihydromethysticin from kava blocks tobacco carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis and differentially reduces DNA damage in A/J mice. Carcinogenesis 2014, 35, 2365–2372. [Google Scholar] [CrossRef]
- Hati, S.; Hu, Q.; Huo, Z.; Lu, J.; Xing, C. In vivo Structure-Activity Relationship of Dihydromethysticin in Reducing Nicotine-Derived Nitrosamine Ketone (NNK)-Induced Lung DNA Damage against Lung Carcinogenesis in A/J Mice. ChemMedChem 2022, 17, e202100727. [Google Scholar] [CrossRef]
- Puppala, M.; Narayanapillai, S.C.; Leitzman, P.; Sun, H.; Upadhyaya, P.; O’Sullivan, M.G.; Hecht, S.S.; Xing, C. Pilot in Vivo Structure-Activity Relationship of Dihydromethysticin in Blocking 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone-Induced O(6)-Methylguanine and Lung Tumor in A/J Mice. J. Med. Chem. 2017, 60, 7935–7940. [Google Scholar] [CrossRef] [PubMed]
- Bian, T.; Ding, H.; Wang, Y.; Hu, Q.; Chen, S.; Fujioka, N.; Aly, F.Z.; Lu, J.; Huo, Z.; Xing, C. Suppressing the activation of protein kinase A as a DNA damage-independent mechanistic lead for dihydromethysticin (DHM) prophylaxis of NNK-induced lung carcinogenesis. Carcinogenesis 2022, 43, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Narayanapillai, S.C.; Lin, S.H.; Leitzman, P.; Upadhyaya, P.; Baglole, C.J.; Xing, C. Dihydromethysticin (DHM) Blocks Tobacco Carcinogen 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-Induced O(6)-Methylguanine in a Manner Independent of the Aryl Hydrocarbon Receptor (AhR) Pathway in C57BL/6 Female Mice. Chem. Res. Toxicol. 2016, 29, 1828–1834. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Wu, X.; Charoensinphon, N.; Wang, M.; Zheng, J.; Gao, Z.; Xu, F.; Li, Z.; Li, F.; Zhou, J.; et al. Dietary 5-demethylnobiletin inhibits cigarette carcinogen NNK-induced lung tumorigenesis in mice. Food Funct. 2017, 8, 954–963. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Masumura, K.-i.; Matsui, K.; Kohno, H.; Sakuma, K.; Tanaka, T.; Nohmi, T. Chemopreventive Effects of Nobiletin against Genotoxicity Induced by 4-(Methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in the Lung of gpt delta Transgenic Mice. Genes Environ. 2006, 28, 84–91. [Google Scholar] [CrossRef]
- Sun, Y.; Han, Y.; Song, M.; Charoensinphon, N.; Zheng, J.; Qiu, P.; Wu, X.; Xiao, H. Inhibitory effects of nobiletin and its major metabolites on lung tumorigenesis. Food Funct. 2019, 10, 7444–7452. [Google Scholar] [CrossRef] [PubMed]
- Gong, C.; Qi, L.; Huo, Y.; Zhang, S.; Ning, X.; Bai, L.; Wang, Z. Anticancer effect of Limonin against benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice and the inhibition of A549 cell proliferation through apoptotic pathway. J. Biochem. Mol. Toxicol. 2019, 33, e22374. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Miao, B.; Li, X.; Hu, K.Q.; Liu, C.; Wang, X.D. beta-Cryptoxanthin Reduced Lung Tumor Multiplicity and Inhibited Lung Cancer Cell Motility by Downregulating Nicotinic Acetylcholine Receptor alpha7 Signaling. Cancer Prev. Res. 2016, 9, 875–886. [Google Scholar] [CrossRef]
- Iskandar, A.R.; Liu, C.; Smith, D.E.; Hu, K.Q.; Choi, S.W.; Ausman, L.M.; Wang, X.D. beta-cryptoxanthin restores nicotine-reduced lung SIRT1 to normal levels and inhibits nicotine-promoted lung tumorigenesis and emphysema in A/J mice. Cancer Prev. Res. 2013, 6, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Bodduluru, L.N.; Kasala, E.R.; Barua, C.C.; Karnam, K.C.; Dahiya, V.; Ellutla, M. Antiproliferative and antioxidant potential of hesperetin against benzo(a)pyrene-induced lung carcinogenesis in Swiss albino mice. Chem.-Biol. Interact. 2015, 242, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Kamaraj, S.; Anandakumar, P.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Hesperidin attenuates mitochondrial dysfunction during benzo(a)pyrene-induced lung carcinogenesis in mice. Fundam. Clin. Pharm. 2011, 25, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Mustra Rakic, J.; Liu, C.; Veeramachaneni, S.; Wu, D.; Paul, L.; Chen, C.O.; Ausman, L.M.; Wang, X.D. Lycopene Inhibits Smoke-Induced Chronic Obstructive Pulmonary Disease and Lung Carcinogenesis by Modulating Reverse Cholesterol Transport in Ferrets. Cancer Prev. Res. 2019, 12, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Rakic, J.M.; Liu, C.; Veeramachaneni, S.; Wu, D.; Paul, L.; Chen, O.; Ausman, L.; Wang, X.-D. Modulation of Reverse Cholesterol Transport by Lycopene Is Associated with Its Protective Role Against Cigarette Smoke Induced COPD and Lung Carcinogenesis in Ferrets (OR05-02-19). Curr. Dev. Nutr. 2019, 3, nzz029-OR05. [Google Scholar] [CrossRef]
- Aizawa, K.; Liu, C.; Tang, S.; Veeramachaneni, S.; Hu, K.Q.; Smith, D.E.; Wang, X.D. Tobacco carcinogen induces both lung cancer and non-alcoholic steatohepatitis and hepatocellular carcinomas in ferrets which can be attenuated by lycopene supplementation. Int. J. Cancer 2016, 139, 1171–1181. [Google Scholar] [CrossRef]
- Lian, F.; Smith, D.E.; Ernst, H.; Russell, R.M.; Wang, X.D. Apo-10′-lycopenoic acid inhibits lung cancer cell growth in vitro, and suppresses lung tumorigenesis in the A/J mouse model in vivo. Carcinogenesis 2007, 28, 1567–1574. [Google Scholar] [CrossRef] [PubMed]
- Monteillier, A.; Voisin, A.; Furrer, P.; Allemann, E.; Cuendet, M. Intranasal administration of resveratrol successfully prevents lung cancer in A/J mice. Sci. Rep. 2018, 8, 14257. [Google Scholar] [CrossRef]
- Revel, A.; Raanani, H.; Younglai, E.; Xu, J.; Rogers, I.; Han, R.; Savouret, J.F.; Casper, R.F. Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects lung from DNA damage and apoptosis caused by benzo[a]pyrene. J. Appl. Toxicol. 2003, 23, 255–261. [Google Scholar] [CrossRef]
- Arimoto-Kobayashi, S.; Sasaki, K.; Hida, R.; Miyake, N.; Fujii, N.; Saiki, Y.; Daimaru, K.; Nakashima, H.; Kubo, T.; Kiura, K. Chemopreventive effects and anti-tumorigenic mechanisms of 2,6-dimethoxy-1,4-benzoquinone, a constituent of Vitis coignetiae Pulliat (crimson glory vine, known as yamabudo in Japan), toward 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced lung tumorigenesis in A/J mice. Food Chem. Toxicol. 2021, 154, 112319. [Google Scholar] [CrossRef]
- Akhtar, S.; Meeran, S.M.; Katiyar, N.; Katiyar, S.K. Grape Seed Proanthocyanidins Inhibit the Growth of Human Non-Small Cell Lung Cancer Xenografts by Targeting Insulin-Like Growth Factor Binding Protein-3, Tumor Cell Proliferation, and Angiogenic Factors. Clin. Cancer Res. 2009, 15, 821–831. [Google Scholar] [CrossRef]
- Staretz, M.E.; Koenig, L.A.; Hecht, S.S. Effects of long term dietary phenethyl isothiocyanate on the microsomal metabolism of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol in F344 rats. Carcinogenesis 1997, 18, 1715–1722. [Google Scholar] [CrossRef] [PubMed]
- Ye, B.; Zhang, Y.X.; Yang, F.; Chen, H.L.; Xia, D.; Liu, M.Q.; Lai, B.T. Induction of lung lesions in Wistar rats by 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone and its inhibition by aspirin and phenethyl isothiocyanate. BMC Cancer 2007, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Kassie, F.; Anderson, L.B.; Scherber, R.; Yu, N.; Lahti, D.; Upadhyaya, P.; Hecht, S.S. Indole-3-carbinol inhibits 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone plus benzo(a)pyrene-induced lung tumorigenesis in A/J mice and modulates carcinogen-induced alterations in protein levels. Cancer Res. 2007, 67, 6502–6511. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Melkamu, T.; Upadhyaya, P.; Kassie, F. Indole-3-carbinol inhibited tobacco smoke carcinogen-induced lung adenocarcinoma in A/J mice when administered during the post-initiation or progression phase of lung tumorigenesis. Cancer Lett. 2011, 311, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kassie, F.; Matise, I.; Negia, M.; Upadhyaya, P.; Hecht, S.S. Dose-dependent inhibition of tobacco smoke carcinogen-induced lung tumorigenesis in A/J mice by indole-3-carbinol. Cancer Prev. Res. 2008, 1, 568–576. [Google Scholar] [CrossRef]
- Shukla, S.; Khan, S.; Kumar, S.; Sinha, S.; Farhan, M.; Bora, H.K.; Maurya, R.; Meeran, S.M. Cucurbitacin B Alters the Expression of Tumor-Related Genes by Epigenetic Modifications in NSCLC and Inhibits NNK-Induced Lung Tumorigenesis. Cancer Prev. Res. 2015, 8, 552–562. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Wang, Z.Y.; Smith, T.J.; Zhou, S.; Shi, S.; Pan, J.; Yang, C.S. Inhibitory effects of diallyl sulfide on the metabolism and tumorigenicity of the tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) in A/J mouse lung. Carcinogenesis 1992, 13, 901–904. [Google Scholar] [CrossRef]
- Hudlikar, R.R.; Sargsyan, D.; Cheng, D.; Kuo, H.D.; Wu, R.; Su, X.; Kong, A.N. Tobacco carcinogen 4-[Methyl(nitroso)amino]-1-(3-pyridinyl)-1-butanone (NNK) drives metabolic rewiring and epigenetic reprograming in A/J mice lung cancer model and prevention with Diallyl Sulphide (DAS). Carcinogenesis 2022, 43, 140–149. [Google Scholar] [CrossRef]
- Yang, C.S.; Chhabra, S.K.; Hong, J.-Y.; Smith, T.J. Mechanisms of Inhibition of Chemical Toxicity and Carcinogenesis by Diallyl Sulfide (DAS) and Related Compounds from Garlic. J. Nutr. 2001, 131, 1041S–1045S. [Google Scholar] [CrossRef]
- Khan, A.; Alhumaydhi, F.A.; Alwashmi, A.S.S.; Allemailem, K.S.; Alsahli, M.A.; Alrumaihi, F.A.; Almatroudi, A.; Mobark, M.A.; Mousa, A.; Khan, M.A. Diallyl Sulfide-Mediated Modulation of the Fatty Acid Synthase (FASN) Leads to Cancer Cell Death in BaP-Induced Lung Carcinogenesis in Swiss Mice. J. Inflamm. Res. 2020, 13, 1075–1087. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Asokkumar, S.; Naveenkumar, C.; Raghunandhakumar, S.; Devaki, T. Capsaicin inhibits benzo(a)pyrene-induced lung carcinogenesis in an in vivo mouse model. Inflamm. Res. 2012, 61, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Devaki, T. Capsaicin provokes apoptosis and restricts benzo(a)pyrene induced lung tumorigenesis in Swiss albino mice. Int. Immunopharmacol. 2013, 17, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Asokkumar, S.; Naveenkumar, C.; Raghunandhakumar, S.; Vanitha, M.K.; Devaki, T. The Anticancer Role of Capsaicin in Experimentallyinduced Lung Carcinogenesis. J. Pharmacopunct. 2015, 18, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Vinodhkumar, R.; Devaki, T. Capsaicin modulates pulmonary antioxidant defense system during benzo(a)pyrene-induced lung cancer in Swiss albino mice. Phytother. Res. 2008, 22, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Miyamoto, S.; Yasui, Y.; Oyama, T.; Murakami, A.; Tanaka, T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int. J. Cancer 2009, 124, 264–271. [Google Scholar] [CrossRef]
- Puliyappadamba, V.T.; Thulasidasan, A.K.; Vijayakurup, V.; Antony, J.; Bava, S.V.; Anwar, S.; Sundaram, S.; Anto, R.J. Curcumin inhibits B[a]PDE-induced procarcinogenic signals in lung cancer cells, and curbs B[a]P-induced mutagenesis and lung carcinogenesis. Biofactors 2015, 41, 431–442. [Google Scholar] [CrossRef]
- Chikara, S.; Mamidi, S.; Sreedasyam, A.; Chittem, K.; Pietrofesa, R.; Zuppa, A.; Moorthy, G.; Dyer, N.; Christofidou-Solomidou, M.; Reindl, K.M. Flaxseed Consumption Inhibits Chemically Induced Lung Tumorigenesis and Modulates Expression of Phase II Enzymes and Inflammatory Cytokines in A/J Mice. Cancer Prev. Res. 2017, 11, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.; Guo, Z.; Zhu, Y.; Kong, M.; Zhang, R.; Lu, L.; Wu, F.; Liu, Z.; Wu, J. Houttuynia cordata Thunb. and its bioactive compound 2-undecanone significantly suppress benzo(a)pyrene-induced lung tumorigenesis by activating the Nrf2-HO-1/NQO-1 signaling pathway. J. Exp. Clin. Cancer Res. 2019, 38, 242. [Google Scholar] [CrossRef]
- He, S.; Ou, R.; Wang, W.; Ji, L.; Gao, H.; Zhu, Y.; Liu, X.; Zheng, H.; Liu, Z.; Wu, P.; et al. Camptosorus sibiricus rupr aqueous extract prevents lung tumorigenesis via dual effects against ROS and DNA damage. J. Ethnopharmacol 2018, 220, 44–56. [Google Scholar] [CrossRef]
- Phutthaphadoong, S.; Yamada, Y.; Hirata, A.; Tomita, H.; Taguchi, A.; Hara, A.; Limtrakul, P.N.; Iwasaki, T.; Kobayashi, H.; Mori, H. Chemopreventive effects of fermented brown rice and rice bran against 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced lung tumorigenesis in female A/J mice. Oncol. Rep. 2009, 21, 321–327. [Google Scholar]
- Wang, Y.; Narayanapillai, S.C.; Tessier, K.M.; Strayer, L.G.; Upadhyaya, P.; Hu, Q.; Kingston, R.; Salloum, R.G.; Lu, J.; Hecht, S.S.; et al. The Impact of One-week Dietary Supplementation with Kava on Biomarkers of Tobacco Use and Nitrosamine-based Carcinogenesis Risk among Active Smokers. Cancer Prev. Res. 2020, 13, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.M.; Stepanov, I.; Murphy, S.E.; Wang, R.; Allen, S.; Jensen, J.; Strayer, L.; Adams-Haduch, J.; Upadhyaya, P.; Le, C.; et al. Clinical Trial of 2-Phenethyl Isothiocyanate as an Inhibitor of Metabolic Activation of a Tobacco-Specific Lung Carcinogen in Cigarette Smokers. Cancer Prev. Res. 2016, 9, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Boldry, E.J.; Yuan, J.M.; Carmella, S.G.; Wang, R.; Tessier, K.; Hatsukami, D.K.; Hecht, S.S.; Tretyakova, N.Y. Effects of 2-Phenethyl Isothiocyanate on Metabolism of 1,3-Butadiene in Smokers. Cancer Prev. Res. 2020, 13, 91–100. [Google Scholar] [CrossRef]
- Kubatka, P.; Kello, M.; Kajo, K.; Samec, M.; Jasek, K.; Vybohova, D.; Uramova, S.; Liskova, A.; Sadlonova, V.; Koklesova, L.; et al. Chemopreventive and Therapeutic Efficacy of Cinnamomum zeylanicum L. Bark in Experimental Breast Carcinoma: Mechanistic In Vivo and In Vitro Analyses. Molecules 2020, 25, 1399. [Google Scholar] [CrossRef] [PubMed]
- Amararathna, M.; Hoskin, D.W.; Rupasinghe, H.P.V. Anthocyanin-rich haskap (Lonicera caerulea L.) berry extracts reduce nitrosamine-induced DNA damage in human normal lung epithelial cells in vitro. Food Chem. Toxicol. 2020, 141, 111404. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Arumuggam, N.; Amararathna, M.; De Silva, A.B.K.H. The potential health benefits of haskap ( Lonicera caerulea L.): Role of cyanidin-3- O -glucoside. J. Funct. Foods 2018, 44, 24–39. [Google Scholar] [CrossRef]
- Kuno, T.; Yokohira, M.; Matsuda, Y.; Suzuki, S.; Hashimoto, N.; Yamakawa, K.; Saoo, K.; Imaida, K. Lack of modifying potential of 8-methoxypsoralen in the promotion or progression stages of lung carcinogenesis in A/J female mice. Oncol. Rep. 2008, 20, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Kandpal, J.B.; Sharma, R.K.; Chitme, H. Nobiletin a Biologically Active Phytoconstituent: Systematic Review. J. Biol. Act. Prod. Nat. 2021, 11, 204–211. [Google Scholar] [CrossRef]
- Roohbakhsh, A.; Parhiz, H.; Soltani, F.; Rezaee, R.; Iranshahi, M. Molecular mechanisms behind the biological effects of hesperidin and hesperetin for the prevention of cancer and cardiovascular diseases. Life Sci. 2015, 124, 64–74. [Google Scholar] [CrossRef]
- Bodduluru, L.N.; Kasala, E.R.; Madhana, R.M.; Barua, C.C.; Hussain, M.I.; Haloi, P.; Borah, P. Naringenin ameliorates inflammation and cell proliferation in benzo(a)pyrene induced pulmonary carcinogenesis by modulating CYP1A1, NFkappaB and PCNA expression. Int. Immunopharmacol. 2016, 30, 102–110. [Google Scholar] [CrossRef]
- Lian, F.; Wang, X.D. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int. J. Cancer 2008, 123, 1262–1268. [Google Scholar] [CrossRef] [PubMed]
- Catalano, A.; Simone, R.E.; Cittadini, A.; Reynaud, E.; Caris-Veyrat, C.; Palozza, P. Comparative antioxidant effects of lycopene, apo-10′-lycopenoic acid and apo-14’-lycopenoic acid in human macrophages exposed to H2O2 and cigarette smoke extract. Food Chem. Toxicol. 2013, 51, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Maritz, G.S.; Mutemwa, M.; Kayigire, A.X. Tomato juice protects the lungs of the offspring of female rats exposed to nicotine during gestation and lactation. Pediatr. Pulmonol. 2011, 46, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Li, Y.; Gao, H.; Yang, D.; He, X.; Fang, Y.; Zhou, G. Phenolic compound ellagic acid inhibits mitochondrial respiration and tumor growth in lung cancer. Food Funct. 2020, 11, 6332–6339. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.; Zhan, J.-C.; Wang, G.-Z.; Zhao, X.-C.; Huang, W.-D.; Zhou, G.-B. The red wine component ellagic acid induces autophagy and exhibits anti-lung cancer activity in vitro and in vivo. J. Cell. Mol. Med. 2019, 23, 143–154. [Google Scholar] [CrossRef]
- Alsharairi, N.A. Insights into the Mechanisms of Action of Proanthocyanidins and Anthocyanins in the Treatment of Nicotine-Induced Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 7905. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Chen, Y.; Cao, K.; Liu, Z.; Wan, Z.; Liao, Z.; Li, B.; Cui, J.; Yang, Y.; et al. Grape Seed Proanthocyanidins play the roles of radioprotection on Normal Lung and radiosensitization on Lung Cancer via differential regulation of the MAPK Signaling Pathway. J. Cancer 2021, 12, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Xu, C.; Shen, G.; Jain, M.R.; Khor, T.O.; Gopalkrishnan, A.; Lin, W.; Reddy, B.; Chan, J.Y.; Kong, A.N. Identification of Nrf2-regulated genes induced by chemopreventive isothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006, 79, 1944–1955. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Smith, T.J.; Yang, C.S.; Pittman, B.; Desai, D.; Amin, S.; Chung, F.L. Chemopreventive activity of thiol conjugates of isothiocyanates for lung tumorigenesis. Carcinogenesis 1997, 18, 2143–2147. [Google Scholar] [CrossRef]
- Hecht, S.S.; Trushin, N.; Rigotty, J.; Carmella, S.G.; Borukhova, A.; Akerkar, S.; Rivenson, A. Complete inhibition of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone-induced rat lung tumorigenesis and favorable modification of biomarkers by phenethyl isothiocyanate. Cancer Epidemiol. Biomark. Prev. 1996, 5, 645–652. [Google Scholar]
- Conaway, C.C.; Wang, C.X.; Pittman, B.; Yang, Y.M.; Schwartz, J.E.; Tian, D.; McIntee, E.J.; Hecht, S.S.; Chung, F.L. Phenethyl isothiocyanate and sulforaphane and their N-acetylcysteine conjugates inhibit malignant progression of lung adenomas induced by tobacco carcinogens in A/J mice. Cancer Res. 2005, 65, 8548–8557. [Google Scholar] [CrossRef] [PubMed]
- Kassie, F.; Melkamu, T.; Endalew, A.; Upadhyaya, P.; Luo, X.; Hecht, S.S. Inhibition of lung carcinogenesis and critical cancer-related signaling pathways by N-acetyl-S-(N-2-phenethylthiocarbamoyl)-l-cysteine, indole-3-carbinol and myo-inositol, alone and in combination. Carcinogenesis 2010, 31, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Dagne, A.; Melkamu, T.; Schutten, M.M.; Qian, X.; Upadhyaya, P.; Luo, X.; Kassie, F. Enhanced inhibition of lung adenocarcinoma by combinatorial treatment with indole-3-carbinol and silibinin in A/J mice. Carcinogenesis 2011, 32, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Liu, X.; Lv, S.; Wang, Z. Protective Effects of Cucurbitacin B on Acute Lung Injury Induced by Sepsis in Rats. Med. Sci. Monit. 2017, 23, 1355–1362. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, K.; Iwasaki, Y.; Narukawa, M.; Iitsuka, Y.; Fukao, T.; Seki, T.; Ariga, T.; Watanabe, T. Diallyl sulfides in garlic activate both TRPA1 and TRPV1. Biochem. Biophys. Res. Commun. 2009, 382, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Sparnins, V.L.; Barany, G.; Wattenberg, L.W. Effects of organosulfur compounds from garlic and onions on benzo[ a ]pyrene-induced neoplasia and glutathione S-transferase activity in the mouse. Carcinogenesis 1988, 9, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.J.; Hu, Y.; Lamy, E.; Mersch-Sundermann, V. Apoptosis induction in human lung adenocarcinoma cells by oil-soluble allyl sulfides: Triggers, pathways, and modulators. Environ. Mol. Mutagen 2009, 50, 266–275. [Google Scholar] [CrossRef]
- Xiao, D.; Zeng, Y.; Hahm, E.R.; Kim, Y.A.; Ramalingam, S.; Singh, S.V. Diallyl trisulfide selectively causes Bax- and Bak-mediated apoptosis in human lung cancer cells. Env. Mol. Mutagen 2009, 50, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Lau, J.K.; Brown, K.C.; Dom, A.M.; Witte, T.R.; Thornhill, B.A.; Crabtree, C.M.; Perry, H.E.; Brown, J.M.; Ball, J.G.; Creel, R.G.; et al. Capsaicin induces apoptosis in human small cell lung cancer via the TRPV6 receptor and the calpain pathway. Apoptosis 2014, 19, 1190–1201. [Google Scholar] [CrossRef]
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Vinodhkumar, R.; Devaki, T. Stabilization of pulmonary mitochondrial enzyme system by capsaicin during benzo(a)pyrene induced experimental lung cancer. Biomed. Pharm. 2008, 62, 390–394. [Google Scholar] [CrossRef]
- Yocum, G.T.; Hwang, J.J.; Mikami, M.; Danielsson, J.; Kuforiji, A.S.; Emala, C.W. Ginger and its bioactive component 6-shogaol mitigate lung inflammation in a murine asthma model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 318, L296–L303. [Google Scholar] [CrossRef] [PubMed]
- Garg, R.; Gupta, S.; Maru, G.B. Dietary curcumin modulates transcriptional regulators of phase I and phase II enzymes in benzo[a]pyrene-treated mice: Mechanism of its anti-initiating action. Carcinogenesis 2008, 29, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
| Nature Products | Bioactive Components | Classification of Compounds | Dose | Model of Animals | Effects and Mechanisms | Ref |
|---|---|---|---|---|---|---|
| Glycyrrhiza | LicA | Monomeric compound | 20 mg/Kg | NNK-induced A/J mice | Reversed NNK-induced miRNA expression | [29] |
| Glycyrrhiza | Monomeric compound | 100 mg/Kg | PDX mice | Inhibited the growth of lung tumor; Suppressed migration and invasion; Decreased the activity of Jak/KSTAT signaling | [30] | |
| Glycyrrhiza + cisplatin | Monomeric compound | 135 mg/Kg + 2.5 mg/Kg 50 mg/Kg/2 d | Nude mice | Reduced expression of thromboxane synthase (TxAS) and PCNA; Rescued damage of liver and kidney | [31,32] | |
| Haskap berry | Cyanidin-3-O-glucoside | Monomeric compound | 0.2 g/mice/d | NNK-induced A/J mice | Reduced tumor multiplicity, tumor area, PCNA and Ki-67 | [33] |
| Cyanidin-3-O-glucoside plus 5-FU | Monomeric compound | 5 mg/Kg + 25 mg/Kg | Nude mice | Decreased inflammatory cytokine levels, such as IL-1β,IL-6 and TNF-α; Decreased COX-2, NF-kpa, PCNA | [34] | |
| Emblic | EmblicL extracts | Mixture | 5 g/kg | BaP-induced A/J mice | Reduced the number of nodes by regulated the IL-1β/miR-i101/Lin28B signaling pathway | [35] |
| Psoralea | 8-MOP | Monomeric compound | 50/12.5 mg/Kg | NNK-induced A/J mice | Inhibited the CYP2A5-mediated metabolic activation of NNK in the mouse lung; Reduced NNK-induced tumor incidence and tumor multiplicity | [36,37,38] |
| 12.5 mg/Kg | NNK-induced C57BL/C mice | Decreased α-hydroxylation of NNK | [39] | |||
| Tea | EGCG | Monomeric compound | 0.5% diet | NNK-induced A/J mice | Attenuated DNMT1 and inhibited lung tumorigenesis | [40] |
| Caffeine | Monomeric compound | 0.044% in drinking water | NNK-induced A/J mice | Inhibited the progression of lung adenoma to adenocarcinoma | [41] | |
| 680 ppm in drinking water | NNK-induced F344 rats | Decreased the incidence of lung tumor | [42] | |||
| PBPs | Mixture | 0.3% in drinking water | NNK-induced A/J mice | Inhibited the progression of adenoma to adenocarcinoma and cell proliferation | [43] | |
| 1.5% in drinking water | NNK- and BaP-induced A/J mice | Inhibited inflammation and induced apoptosis via p38 and Akt | [44] | |||
| 3% in drinking water | Anti-initiating and anti-promoting and inhibited the expression of CYP1A2 and increased the activity of GSTs and decreased tumor incidence and multiplicity | [45] | ||||
| Polyphenon E | Mixture | 0.5% in drinking water | NNK-induced A/J mice | Reduced the incidence and multiplicity; Inhibited cell proliferation and enhanced apoptosis and lowered levels of c-Jun and extracellular signal-regulated kinase (Erk) ½ phosphorylation | [41] | |
| Polyohenon E plus Eatorvastatin | Mixture | 0.5% in drinking water + 200 ppm diet | NNK-induced A/J mice | Suppressed myeloid cell leukemia 1; Increasing the cleaved caspase-3 and cleaved poly (ADP)-ribose polymerase level | [46] | |
| Catechin hydrate | Mixture | 20 mg/Kg | BaP-induced albino rats | Reduced Bcl-2, Bax and Capase-3 expression | [47] | |
| Tea preparation | Mixture | 0.6%, 2%, 0.3% in drinking water | NNK-induced A/J mice | Reduced lung tumor multiplicity; Inhibited angiogenesis and enhanced apoptosis index; Reduced the number of lung tumors; Inhibited the increase of 8-OH-dGuo level; Inhibited the percentage of PD-L1 | [48,49,50] | |
| 1% in drinking water | Bap-induced SD rats | Decreased the level of ROS, LPO and NO | [51] | |||
| Kava | Kava | Mixture | 5 mg/g 10 mg/g | NNK-induced A/J mice | Reduced tumor multiplicities; Decreased O6-mG; Suppressed NF-κB; Enhanced apoptosis | [52,53,54] |
| 10 mg/g | NNK plus Bap-induced A/J mice | Reduced PCNA; Increase in caspase-3, and cleavage of poly (ADP-ribose) polymerase (PARP); Inhibited the activation of nuclear factor κBNF-κB | [55,56] | |||
| 500 mg/Kg | Acetaminophen induced C57BL/6 mice | Increased AST and ALT; Increased severity of liver lesions | [57] | |||
| DHM | Monomeric compound | 2 mg/d 32 mg/Kg 1 mg/g 0.05 mg/g 200 ppm diet | NNK-induced A/J mice | Decreased the formation of O6-mG; Increased the urinary excretion of NNK metabolites; Increased NNAL-O-glue; Decreased adenoma multiplicity; Reduced DNA adducts; Suppressed PKA pathway | [58,59,60,61,62,63] | |
| 1 mg/g | NNK-induced C57BL/6 mice | Reduced the formation of O6-mG adducts | [64] | |||
| Citrus | 5-Demethylnobiletin | Monomeric compound | 0.05% diet | NNK-induced A/J mice | Decreased lung tumor multiplicity and tumor volume | [65] |
| Nobiletin | Monomeric compound | 500 ppm diet | NNK-induced gpt delte mice | Inhibited CYP enzymes that involved in the metabolic activation of NNK | [66] | |
| 0.05% diet | NNK-induced A/J mice | Decreased tumor volume; Decreased the expression level of PCNA | [67] | |||
| Limonin | Monomeric compound | 50 mg/Kg | Bap-induced A/J mice | Decreased lipid peroxidation, serum marker enzymes and inflammatory cytokines levels; Enhanced apoptosis | [68] | |
| β-Cryptoxanthin | Monomeric compound | 10 mg/Kg | NNK-induced A/J mice | Down-regulated α7-nAChR/PI3K signaling | [69] | |
| 20 mg/Kg | NNK-induced A/J mice | Restored the nicotine-suppressed expression of lung SIRT1, p53, and RAR-β; Decreased the levels of lung il-6 mRNA and phosphorylation of AKT | [70] | |||
| Hesperetin/Naringenin /Hesperidin | Monomeric compound | 25 mg/Kg 50 mg/Kg | BaP-induced Swiss albino mice | Alleviated LPO, modulated antioxidants and decreased the expression of NF-kB, PCNA and CYP1A1; Attenuated mitochondrial dysfunction | [71,72] | |
| Tomato | Lycopene | Monomeric compound | 6.6 mg/Kg | NNK/CS-induced ferrets | Inhibited chronic bronchitis, emphysema, and preneoplastic lesions; Increased mRNA expression of critical genes related to RCT in the lungs | [73] [74] |
| 6.6 mg/Kg | NNK-induced ferrets | Prevented the expression of α7 nicotinic acetylcholine receptor in the lung; Decreased the mortality rate of ferrets | [75] | |||
| apo-10′-lycopenoids | Monomeric compound | 120 mg/Kg | NNK-induced A/J mice | Decreased lung tumor multiplicity | [76] | |
| Grape | Resveratrol | Monomeric compound | 60 mg/Kg | NNK-induced A/J mice | Inhibited the level of CYP450 | [77] |
| 50 mg/Kg | BaP-induced Balb/C mice | Prevented CYP 1A1 expression | [78] | |||
| DBQ | Monomeric compound | 0.1 g/L drinking water | NNK-induced A/J mice | Decreased lipid peroxidation | [79] | |
| Grape seed proanthocyanidins | Mixture | 0.5% diet | Nude mice | Reduce Bax, capase-3 | [80] | |
| Cruciferous vegetables | PEITC | Monomeric compound | 3 μMol/g diet 0.5 g/Kg diet | NNK-induced F344/Wistar rats | Inhibited metabolic activation of NNK; inhibited lung α-hydroxylation of NNAL; Decreased COX-2 expression and PCNA expression | [81,82] |
| I3C | Monomeric compound | 112 mM/g diet 10 μM/g diet | NNK-induced A/J mice | Reduced tumor multiplicity; Reduced the level of tumor-associated signature proteins; Inhibited PI3K/Akt signaling | [83,84] | |
| 112 mM/g diet | NNK plus BaP-induced mice | Reduced the number of Ki-67–positive cells and expression of proliferating cell nuclear antigen, phospho-Akt, and phospho-BAD | [85] | |||
| Cucurbitacin B | Monomeric compound | 0.2 mg/Kg | NNK-induced A/J mice | Inhibited tumor incidence and multiplicity; Inhibited DNA methyltransferase (DNMTs) and histone deacetylase (HDACs) | [86] | |
| Garlic | DAS | Monomeric compound | 200 mg/Kg | NNK-induced A/J mice | Decreased the incidence of lung tumors and multiplicity; inhibited the metabolism of NNK in mouse lung microsomes Reversed mitochondrial metabolic pathways, global methylation and transcriptomic changes Inhibited the bioactivation of NNK by inhibition of other CYP enzymes active | [87,88,89] |
| 100 mg/Kg | BaP-induced Swiss mice | Decreased tumor marker enzymes and recovered antioxidant enzymes, SOD and CAT | [90] | |||
| Garlic oil | Mixture | 50 mg/Kg | NNK-induced A/J mice | Induced the expressions of the phase II drug-metabolizing enzymes, and HO-1 | [7] | |
| Chilli | Capsaicin | Monomeric compound | 10 mg/Kg | BaP treated A/J mice | Prevented the increasing of TNF-α, IL-6, COX-2 and NF-κB | [91] |
| 10 mg/Kg | BaP-induced Swiss albino mice | Inhibited the development lung carcinogenesis; Induced apoptosis, through inducing increase DNA fragmentation and the expressions of p53, Bax and caspase-3 and decreasing the level of Bcl-2 | [92] [93] | |||
| 10 mg/Kg | Inversed BaP-induced oxidative stress | [94] | ||||
| Zinger | Zerumbone | Monomeric compound | 500 ppm diet | NNK-induced A/J mice | Inhibited the multiplicity of lung adenomas Induced apoptosis, and suppressed NF-κB and HO-1 expression | [95] |
| Turmeric | Curcumin | Monomeric compound | 2% diet | BaP-induced Swiss albino mice | Decreased lung nodes; Reduced the activation of NF-κB and MAPK signaling and Cox-2 transcription | [96] |
| Flax | Flaxseed | Mixture | 10% diet | NNK-induced A/J mice | Reduced lung tumor incidence and multiplicity; Suppressed the phosphorylation (activation) of p-AKT, p-ERK, and p-JNK kinases | [97] |
| HCT | HCT extracts | Mixture | 50 g/Kg | BaP-induced A/J mice | Activated the Nrf2-HO-1/NQO-1 signaling pathway intracellular ROS generation, attenuated DNA damage and inflammation | [98] |
| CSR | CSR extracts | Mixture | 3 g/Kg | BaP-induced A/J mice | Suppressed ROS production by re-activating Nrf2-mediated reductases HO-1 and NQO-1 | [99] |
| FBRA | FBR | Mixture | 10% diet | NNK-induced A/J mice | Decreased the expression of CYP-450 and Ki-67 positivity | [100] |
| Nature Products | Bioactive Components | Classification of Compounds | Dose | Effects and Mechanisms | Ref |
|---|---|---|---|---|---|
| Kava | Kava | Mixture | 225 mg/d | Increased urinary excretion of total NNAL; Reduced 3-mA | [101] |
| Cruciferous vegetables | PEITC | Monomeric compound | 10 mg dissolved in 1 ml olive oil 4 times/d, lasts 5 days | Reduced NNK metabolic activation ratio; Increased urinary levels of BD-mercapturic acids | [102,103] |
| Plants | The Structure of Nature Products | |||
|---|---|---|---|---|
 Tea | 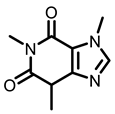 Caffeine | 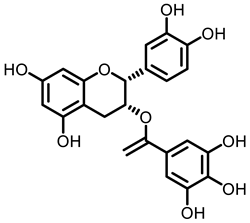 EGCG | ||
 Kava |  Dihydromethysticin | |||
 | 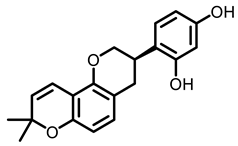 LicA |  Glycyrrhizin | ||
 |  C3G | |||
 Psoralea corylifolia L. |  8-MOP | |||
Grape | 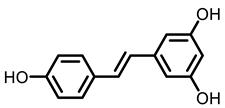 Resveratrol |  EA | 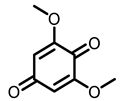 DBQ | |
Citrus | 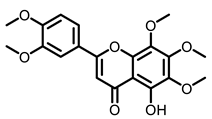 5-Demethylnobiletin | 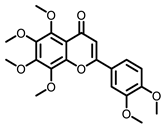 Nobiletin | 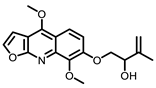 Limonin | 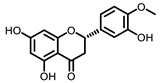 Hesperetin |
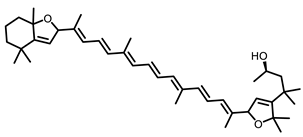 BCX |  Hesperidin |  Naringenin | ||
 Tomato |  Lycopene | |||
Cruciferous vegetables | 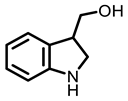 I3C |  PEITC | ||
Cucurbitaceae | 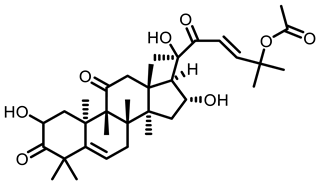 Cucurbitacin B | |||
Garlic |  DAS | 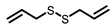 DADS | 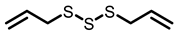 DATS | |
 Chilli | 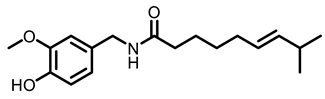 Capsaicin | |||
Ginger | 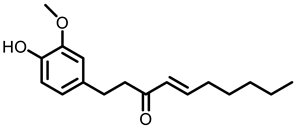 6-shogaol | 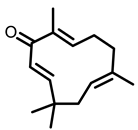 Zerumbone | ||
Turmeric | 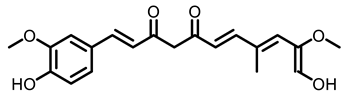 Curcumin | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Hou, R.; Yu, J.; Xing, C.; Zhuang, C.; Qu, Z. Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis. Nutrients 2023, 15, 491. https://doi.org/10.3390/nu15030491
Ding Y, Hou R, Yu J, Xing C, Zhuang C, Qu Z. Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis. Nutrients. 2023; 15(3):491. https://doi.org/10.3390/nu15030491
Chicago/Turabian StyleDing, Yan, Ruilin Hou, Jianqiang Yu, Chengguo Xing, Chunlin Zhuang, and Zhuo Qu. 2023. "Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis" Nutrients 15, no. 3: 491. https://doi.org/10.3390/nu15030491
APA StyleDing, Y., Hou, R., Yu, J., Xing, C., Zhuang, C., & Qu, Z. (2023). Dietary Phytochemicals as Potential Chemopreventive Agents against Tobacco-Induced Lung Carcinogenesis. Nutrients, 15(3), 491. https://doi.org/10.3390/nu15030491






