Early Naso-Gastric Feeding and Outcomes of Anorexia Nervosa Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cuerda, C.; Vasiloglou, M.F.; Arhip, L. Nutritional management and outcomes in malnourished medical inpatients: Anorexia Nervosa. J. Clin. Med. 2019, 8, 1042. [Google Scholar] [CrossRef] [PubMed]
- Treasure, J.; Tiago, A.D.; Schmidt, U. Eating disorders. Lancet 2020, 395, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Mehler, P.S.; Watters, A.; Joiner, T.; Krantz, M.J. What accounts for the high mortality of anorexia nervosa? Int. J. Eat. Disord. 2022, 55, 633–636. [Google Scholar] [CrossRef]
- Arcelus, J.M.A. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta analysis of 36 studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef]
- Khalsa, S.S.; Portnoff, L.C.; McCurdy-McKinnon, D.; Feusner, J.D. What happens after treatment? A systematic review of relapse, remission, and recovery in anorexia nervosa. J. Eat. Disord. 2017, 5, 20. [Google Scholar] [CrossRef]
- Frank, G.K.W. Pharmacotherapeutic strategies for the treatment of anorexia nervosa—Too much for one drug? Expert Opin. Pharmacother. 2020, 21, 1045–1058. [Google Scholar] [CrossRef]
- Harrington, B.C.; Jimerson, M.; Haxton, C.; Jimerson, D.C. Initial evaluation, diagnosis, and treatment of anorexia nervosa and bulimia nervosa. Am. Fam. Physician 2015, 91, 46–52. [Google Scholar]
- Bargiacchi, A.; Clarke, J.; Paulsen, A.; Leger, J. Refeeding in anorexia nervosa. Eur. J. Pediatr. 2018, 178, 413–422. [Google Scholar] [CrossRef]
- Rizzo, S.M.; Douglas, J.W.; Lawrence, J.C. Enteral Nutrition via Nasogastric Tube for Refeeding Patients with Anorexia Nervosa: A Systematic Review. Nutr. Clin. Pract. 2018, 34, 359–370. [Google Scholar] [CrossRef]
- Shafii, T.; Morrison, A.; Qu, P.; Rutman, L.; Kaplan, R. Implementation of Standardized Care for the Medical Stabilization of Patients with Anorexia Nervosa Taraneh. Pediatr. Qual. Saf. 2022, 7, e582. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. Parenteral Nutrition Safety and Clinical Practice Committees, American Society for Parenteral and Enteral Nutrition. ASPEN Consensus Recommendations for Refeeding Syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [PubMed]
- Trovato, C.M.; Capriati, T.; Bolasco, G.; Campana, C.; Papa, V.; Mazzoli, B. Five-Year Inpatient Management of Teenagers with Anorexia Nervosa: Focus on Nutritional Issues. J. Craniofacial Surg. 2022, 74, 674–680. [Google Scholar] [CrossRef]
- Pruccoli, J.; Pelusi, M.; Romagnoli, G.; Malaspina, E.; Moscano, F.; Parmeggiani, A. Timing of Psychopharmacological and Nutritional Interventions in the Inpatient Treatment of Anorexia Nervosa: An Observational Study. Brain Sci. 2021, 11, 1242. [Google Scholar] [CrossRef] [PubMed]
- Kells, M.; Kell-weeder, S. Nasogastric Tube Feeding for Individuals with Anorexia Nervosa: An Integrative Review. J. Am. Psychiatr. Nurses Assoc. 2016, 22, 449–468. [Google Scholar] [CrossRef]
- Hindley, K.; Fenton, C.; McIntosh, J. A systematic review of enteral feeding by nasogastric tube in young people with eating disorders. J. Eat. Disord. 2021, 9, 90. [Google Scholar] [CrossRef]
- Battle, D.E. Diagnostic and Statistical Manual of Mental Disorders (DSM). Codas 2013, 25, 191–192. [Google Scholar]
- Cole, T.J.; Flegal, K.M.; Nicholls, D.; Jackson, A.A. Body mass index cut offs to define thinness in children and adolescents: International survey. BMJ 2007, 335, 194. [Google Scholar] [CrossRef]
- Eating Disorders: Recognition and Treatment; National Institute for Health and Care Excellence (NICE): London, UK, 2020.
- Nehring, I.; Kewitz, K.; von Kries, R.; Thyen, U. Long-term effects of enteral feeding on growth and mental health in adolescents with anorexia nervosa--results of a retrospective German cohort study. Eur. J. Clin. Nutr. 2014, 68, 171–177. [Google Scholar] [CrossRef]
- Strik, L.L.; Curt, F.W.J.; Perdereau, F.; Rein, Z.; Jeammet, P.; Godart, N. Predictive factors of length of inpatient treatment in anorexia nervosa. Eur. Child Adolesc. Psychiatry 2008, 18, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Maginot, T.R.; Kumar, M.M.; Shiels, J.; Kaye, W.; Rhee, K.E. Outcomes of an inpatient refeeding protocol in youth with anorexia nervosa: Rady Children’s Hospital San Diego/University of California, San Diego. J. Eat. Disord. 2017, 5, 1. [Google Scholar] [CrossRef]
- Agostino, H.; Erdstein, J.; Di Meglio, G. Shifting paradigms: Continuous nasogastric feeding with high caloric intakes in anorexia nervosa. J. Adolesc. Health 2013, 53, 590–594. [Google Scholar] [CrossRef] [PubMed]
- Golden, N.H.; Keane-Miller, C.; Sainani, K.L.; Kapphahn, C.J. Higher caloric intake in hospitalized adolescents with anorexia nervosa is associated with reduced length of stay and no increased rate of refeeding syndrome. J. Adolesc. Health 2013, 53, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.K.; Mauldin, K.; Michihata, N.; Buckelew, S.M.; Shafer, M.A.; Moscicki, A.B. Higher calorie diets increase rate of weight gain and shorten hospital stay in hospitalized adolescents with anorexia nervosa. J. Adolesc. Health 2013, 53, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Garber, A.K.; Sawyer, S.M.; Golden, N.H.; Guarda, A.S.; Katzman, D.K.; Kohn, M.R.; Le Grange, D.; Madden, S.; Whitelaw, M.; Redgrave, G.W. A systematic review of approaches to refeeding in patients with anorexia nervosa. Int. J. Eat. Disord. 2015, 49, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Lock, J.; Le Grange, D.; Agras, W.S.; Moye, A.; Bryson, S.W.; Jo, B. Randomized clinical trial comparing family-based treatment with adolescent-focused individual therapy for adolescents with anorexia nervosa. Arch. Gen. Psychiatry 2010, 67, 1025–1032. [Google Scholar] [CrossRef]
- Hay, P.J.; Claudino, A.M. Clinical psychopharmacology of eating disorders: A research update. Int. J. Neuropsychopharmacol. 2011, 15, 209–222. [Google Scholar] [CrossRef]
- Zanna, V. Improvements on Clinical Status of Adolescents with Anorexia Nervosa in Inpatient and Day Hospital Treatment: A Retrospective Pilot Study. Front. Psychiatry 2021, 12, 653482. [Google Scholar] [CrossRef]
- Santos, D.C.D.; Ataide, C.D.G.; Mota da Costa, N.; Oliveira Junior, V.P.; Egea, M.B. Blenderized formulations in home enteral nutrition: A narrative review about challenges in nutritional security and food safety. Nutr. Rev. 2022, 80, 1580–1598. [Google Scholar] [CrossRef]
- Attia, E. Anorexia nervosa: Current status and future directions. Annu. Rev. Med. 2010, 61, 425–435. [Google Scholar] [CrossRef]
- Bulik, C.M.; Hebebrand, J.; Keski-Rahkonen, A.; Klump, K.L.; Reichborn-Kjennerud, T.; Mazzeo, S.E.; Wade, T.D. Genetic epidemiology, endophenotypes, and eating disorder classification. Int. J. Eat. Disord. 2007, 40, S52–S60. [Google Scholar] [CrossRef]


| Total | 315 | N/A |
|---|---|---|
| Age (years)—mean ± SD (range) | 14.4 ± 1.2 (5.8–17.9) | 0 |
| Females—no. (%) | 281 (89.2) | 0 |
| Before COVID-19 pandemic—no. (%) | 77 (24.4) | 0 |
| Triage coding (blue or higher)—no. (%) | 245 (77.8) | 29 |
| Weight loss on admission (kg)—mean ± SD (range) | 11.4 ± 7.7 (0.5–40.0) | 46 |
| Weight loss to admission (months)—median ± IQR (5°–95°) | 4 ± 6 (0.7–12.0) | 40 |
| BMI on admission (value)—mean ± SD (range) | 15.5 ± 2.6 (9.0–32.0) | 1 |
| BMI on admission (percentile)—median ± IQR (5°–95°) | 0.7 ± 8.7 (0.1–55.0) | 1 |
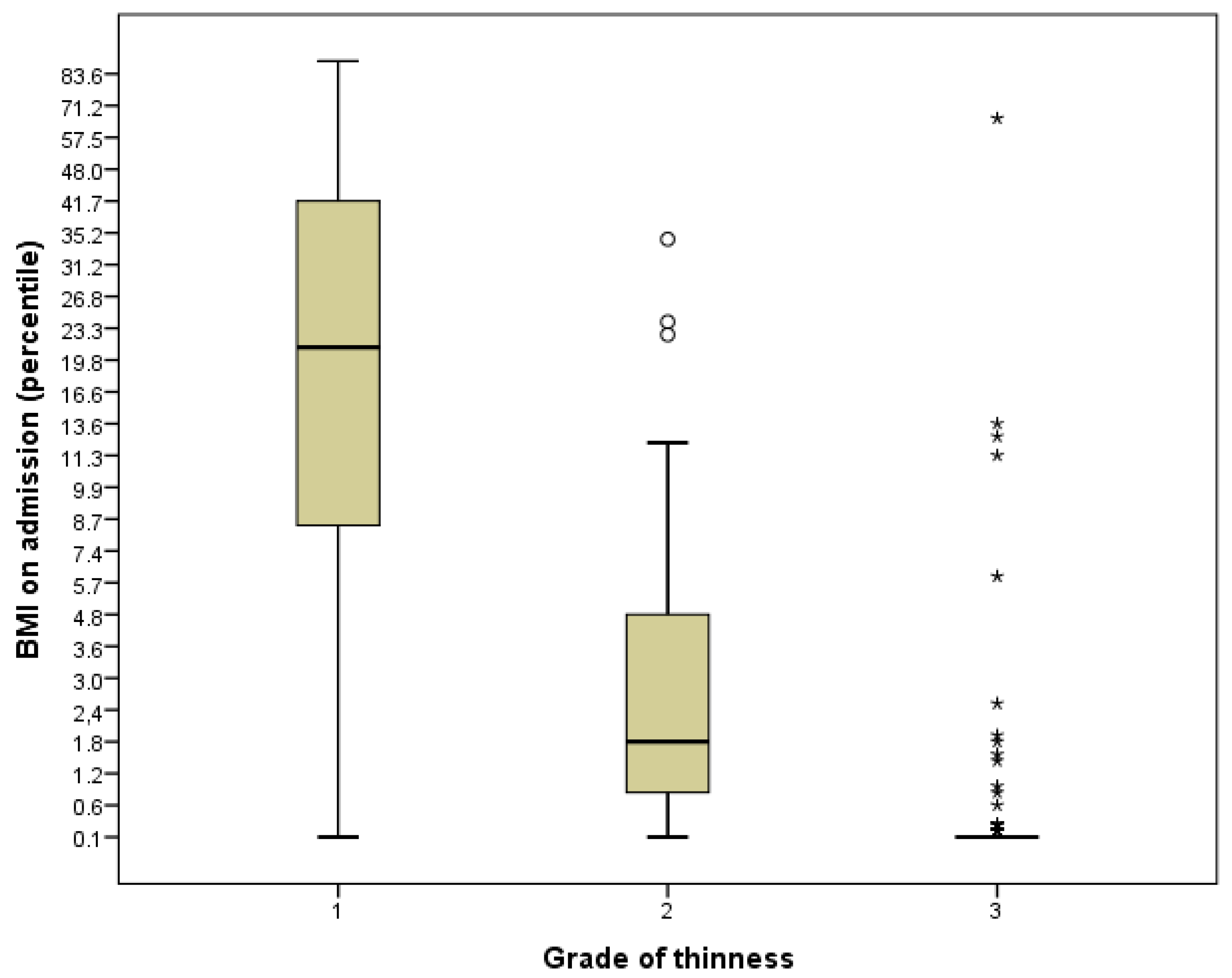
Grade of thinness—no. (%)
| 109 (34.6) 70 (22.2) 136 (43.2) | 0 0 0 |
| LOS (days)—mean ± SD (range) | 21 ± 12 (1–70) | 39 |
| BMI at discharge (value)—mean ± SD (range) | 16.3 ± 2.6 (11.9–32.3) | 27 |
| BMI at discharge (percentile)—median ± IQR (5°–95°) | 2.5 ± 16.8 (0.1–56.2) | 27 |
| Follow-up (months)—median ± IQR (5°–95°) | 3 ± 5 (1–12) | - |
| Relapse—no. (%) Time to relapse (months)—median ± IQR (5°–95°) | 47 (14.9) 3.6 ± 8.0 (0.4–14.9) | - - |
| 2° relapse—no. (%) Time to 2° relapse (months)—median ± IQR (5°–95°) | 6 (1.9) 5.0 ± 4.0 (0.6–12.9) | - - |
| Menarche—no. (%) | 177 (56.2) | - |
| Amenorrhea—no. (%) Amenorrhea (months)—median ± IQR (5°–95°) | 164 (52.1) 5.4 ± 5.6 (0.3–16.2) | - - |
| Psychiatric disorder *—no. (%) | 57 (18.1) | - |
| Suicidal ideation—no. (%) | 16 (5.1) | - |
| Depression—no. (%) | 26 (8.3) | - |
| Pericardial effusion—no. (%) | 19 (6.0) | - |
| Any comorbidity—no. (%) | 124 (39.4) | - |
| Vital Signs | ||
| Heart rate (bpm)—mean ± SD (range) | 63.7 ± 18.2 (33–128) | 14 |
| Bradycardic—no. (%) | 104 (33.0) | - |
| Systolic Blood Pressure (mmHg)—mean ± SD (range) | 104 ± 12 (69–167) | 27 |
| Diastolic Blood Pressure (mmHg)—mean ± SD (range) | 66 ± 9 (39–94) | 27 |
| Respiratory Rate (rpm)—mean ± SD (range) | 17 ± 3 (12–28) | 79 |
| Blood Gas Analysis | ||
| pH (value)—mean ± SD (range) | 7.36 ± 0.05 (7.20–7.52) | 127 |
| Base Excess (value)—mean ± SD (range) | 1.6 ± 4 (−15–13) | 130 |
| Lactate (mmol/L)—mean ± SD (range) | 1.2 ± 0.5 (0.2–3.7) | 214 |
| Laboratory workup | ||
| Azotemia (mg/dL)—mean ± SD (range) | 13 ± 5 (2–40) | 17 |
| Creatinine (mg/dL)—mean ± SD (range) | 0.7 ± 0.3 (0.03–1.4) | 16 |
| Hb (g/dL)—mean ± SD (range) | 13.5 ± 1.1 (9.1–16.7) | 16 |
| Albumin (g/dL)—mean ± SD (range) | 4.8 ± 0.4 (3.8–6.0) | 46 |
| Vitamin A (0.7–2.8 μM/mL)—mean ± SD (range) | 1.4 ± 0.7 (0.4–4.1) | 156 |
| Vitamin B1 (32–95 ng/mL)—mean ± SD (range) | 60.1 ± 36.0 (5.0–289) | 149 |
| Vitamin B6 (8.7–27.2 ng/mL)—mean ± SD (range) | 36.2 ± 26.5 (5.2–203) | 148 |
| Vitamin B12 (197–711 pg/mL)—mean ± SD (range) | 739 ± 422 (4–3100) | 106 |
| Folic acid (5–27.2 ng/mL)—mean ± SD (range) | 8.4 ± 5.5 (1.8–40.3) | 127 |
| Vitamin C (26.1–84.6 μM/L)—mean ± SD (range) | 55.0 ± 46.5 (0.3–349) | 126 |
| Vitamin D (30–100 ng/mL)—mean ± SD (range) | 26.9 ± 8.1 (5.3–54.8) | 98 |
| Vitamin E (12.7–39.4 μM/mL)—mean ± SD (range) | 28.0 ± 26.5 (9.0–310) | 178 |
| TSH (0.51–4.30 μIU/mL)—mean ± SD (range) | 2.20 ± 1.16 (0.06–6.70) | 76 |
| FT4 (0.98–1.64 ng/dL)—mean ± SD (range) | 1.13 ± 0.23 (0.57–2.11) | 77 |
| PRL (4–15 ng/mL)—mean ± SD (range) | 14 ± 13 (0.6–74.1) | 182 |
| FSH (1.5–8.9 ng/mL)—mean ± SD (range) | 3.2 ± 3.2 (0.3–18.1) | 228 |
| LH (0.7–17.8 mIU/L)—mean ± SD (range) | 2.0 ± 6.7 (0.3–66.8) | 200 |
| Cortisol (6.0–18.4 μg/dL)—mean ± SD (range) | 16.4 ± 5.2 (3.7–27.3) | 222 |
| ACTH (7.3–63.3 pg/mL)—mean ± SD (range) | 23.5 ± 38.9 (2.3–357) | 228 |
| β-estradiol (12.4–398 ng/mL)—mean ± SD (range) | 17.5 ± 36.0 (5.00–325) | 215 |
| Progesterone (0.05–14.5 ng/mL)—mean ± SD (range) | 0.37 ± 1.07 (0.05–9.31) | 240 |
| Testosterone (4.6–38.3 ng/dL)—mean ± SD (range) | 61.6 ± 126 (5.4–440) | 304 |
| Management | ||
| Intravenous fluids—no. (%) | 261 (82.9) | - |
| NGF—no. (%) Timing to NGF—median ± IQR (5°–95°) NGF duration—median ± IQR (5°–95°) NGF overnight—no. (%) NGF during daytime– no. (%) NGF all day—no. (%) | 101 (32.1) 5 ± 5 (0–17) 21 ± 13 (9–44) 60 (19.0) 26 (8.3) 2 (0.6) | - - - - - |
| Outpatient psychotropic drug therapy—no. (%) | 73 (23.2) | - |
| Inpatient psychotropic drug therapy—no. (%) | 254 (80.6) | - |
| Aripiprazole—no. (%) | 215 (68.3) | - |
| Sertraline—no. (%) | 141 (44.8) | - |
| Fluoxetine—no. (%) | 29 (9.2) | - |
| Diazepam—no. (%) | 19 (6.0) | - |
| Other—no. (%) ** | 49 (15.7) | - |
| Guarding—no. (%) | 17 (5.4) | - |
| Brain MRI—no. (%) | 134 (42.5) | - |
| Pathologic brain MRI—no. (%) | 17 (5.4) | - |
| NGF (Group A) | No NGF (Group B) | p-Value | |
|---|---|---|---|
| Total | 101 | 214 | |
| Age (years)—mean ± SD (range) | 14.6 ± 1.8 (9.1–17.7) | 14.4 ± 2.3 (5.8–17.9) | 0.369 |
| Females—no. (%) | 92 (91.1) | 189 (88.3) | 0.459 |
| Weight loss on admission (kg)—mean ± SD (range) | 11.3 ± 7.9 (1.2–32.5) | 11.4 ± 7.6 (0.5–40.0) | 0.934 |
| Weight loss to admission (months)—median ± IQR (5°–95°) | 6 ± 6 (1–21) | (0.5–12) | 0.151 |
| BMI on admission (value)—mean ± SD (range) | 14.5 ± 1.9 (10.6–19.0) | 16.1 ± 2.8 (9.0–32.0) | <0.001 |
| BMI on admission (percentile)—median ± IQR (5°–95°) | 0.2 ± 5.1 (0.1–22.1) | 1.9 ± 19.5 (0.1–64.7) | <0.001 |
| Grade of thinness—no. (%) Grade 1 Grade 2 Grade 3 | 27 (38.6) 16 (22.9) 27 (38.6) | 82 (33.5) 54 (22.0) 109 (44.5) | 0.429 0.885 0.378 |
| LOS (days)—mean ± SD (range) | 30 ± 11 (11–65) | 16 ± 9 (1–70) | <0.001 |
| BMI at discharge (value)—mean ± SD (range) | 15.5 ± 1.7 (12.0—20.0) | 16.7 ± 2.8 (12.0 -32.0) | <0.001 |
| BMI at discharge (percentile)—median ± IQR (5°–95°) | 1.8 ± 10 (0.1–43.7) | 6.7 ± 31.0 (0.1–74.6) | 0.001 |
| Follow-up (months)—median ± IQR (5°–95°) | (1–14) | (1–12) | 0.921 |
| Relapse—no. (%) Time to relapse (months)—median ± IQR (5°–95°) | 14 (13.9) 8.2 ± 9.7 (–) | 33 (15.4) 3.0 ± 6.0 (–) | 0.717 0.035 |
| 2° relapse—no. (%) | 1 (1.0) | 5 (2.3) | 0.668 |
| Menarche—no. (%) | 61 (60.4) | 119 (55.6) | 0.423 |
| Amenorrhea—no. (%) Amenorrhea (months)—median ± IQR (5°–95°) | 56 (91.8) 6 ± 5 (0.7–38.5) | 108 (90.8) 5 ± 5 (0.03–16) | 0.815 0.049 |
| Psychiatric disorder **—no. (%) | 20 (19.8) | 37 (17.3) | 0.589 |
| Suicidal ideation—no. (%) | 9 (8.9) | 7 (3.3) | 0.033 |
| Depression—no. (%) | 10 (9.9) | 16 (7.5) | 0.466 |
| Pericardial effusion—no. (%) | 11 (10.9) | 8 (3.7) | 0.013 |
| Any comorbidity—no. (%) | 48 (47.5) | 76 (35.5) | 0.042 |
| Vital Signs | |||
| Heart rate (bpm)—mean ± SD (range) | 61 ± 17 (33–109) | 65 ± 19 (33–128) | 0.053 |
| Bradycardic—no. (%) | 47 (46.5) | 57 (26.6) | <0.001 |
| Blood Gas Analysis | |||
| pH (value)—mean ± SD (range) | 7.36 ± 0.05 (7.24–7.50) | 7.35 ± 0.04 (7.20–7.52) | 0.084 |
| Base Excess (value)—mean ± SD (range) | 1.6 ± 4.6 (−13.0–13.3) | 1.6 ± 3.8 (−15.0–13.0) | 0.916 |
| Lactate (mmol/L)—mean ± SD (range) | 1.2 ± 0.6 (0.2–3.7) | 1.2 ± 0.4 (0.4–2.4) | 0.939 |
| Laboratory workup | |||
| Azotemia (mg/dL)—mean ± SD (range) | 14 ± 6 (2–39) | 13 ± 5 (4–40) | 0.126 |
| Creatinine (mg/dL)—mean ± SD (range) | 0.7 ± 0.2 (0.04–1.3) | 0.7 ± 0.3 (0.03–1.4) | 0.039 |
| Hb (g/dL)—mean ± SD (range) | 13.5 ± 1.1 (11.3–16.0) | 13.5 ± 1.1 (9.1–16.7) | 0.634 |
| Albumin (g/dL)—mean ± SD (range) | 4.8 ± 0.4 (3.8–5.8) | 4.8 ± 0.4 (3.9–6.0) | 0.075 |
| Management | |||
| Intravenous fluids | 101 (100) | 160 (74.8) | <0.001 |
| Outpatient psychotropic drug therapy—no. (%) | 27 (26.7) | 46 (21.6) | 0.314 |
| Inpatient psychotropic drug therapy—no. (%) | 99 (98.0) | 149 (69.6) | <0.001 |
| Aripiprazole—no. (%) | 93 (92.1) | 122 (57.0) | <0.001 |
| Sertraline—no. (%) | 63 (62.4) | 78 (36.4) | <0.001 |
| Fluoxetine—no. (%) | 21 (20.8) | 8 (3.7) | <0.001 |
| Diazepam—no. (%) | 9 (8.9) | 10 (4.7) | 0.140 |
| Other—no. (%) | 20 (19.8) | 29 (13.6) | 0.153 |
| Guarding—no. (%) | 10 (9.9) | 7 (3.3) | 0.016 |
| Brain MRI—no. (%) | 55 (54.5) | 79 (36.9) | 0.003 |
| Pathologic brain MRI—no. (%) | 5 (9.3) | 12 (15.4) | 0.302 |
| Total | OR | C.I. 95% | p-Value |
|---|---|---|---|
| Before COVID-19 pandemic (yes) | 1.891 | 0.285–12.561 | 0.510 |
| BMI on admission (percentile) | 0.870 | 0.736–1.029 | 0.104 |
| Grade of thinness (value) | 0.669 | 0.209–2.141 | 0.499 |
| BMI at discharge (percentile) | 1.031 | 0.973–1.093 | 0.299 |
| Time to relapse (months) | 1.198 | 1.015–1.415 | 0.033 |
| Bradycardic (yes) | 0.692 | 0.110–4.334 | 0.694 |
| p-Value | ||
|---|---|---|
| All subjects—mean ± SD (range) | 4.7 ± 12.0 (−31.2–92.4) | - |
| NGF—mean ± SD (range) No NGF—mean ± SD (range) | 3.7 ± 7.8 (−6.8–48.7) 5.2 ± 13.6 (−31.2–92.4) | 0.314 |
| IPDT– mean ± SD (range) No IPDT—mean ± SD (range) | 5.0 ± 11.7 (−23.3–92.4) 3.5 ± 13.1 (−31.2–56.3) | 0.419 |
| p-Value | ||
|---|---|---|
| All subjects—mean ± SD (range) | 21 ± 12 (1–70) | - |
| NGF–mean ± SD (range) No NGF—mean ± SD (range) | 31 ± 11 (11–65) 16 ± 9 (1–70) | <0.001 |
| IPDT—mean ± SD (range) No IPDT—mean ± SD (range) | 22 ± 12 (2–70) 12 ± 8 (1—38) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchili, M.R.; Diamanti, A.; Zanna, V.; Spina, G.; Mascolo, C.; Roversi, M.; Guarnieri, B.; Mirra, G.; Testa, G.; Raucci, U.; et al. Early Naso-Gastric Feeding and Outcomes of Anorexia Nervosa Patients. Nutrients 2023, 15, 490. https://doi.org/10.3390/nu15030490
Marchili MR, Diamanti A, Zanna V, Spina G, Mascolo C, Roversi M, Guarnieri B, Mirra G, Testa G, Raucci U, et al. Early Naso-Gastric Feeding and Outcomes of Anorexia Nervosa Patients. Nutrients. 2023; 15(3):490. https://doi.org/10.3390/nu15030490
Chicago/Turabian StyleMarchili, Maria Rosaria, Antonella Diamanti, Valeria Zanna, Giulia Spina, Cristina Mascolo, Marco Roversi, Benedetta Guarnieri, Gianluca Mirra, Giulia Testa, Umberto Raucci, and et al. 2023. "Early Naso-Gastric Feeding and Outcomes of Anorexia Nervosa Patients" Nutrients 15, no. 3: 490. https://doi.org/10.3390/nu15030490
APA StyleMarchili, M. R., Diamanti, A., Zanna, V., Spina, G., Mascolo, C., Roversi, M., Guarnieri, B., Mirra, G., Testa, G., Raucci, U., Reale, A., & Villani, A. (2023). Early Naso-Gastric Feeding and Outcomes of Anorexia Nervosa Patients. Nutrients, 15(3), 490. https://doi.org/10.3390/nu15030490






