Vitamin D and Bone: A Story of Endocrine and Auto/Paracrine Action in Osteoblasts
Abstract
1. Introduction
2. Literature Search Strategy
3. Osteoblasts
3.1. Proliferation and Apoptosis
3.2. Differentiation
3.3. Mineralization

3.4. Gene Expression
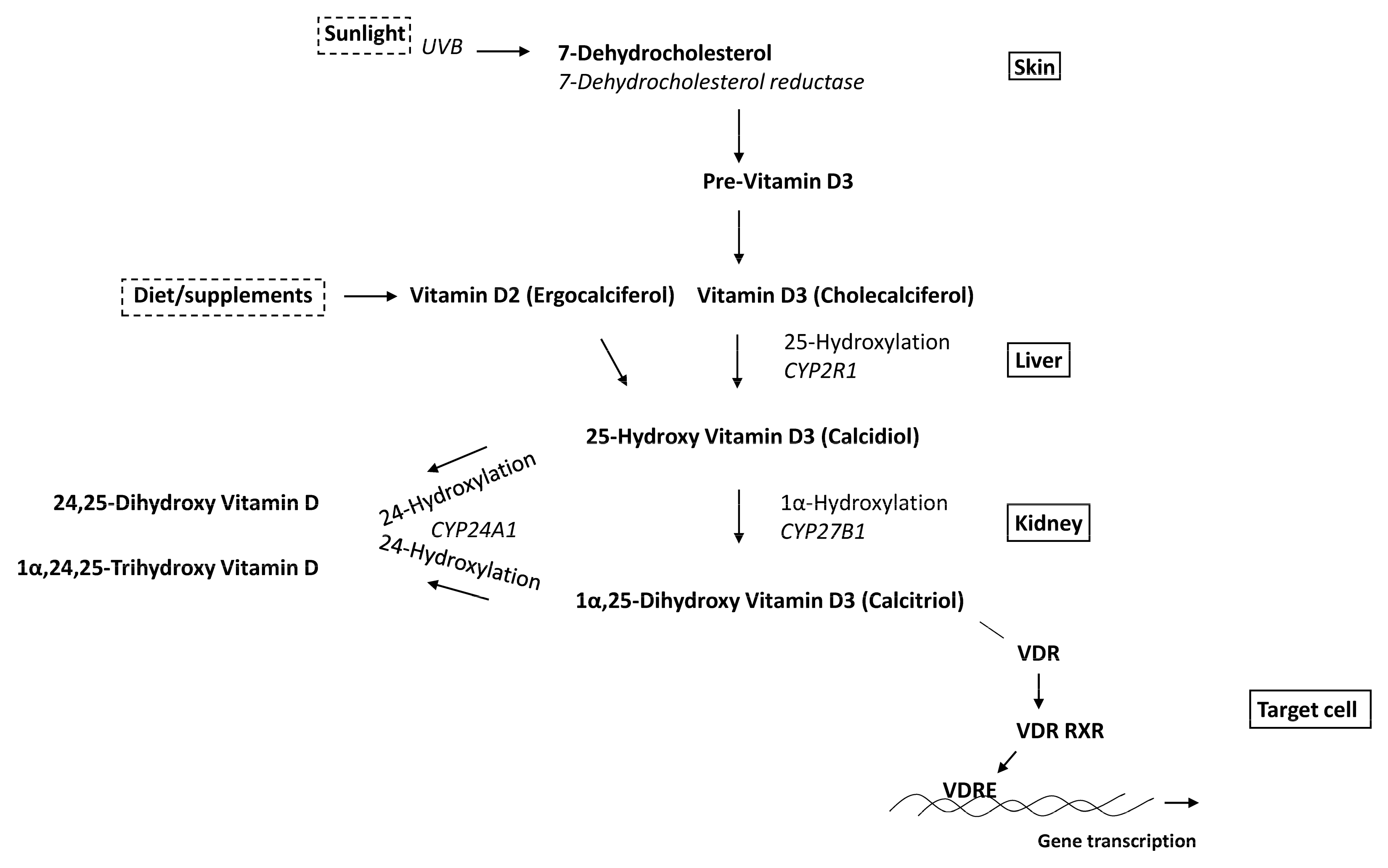
4. Vitamin D Metabolism
4.1. CYP27B1
4.2. CYP24A1
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, H.K.; Ng, W.-F.; Walker, J.A.; Tuck, S.P.; Varanasi, S.S. The cell biology of bone metabolism. J. Clin. Pathol. 2008, 61, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D. Vitamin D and Bone. Curr. Osteoporos. Rep. 2012, 10, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Christakos, S.; Li, S.; DeLa Cruz, J.; Verlinden, L.; Carmeliet, G. Vitamin D and Bone. Handb. Exp. Pharmacol. 2020, 262, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Amling, M.; Pirro, A.E.; Priemel, M.; Meuse, J.; Baron, R.; Delling, G.; Demay, M.B. Normalization of Mineral Ion Homeostasis by Dietary Means Prevents Hyperparathyroidism, Rickets, and Osteomalacia, But Not Alopecia in Vitamin D Receptor-Ablated Mice. Endocrinology 1998, 139, 4391–4396. [Google Scholar] [CrossRef]
- Amling, M.; Priemel, M.; Holzmann, T.; Chapin, K.; Rueger, J.M.; Baron, R.; DeMay, M.B. Rescue of the Skeletal Phenotype of Vitamin D Receptor-Ablated Mice in the Setting of Normal Mineral Ion Homeostasis: Formal Histomorphometric and Biomechanical Analyses. Endocrinology 1999, 140, 4982–4987. [Google Scholar] [CrossRef]
- Panda, D.K.; Miao, D.; Bolivar, I.; Li, J.; Huo, R.; Hendy, G.N.; Goltzman, D. Inactivation of the 25-Hydroxyvitamin D 1α-Hydroxylase and Vitamin D Receptor Demonstrates Independent and Interdependent Effects of Calcium and Vitamin D on Skeletal and Mineral Homeostasis. J. Biol. Chem. 2004, 279, 16754–16766. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Udagawa, N.; Horibe, K.; Mizoguchi, T.; Yamamoto, Y.; Nakamura, T.; Hosoya, A.; Kato, S.; Suda, T.; Takahashi, N. VDR in Osteoblast-Lineage Cells Primarily Mediates Vitamin D Treatment-Induced Increase in Bone Mass by Suppressing Bone Resorption. J. Bone Miner. Res. 2017, 32, 1297–1308. [Google Scholar] [CrossRef]
- Zarei, A.; Morovat, A.; Javaid, K.; Brown, C.P. Vitamin D receptor expression in human bone tissue and dose-dependent activation in resorbing osteoclasts. Bone Res. 2016, 4, 16030. [Google Scholar] [CrossRef]
- Pols, H.A.P.A.; van Leeuwen, J.P.T.M.P.; Schilte, J.P.P.; Visser, T.J.J.; Birkenhäger, J.C.C. Heterologous up-regulation of the 1,25-dihydroxyvitamin D3 receptor by parathyroid hormone (PTH) and PTH-like peptide in osteoblast-like cells. Biochem. Biophys. Res. Commun. 1988, 156, 588–594. [Google Scholar] [CrossRef]
- Van Leeuwen, J.P.; Birkenhäger, J.C.; Buurman, C.J.; Bemd, G.J.V.D.; Bos, M.P.; A Pols, H. Bidirectional regulation of the 1,25-dihydroxyvitamin D3 receptor by phorbol ester-activated protein kinase-C in osteoblast-like cells: Interaction with adenosine 3′,5′-monophosphate-induced up-regulation of the 1,25-dihydroxyvitamin D3 receptor. Endocrinology 1992, 130, 2259–2266. [Google Scholar] [CrossRef]
- van Leeuwen, J.P.; Pols, H.A.; Schilte, J.P.; Visser, T.J.; Birkenhäger, J.C. Modulation by epidermal growth factor of the basal 1,25(OH)2D3 receptor level and the heterologous up-regulation of the 1,25(OH)2D3 receptor in clonal osteoblast-like cells. Calcif. Tissue Int. 1991, 49, 35–42. [Google Scholar] [CrossRef]
- Godschalk, M.; Levy, J.R.; Downs, R.W., Jr. Glucocorticoids decrease vitamin D receptor number and gene expression in human osteosarcoma cells. J. Bone Miner. Res. 1992, 7, 21–27. [Google Scholar] [CrossRef]
- Reinhardt, T.A.; Horst, R.L. Parathyroid Hormone Down-Regulates 1,25-Dihydroxyvitamin D Receptors (VDR) and VDR Messenger Ribonucleic Acid in Vitro and Blocks Homologous Up-Regulation of VDR in Vivo. Endocrinology 1990, 127, 942–948. [Google Scholar] [CrossRef]
- Gardiner, E.M.; Baldock, P.A.; Thomas, G.P.; Sims, N.A.; Henderson, N.K.; Hollis, B.; White, C.P.; Sunn, K.L.; Morrison, N.A.; Walsh, W.R.; et al. Increased formation and decreased resorption of bone in mice with elevated vitamin D receptor in mature cells of the osteoblastic lineage. FASEB J. 2000, 14, 1908–1916. [Google Scholar] [CrossRef]
- Triliana, R.; Lam, N.N.; Sawyer, R.K.; Atkins, G.J.; Morris, H.A.; Anderson, P.H. Skeletal characterization of an osteoblast-specific vitamin D receptor transgenic (ObVDR-B6) mouse model. J. Steroid Biochem. Mol. Biol. 2016, 164, 331–336. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Yoshizawa, T.; Fukuda, T.; Shirode-Fukuda, Y.; Yu, T.; Sekine, K.; Sato, T.; Kawano, H.; Aihara, K.-I.; Nakamichi, Y.; et al. Vitamin D Receptor in Osteoblasts Is a Negative Regulator of Bone Mass Control. Endocrinology 2013, 154, 1008–1020. [Google Scholar] [CrossRef]
- Lieben, L.; Carmeliet, G. The delicate balance between vitamin D, calcium and bone homeostasis: Lessons learned from intestinal- and osteocyte-specific VDR null mice. J. Steroid Biochem. Mol. Biol. 2013, 136, 102–106. [Google Scholar] [CrossRef]
- Verlinden, L.; Janssens, I.; Doms, S.; Vanhevel, J.; Carmeliet, G.; Verstuyf, A. Vdr expression in osteoclast precursors is not critical in bone homeostasis. J. Steroid Biochem. Mol. Biol. 2019, 195, 105478. [Google Scholar] [CrossRef]
- Atkins, G.J.; Anderson, P.; Findlay, D.M.; Welldon, K.J.; Vincent, C.; Zannettino, A.; O’Loughlin, P.D.; Morris, H.A. Metabolism of vitamin D3 in human osteoblasts: Evidence for autocrine and paracrine activities of 1α,25-dihydroxyvitamin D3. Bone 2007, 40, 1517–1528. [Google Scholar] [CrossRef]
- van den Bemd, G.J.; Pols, H.A.; Birkenhäger, J.C.; Kleinekoort, W.M.; van Leeuwen, J.P. Differential effects of 1,25-dihydroxyvitamin D3-analogs on osteoblast-like cells and on in vitro bone resorption. J. Steroid Biochem. Mol. Biol. 1995, 55, 337–346. [Google Scholar] [CrossRef]
- Döhla, J.; Kuuluvainen, E.; Gebert, N.; Amaral, A.; Englund, J.I.; Gopalakrishnan, S.; Konovalova, S.; Nieminen, A.I.; Salminen, E.S.; Torregrosa Muñumer, R.; et al. Metabolic determination of cell fate through selective inheritance of mitochondria. Nat. Cell Biol. 2022, 24, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Eelen, G.; Verlinden, L.; Van Camp, M.; Mathieu, C.; Carmeliet, G.; Bouillon, R.; Verstuyf, A. Microarray analysis of 1α,25-dihydroxyvitamin D3-treated MC3T3-E1 cells. J. Steroid Biochem. Mol. Biol. 2004, 89–90, 405–407. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.L.; Cone, C.M.; Feldman, D. Effects of 1α,25-dihydroxyvitamin D3 and glucocorticoids on the growth of rat and mouse osteoblast-like bone cells. Calcif. Tissue Int. 1983, 35, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.S.; A Glackin, C.; Murray, E.J.B. Variation in 1,25-dihydroxyvitamin D3 regulation of proliferation and alkaline phosphatase activity in late-passage rat osteoblastic cell lines. J. Steroid Biochem. Mol. Biol. 1993, 46, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Kanatani, M.; Sugimoto, T.; Fukase, M.; Chihara, K. Effect of 1.25-Dihydroxyvitamin D3 on the Proliferation of Osteoblastic MC3T3-E1 Cells by Modulating the Release of Local Regulators from Monocytes. Biochem. Biophys. Res. Commun. 1993, 190, 529–535. [Google Scholar] [CrossRef]
- Rubin, J.; Fan, X.; Thornton, D.; Bryant, R.; Biskobing, D. Regulation of Murine Osteoblast Macrophage Colony-Stimulating Factor Production by 1,25(OH)2D3. Calcif. Tissue Int. 1996, 59, 291–296. [Google Scholar] [CrossRef]
- Maehata, Y.; Takamizawa, S.; Ozawa, S.; Kato, Y.; Sato, S.; Kubota, E.; Hata, R.-I. Both direct and collagen-mediated signals are required for active vitamin D3-elicited differentiation of human osteoblastic cells: Roles of osterix, an osteoblast-related transcription factor. Matrix Biol. 2006, 25, 47–58. [Google Scholar] [CrossRef]
- Ishida, H.; Bellows, C.G.; E Aubin, J.; Heersche, J.N. Characterization of the 1,25-(OH)2D3-induced inhibition of bone nodule formation in long-term cultures of fetal rat calvaria cells. Endocrinology 1993, 132, 61–66. [Google Scholar] [CrossRef]
- Wang, D.; Song, J.; Ma, H. An in vitro Experimental Insight into the Osteoblast Responses to Vitamin D3 and Its Metabolites. Pharmacology 2018, 101, 225–235. [Google Scholar] [CrossRef]
- van Driel, M.; Koedam, M.; Buurman, C.J.J.; Hewison, M.; Chiba, H.; Uitterlinden, A.G.; Pols, H.A.P.; Van Leeuwen, J.P.T.M. Evidence for auto/paracrine actions of vitamin D in bone: 1a-hydroxylase expression and activity in human bone cells. FASEB J. 2006, 20, 2417–2419. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Worton, L.; Esteban, L.; Baldock, P.; Fong, C.; Eisman, J.A.; Gardiner, E.M. Effects of continuous activation of vitamin D and Wnt response pathways on osteoblastic proliferation and differentiation. Bone 2007, 41, 87–96. [Google Scholar] [CrossRef]
- Hansen, C.M.; Hansen, D.; Holm, P.K.; Binderup, L. Vitamin D compounds exert anti-apoptotic effects in human osteosarcoma cells in vitro. J. Steroid Biochem. Mol. Biol. 2001, 77, 1–11. [Google Scholar] [CrossRef]
- Thompson, L.; Wang, S.; Tawfik, O.; Templeton, K.; Tancabelic, J.; Pinson, D.; Anderson, H.C.; Keighley, J.; Garimella, R. Effect of 25-hydroxyvitamin D3 and 1 α,25 dihydroxyvitamin D3 on differentiation and apoptosis of human osteosarcoma cell lines. J. Orthop. Res. 2011, 30, 831–844. [Google Scholar] [CrossRef]
- Van Leeuwen, J.P.T.M.; van Driel, M.; van den Bemd, G.J.C.M.; Pols, H.A.P. Vitamin D Control of Osteoblast Function and Bone Extracellular Matrix Mineralization. Crit. Rev. Eukaryot. Gene Expr. 2001, 11, 199–226. [Google Scholar] [CrossRef]
- Skjødt, H.; Gallagher, J.A.; Beresford, J.N.; Couch, M.; Poser, J.W.; Russell, R.G.G. Vitamin D metabolites regulate osteocalcin synthesis and proliferation of human bone cells in vitro. J. Endocrinol. 1985, 105, 391–396. [Google Scholar] [CrossRef]
- Uranoa, T.; Hosoib, T.; Shirakic, M.; Toyoshimad, H.; Ouchia, Y.; Inoue, S. Possible Involvement of the p57Kip2 Gene in Bone Metabolism. Biochem. Biophys. Res. Commun. 2000, 269, 422–426. [Google Scholar] [CrossRef]
- van Driel, M.; van Leeuwen, J.P. Vitamin D endocrinology of bone mineralization. Mol. Cell. Endocrinol. 2017, 453, 46–51. [Google Scholar] [CrossRef]
- van de Peppel, J.; van Leeuwen, J.P.T.M. Vitamin D and gene networks in human osteoblasts. Front. Physiol. 2014, 5, 137. [Google Scholar] [CrossRef]
- Zhou, S.; Glowacki, J.; Kim, S.W.; Hahne, J.; Geng, S.; Mueller, S.M.; Shen, L.; Bleiberg, I.; LeBoff, M.S. Clinical characteristics influence in vitro action of 1,25-dihydroxyvitamin D3in human marrow stromal cells. J. Bone Miner. Res. 2012, 27, 1992–2000. [Google Scholar] [CrossRef]
- Geng, S.; Zhou, S.; Bi, Z.; Glowacki, J. Vitamin D metabolism in human bone marrow stromal (mesenchymal stem) cells. Metabolism 2013, 62, 768–777. [Google Scholar] [CrossRef]
- Piek, E.; Sleumer, L.S.; van Someren, E.P.; Heuver, L.; de Haan, J.R.; de Grijs, I.; Gilissen, C.; Hendriks, J.M.; van Ravestein-van Os, R.I.; Bauerschmidt, S.; et al. Osteo-transcriptomics of human mesenchymal stem cells: Accelerated gene expression and osteoblast differentiation induced by vitamin D reveals c-MYC as an enhancer of BMP2-induced osteogenesis. Bone 2010, 46, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Ochiai-Shino, H.; Onodera, S.; Saito, A.; Shibahara, T.; Azuma, T. Promoting effect of 1,25(OH)2 vitamin D3 in osteogenic differentiation from induced pluripotent stem cells to osteocyte-like cells. Open Biol. 2015, 5, 140201. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Banerjee, C.; Javed, A.; Green, J.; Lian, J.B.; Stein, G.S.; Bodine, P.V.; Komm, B.S. Expression and regulation of Runx2/Cbfa1 and osteoblast phenotypic markers during the growth and differentiation of human osteoblasts. J. Cell. Biochem. 2001, 80, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, N.R.; Henriksen, Z.; Sorensen, O.H.; Civitelli, R. Dexamethasone, BMP-2, and 1,25-dihydroxyvitamin D enhance a more differentiated osteoblast phenotype: Validation of an in vitro model for human bone marrow-derived primary osteoblasts. Steroids 2004, 69, 219–226. [Google Scholar] [CrossRef] [PubMed]
- van Driel, M.; Koedam, M.; Buurman, C.J.J.; Roelse, M.; Weyts, F.; Chiba, H.; Uitterlinden, A.G.G.; Pols, H.A.P.; Van Leeuwen, J.P.T.M. Evidence that both 1α,25-dihydroxyvitamin D3 and 24-hydroxylated D3 enhance human osteoblast differentiation and mineralization. J. Cell. Biochem. 2006, 99, 922–935. [Google Scholar] [CrossRef]
- van der Meijden, K.; Lips, P.; van Driel, M.; Heijboer, A.C.; Schulten, E.A.J.M.; Heijer, M.D.; Bravenboer, N. Primary Human Osteoblasts in Response to 25-Hydroxyvitamin D3, 1,25-Dihydroxyvitamin D3 and 24R,25-Dihydroxyvitamin D3. PLoS ONE 2014, 9, e110283. [Google Scholar] [CrossRef]
- van Driel, M.; van Leeuwen, J.P.T.M. Vitamin D endocrine system and osteoblasts. BoneKEy Rep. 2014, 3, 493. [Google Scholar] [CrossRef]
- Siggelkow, H.; Rebenstorff, K.; Kurre, W.; Niedhart, C.; Engel, I.; Schulz, H.; Atkinson, M.J.; Hüfner, M. Development of the osteoblast phenotype in primary human osteoblasts in culture: Comparison with rat calvarial cells in osteoblast differentiation. J. Cell. Biochem. 1999, 75, 22–35. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Yamaguchi, M. High dose 1,25(OH)2D3 inhibits osteoblast mineralization in vitro. Int. J. Mol. Med. 2012, 29, 934–938. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Ninomiya, T.; Hosoya, A.; Hiraga, T.; Miyazawa, H.; Nakamura, H. 1α,25-Dihydroxyvitamin D3 inhibits osteoblastic differentiation of mouse periodontal fibroblasts. Arch. Oral Biol. 2012, 57, 453–459. [Google Scholar] [CrossRef]
- Matsumoto, T.; Igarashi, C.; Takeuchi, Y.; Harada, S.; Kikuchi, T.; Yamato, H.; Ogata, E. Stimulation by 1,25-Dihydroxyvitamin D3 of in vitro mineralization induced by osteoblast-like MC3T3-E1 cells. Bone 1991, 12, 27–32. [Google Scholar] [CrossRef]
- Yang, D.; Atkins, G.J.; Turner, A.G.; Anderson, P.H.; Morris, H.A. Differential effects of 1,25-dihydroxyvitamin D on mineralisation and differentiation in two different types of osteoblast-like cultures. J. Steroid Biochem. Mol. Biol. 2012, 136, 166–170. [Google Scholar] [CrossRef]
- Shevde, N.K.; Plum, L.A.; Clagett-Dame, M.; Yamamoto, H.; Pike, J.W.; DeLuca, H.F. A potent analog of 1α,25-dihydroxyvitamin D3 selectively induces bone formation. Proc. Natl. Acad. Sci. USA 2002, 99, 13487–13491. [Google Scholar] [CrossRef]
- Jeffery, E.C.; Mann, T.L.; Pool, J.A.; Zhao, Z.; Morrison, S.J. Bone marrow and periosteal skeletal stem/progenitor cells make distinct contributions to bone maintenance and repair. Cell Stem Cell 2022, 29, 1547–1561.e6. [Google Scholar] [CrossRef]
- Eisman, J.A.; Bouillon, R. Vitamin D: Direct effects of vitamin D metabolites on bone: Lessons from genetically modified mice. BoneKEy Rep. 2014, 3, 499. [Google Scholar] [CrossRef]
- Yan, X.-Z.; Yang, W.; Yang, F.; Kersten-Niessen, M.; Jansen, J.A.; Both, S.K. Effects of Continuous Passaging on Mineralization of MC3T3-E1 Cells with Improved Osteogenic Culture Protocol. Tissue Eng. Part C Methods 2014, 20, 198–204. [Google Scholar] [CrossRef]
- Viereck, V.; Siggelkow, H.; Tauber, S.; Raddatz, D.; Schutze, N.; Hüfner, M. Differential regulation of Cbfa1/Runx2 and osteocalcin gene expression by vitamin-D3, dexamethasone, and local growth factors in primary human osteoblasts. J. Cell. Biochem. 2002, 86, 348–356. [Google Scholar] [CrossRef]
- Zhang, R.; Ducy, P.; Karsenty, G. 1,25-Dihydroxyvitamin D3 Inhibits Osteocalcin Expression in Mouse through an Indirect Mechanism. J. Biol. Chem. 1997, 272, 110–116. [Google Scholar] [CrossRef]
- Lian, J.B.; Shalhoub, V.; Aslam, F.; Frenkel, B.; Green, J.; Hamrah, M.; Stein, G.S.; Stein, J.L. Species-Specific Glucocorticoid and 1,25-Dihydroxyvitamin D Responsiveness in Mouse MC3T3-E1 Osteoblasts: Dexamethasone Inhibits Osteoblast Differentiation and Vitamin D Down-Regulates Osteocalcin Gene Expression. Endocrinology 1997, 138, 2117–2127. [Google Scholar] [CrossRef]
- Drissi, H.; Pouliot, A.; Koolloos, C.; Stein, J.L.; Lian, J.B.; Stein, G.S.; van Wijnen, A.J. 1,25-(OH)2-Vitamin D3 Suppresses the Bone-Related Runx2/Cbfa1 Gene Promoter. Exp. Cell Res. 2002, 274, 323–333. [Google Scholar] [CrossRef]
- Thomas, G.P.; Bourne, A.; Eisman, J.A.; Gardiner, E.M. Species-Divergent Regulation of Human and Mouse Osteocalcin Genes by Calciotropic Hormones. Exp. Cell Res. 2000, 258, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Ma, C.; Qiu, J.; Ma, X.; Wang, X.; Chen, H.; Huang, B. A novel interaction between insulin-like growth factor binding protein-6 and the vitamin D receptor inhibits the role of vitamin D3 in osteoblast differentiation. Mol. Cell. Endocrinol. 2011, 338, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Ito, N.; Findlay, D.M.; Anderson, P.H.; Bonewald, L.F.; Atkins, G.J. Extracellular phosphate modulates the effect of 1α,25-dihydroxy vitamin D3 (1,25D) on osteocyte like cells. J. Steroid Biochem. Mol. Biol. 2013, 136, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Staal, A.; Birkenhäger, J.C.; Pols, H.A.; Buurman, C.J.; Vink-van Wijngaarden, T.; Kleinekoort, W.M.; van den Bemd, G.J.; van Leeuwen, J.P. Transforming growth factor beta-induced dissociation between vitamin D receptor level and 1,25-dihydroxyvitamin D3 action in osteoblast-like cells. Bone Miner. 1994, 26, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Staal, A.; Geertsma-Kleinekoort, W.M.C.; Van Den Bemd, G.J.C.M.; Buurman, C.J.; Birkenhäger, J.C.; Pols, H.A.P.; Van Leeuwen, J.P.T.M. Regulation of Osteocalcin Production and Bone Resorption by 1,25-Dihydroxyvitamin D3 in Mouse Long Bones: Interaction with the Bone-Derived Growth Factors TGF-β and IGF-I. J. Bone Miner. Res. 1998, 13, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Staal, A.; VanWijnen, A.; Desai, R.; Pols, H.; Birkenhager, J.; Deluca, H.; Denhardt, D.; Stein, J.; Van Leeuwen, J.; Stein, G.; et al. Antagonistic effects of transforming growth factor-beta on vitamin D3 enhancement of osteocalcin and osteopontin transcription: Reduced interactions of vitamin D receptor/retinoid X receptor complexes with vitamin E response elements. Endocrinology 1996, 137, 2001–2011. [Google Scholar] [CrossRef]
- Wergedal, J.E.; Matsuyama, T.; Strong, D.D. Differentiation of normal human bone cells by transforming growth factor-β and 1,25(OH)2 vitamin D3. Metabolism 1992, 41, 42–48. [Google Scholar] [CrossRef]
- Chen, J.; Dosier, C.R.; Park, J.H.; De, S.; Guldberg, R.E.; Boyan, B.D.; Schwartz, Z. Mineralization of three-dimensional osteoblast cultures is enhanced by the interaction of 1α,25-dihydroxyvitamin D3 and BMP2 via two specific vitamin D receptors. J. Tissue Eng. Regen. Med. 2013, 10, 40–51. [Google Scholar] [CrossRef]
- Woeckel, V.J.J.; Koedam, M.; van de Peppel, J.; Chiba, H.; van der Eerden, B.C.J.; van Leeuwen, J.P.T.M. Evidence of vitamin D and interferon-β cross-talk in human osteoblasts with 1α,25-dihydroxyvitamin D3 being dominant over interferon-β in stimulating mineralization. J. Cell. Physiol. 2012, 227, 3258–3266. [Google Scholar] [CrossRef]
- Fretz, J.A.; Zella, L.A.; Kim, S.; Shevde, N.K.; Pike, J.W. 1,25-Dihydroxyvitamin D3 induces expression of the Wnt signaling co-regulator LRP5 via regulatory elements located significantly downstream of the gene’s transcriptional start site. J. Steroid Biochem. Mol. Biol. 2007, 103, 440–445. [Google Scholar] [CrossRef]
- Haussler, M.R.; Haussler, C.A.; Whitfield, G.K.; Hsieh, J.-C.; Thompson, P.D.; Barthel, T.K.; Bartik, L.; Egan, J.B.; Wu, Y.; Kubicek, J.L.; et al. The nuclear vitamin D receptor controls the expression of genes encoding factors which feed the “Fountain of Youth” to mediate healthful aging. J. Steroid Biochem. Mol. Biol. 2010, 121, 88–97. [Google Scholar] [CrossRef]
- Guler, E.; Baripoglu, Y.E.; Alenezi, H.; Arikan, A.; Babazade, R.; Unal, S.; Duruksu, G.; Alfares, F.S.; Yazir, Y.; Oktar, F.N.; et al. Vitamin D3/vitamin K2/magnesium-loaded polylactic acid/tricalcium phosphate/polycaprolactone composite nanofibers demonstrated osteoinductive effect by increasing Runx2 via Wnt/β-catenin pathway. Int. J. Biol. Macromol. 2021, 190, 244–258. [Google Scholar] [CrossRef]
- Doroudi, M.; Olivares-Navarrete, R.; Hyzy, S.L.; Boyan, B.D.; Schwartz, Z. Signaling components of the 1α,25(OH)2D3-dependent Pdia3 receptor complex are required for Wnt5a calcium-dependent signaling. Biochim. Biophys. Acta BBA Mol. Cell Res. 2014, 1843, 2365–2375. [Google Scholar] [CrossRef]
- Jo, S.; Yoon, S.; Lee, S.Y.; Kim, S.Y.; Park, H.; Han, J.; Choi, S.H.; Han, J.-S.; Yang, J.-H.; Kim, T.-H. DKK1 Induced by 1,25D3 Is Required for the Mineralization of Osteoblasts. Cells 2020, 9, 236. [Google Scholar] [CrossRef]
- Chen, C.-T.; Shih, Y.-R.V.; Kuo, T.K.; Lee, O.K.; Wei, Y.-H. Coordinated Changes of Mitochondrial Biogenesis and Antioxidant Enzymes during Osteogenic Differentiation of Human Mesenchymal Stem Cells. Stem Cells 2008, 26, 960–968. [Google Scholar] [CrossRef]
- Weivoda, M.M.; Chew, C.K.; Monroe, D.G.; Farr, J.N.; Atkinson, E.J.; Geske, J.R.; Eckhardt, B.; Thicke, B.; Ruan, M.; Tweed, A.J.; et al. Identification of osteoclast-osteoblast coupling factors in humans reveals links between bone and energy metabolism. Nat. Commun. 2020, 11, 87. [Google Scholar] [CrossRef]
- Shen, L.; Hu, G.; Karner, C.M. Bioenergetic Metabolism In Osteoblast Differentiation. Curr. Osteoporos. Rep. 2022, 20, 53–64. [Google Scholar] [CrossRef]
- Pal, S.; Singh, M.; Porwal, K.; Rajak, S.; Das, N.; Rajput, S.; Trivedi, A.K.; Maurya, R.; Sinha, R.A.; Siddiqi, M.I.; et al. Adiponectin receptors by increasing mitochondrial biogenesis and respiration promote osteoblast differentiation: Discovery of isovitexin as a new class of small molecule adiponectin receptor modulator with potential osteoanabolic function. Eur. J. Pharmacol. 2021, 913, 174634. [Google Scholar] [CrossRef]
- Bruedigam, C.; Eijken, M.; Koedam, M.; Pols, H.A.P.; van Leeuwen, J.P.T. New insights into peroxisome proliferatoractivated receptor gamma action: Stimulation of human osteoblast differentiation. Calcif. Tissue Int. 2007, 80, S73. [Google Scholar]
- Bruedigam, C.; Eijken, M.; Koedam, M.; van de Peppel, J.; Drabek, K.; Chiba, H.; van Leeuwen, J.P.T.M. A New Concept Underlying Stem Cell Lineage Skewing That Explains the Detrimental Effects of Thiazolidinediones on Bone. Stem Cells 2010, 28, 916–927. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G.; Lieben, L.; Watanabe, M.; Perino, A.; Auwerx, J.; Schoonjans, K.; Verstuyf, A. Vitamin D and energy homeostasis—Of mice and men. Nat. Rev. Endocrinol. 2013, 10, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Abu el Maaty, M.A.; Wölfl, S. Vitamin D as a Novel Regulator of Tumor Metabolism: Insights on Potential Mechanisms and Implications for Anti-Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 2184. [Google Scholar] [CrossRef] [PubMed]
- Sheeley, M.P.; Andolino, C.; Kiesel, V.A.; Teegarden, D. Vitamin D regulation of energy metabolism in cancer. Br. J. Pharmacol. 2021, 179, 2890–2905. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Schatz, A.; Adeyemi, B.; Kozminski, D.; Welsh, J.; Tenniswood, M.; Wang, W.-L.W. Vitamin D and testosterone co-ordinately modulate intracellular zinc levels and energy metabolism in prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2019, 189, 248–258. [Google Scholar] [CrossRef]
- Eelen, G.; Verlinden, L.; Meyer, M.B.; Gijsbers, R.; Pike, J.W.; Bouillon, R.; Verstuyf, A. 1,25-Dihydroxyvitamin D3 and the aging-related Forkhead Box O and Sestrin proteins in osteoblasts. J. Steroid Biochem. Mol. Biol. 2013, 136, 112–119. [Google Scholar] [CrossRef]
- Komarova, S.V.; Ataullakhanov, F.I.; Globus, R.K. Bioenergetics and mitochondrial transmembrane potential during differentiation of cultured osteoblasts. Am. J. Physiol. Cell. Physiol. 2000, 279, C1220–C1229. [Google Scholar] [CrossRef]
- Wu, Y.-Y.; Yu, T.; Zhang, X.-H.; Liu, Y.-S.; Li, F.; Wang, Y.-Y.; Wang, Y.-Y.; Gong, P. 1,25(OH)2D3 inhibits the deleterious effects induced by high glucose on osteoblasts through undercarboxylated osteocalcin and insulin signaling. J. Steroid Biochem. Mol. Biol. 2012, 132, 112–119. [Google Scholar] [CrossRef]
- Woeckel, V.J.; Bruedigam, C.; Koedam, M.; Chiba, H.; van der Eerden, B.C.; van Leeuwen, J.P. 1α,25-Dihydroxyvitamin D3 and rosiglitazone synergistically enhance osteoblast-mediated mineralization. Gene 2012, 512, 438–443. [Google Scholar] [CrossRef]
- Ali, S.Y.; Sajdera, S.W.; Anderson, H.C. Isolation and Characterization of Calcifying Matrix Vesicles from Epiphyseal Cartilage. Proc. Natl. Acad. Sci. USA 1970, 67, 1513–1520. [Google Scholar] [CrossRef]
- Anderson, H.C. Molecular biology of matrix vesicles. Clin. Orthop. Relat. Res. 1995, 314, 266–280. [Google Scholar] [CrossRef]
- Anderson, H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003, 5, 222–226. [Google Scholar] [CrossRef]
- Xiao, Z.; Camalier, C.E.; Nagashima, K.; Chan, K.C.; Lucas, D.A.; de la Cruz, M.J.; Gignac, M.; Lockett, S.; Issaq, H.J.; Veenstra, T.D.; et al. Analysis of the extracellular matrix vesicle proteome in mineralizing osteoblasts. J. Cell. Physiol. 2006, 210, 325–335. [Google Scholar] [CrossRef]
- Thouverey, C.; Malinowska, A.; Balcerzak, M.; Strzelecka-Kiliszek, A.; Buchet, R.; Dadlez, M.; Pikula, S. Proteomic characterization of biogenesis and functions of matrix vesicles released from mineralizing human osteoblast-like cells. J. Proteom. 2011, 74, 1123–1134. [Google Scholar] [CrossRef]
- Staines, K.A.; Zhu, D.; Farquharson, C.; MacRae, V.E. Identification of novel regulators of osteoblast matrix mineralization by time series transcriptional profiling. J. Bone Miner. Metab. 2013, 32, 240–251. [Google Scholar] [CrossRef]
- Tye, C.E.; Hunter, G.K.; Goldberg, H.A. Identification of the Type I Collagen-binding Domain of Bone Sialoprotein and Characterization of the Mechanism of Interaction. J. Biol. Chem. 2005, 280, 13487–13492. [Google Scholar] [CrossRef]
- Orimo, H. The Mechanism of Mineralization and the Role of Alkaline Phosphatase in Health and Disease. J. Nippon Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef]
- Kim, H.J.; Minashima, T.; McCarthy, E.F.; A Winkles, J.; Kirsch, T. Progressive ankylosis protein (ANK) in osteoblasts and osteoclasts controls bone formation and bone remodeling. J. Bone Miner. Res. 2010, 25, 1771–1783. [Google Scholar] [CrossRef]
- Millán, J.L. The Role of Phosphatases in the Initiation of Skeletal Mineralization. Calcif. Tissue Int. 2012, 93, 299–306. [Google Scholar] [CrossRef]
- Woeckel, V.J.; Alves, R.D.; Swagemakers, S.M.; Eijken, M.; Chiba, H.; van der Eerden, B.C.; van Leeuwen, J.P. 1α,25-(OH)2D3 acts in the early phase of osteoblast differentiation to enhance mineralization via accelerated production of mature matrix vesicles. J. Cell. Physiol. 2010, 225, 593–600. [Google Scholar] [CrossRef]
- Franceschi, R.T.; Romano, P.R.; Park, K.Y. Regulation of type I collagen synthesis by 1,25-dihydroxyvitamin D3 in human osteosarcoma cells. J. Biol. Chem. 1988, 263, 18938–18945. [Google Scholar] [CrossRef]
- Hicok, K.C.; Thomas, T.; Gori, F.; Rickard, D.J.; Spelsberg, T.C.; Riggs, B.L. Development and Characterization of Conditionally Immortalized Osteoblast Precursor Cell Lines from Human Bone Marrow Stroma. J. Bone Miner. Res. 1998, 13, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Ingram, R.T.; Bonde, S.K.; Riggs, B.L.; Fitzpatrick, L.A. Effects of transforming growth factor beta (TGFβ) and 1,25 dihydroxyvitamin D3 on the function, cytochemistry and morphology of normal human osteoblast-like cells. Differentiation 1994, 55, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Siggelkow, H.; Schulz, H.; Kaesler, S.; Benzler, K.; Atkinson, M.J.; Hüfner, M. 1,25 Dihydroxyvitamin-D3 Attenuates the Confluence-Dependent Differences in the Osteoblast Characteristic Proteins Alkaline Phosphatase, Procollagen I Peptide, and Osteocalcin. Calcif. Tissue Int. 1999, 64, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.T.; Chen, T.L. 1,25-Dihydroxyvitamin D3Interaction with Dexamethasone and Retinoic Acid: Effects on Procollagen Messenger Ribonucleic Acid Levels in Rat Osteoblast-Like Cells. Mol. Endocrinol. 1989, 3, 97–104. [Google Scholar] [CrossRef]
- Harrison, J.R.; Petersen, D.N.; Lichtler, A.C.; Mador, A.T.; Rowe, D.W.; Kream, B.E. 1,25-Dihydroxyvitamin D3Inhibits Transcription of Type I Collagen Genes in the Rat Osteosarcoma Cell Line ROS 17/2.8. Endocrinology 1989, 125, 327–333. [Google Scholar] [CrossRef]
- Yang, D.; Turner, A.G.; Wijenayaka, A.R.; Anderson, P.H.; Morris, H.A.; Atkins, G.J. 1,25-Dihydroxyvitamin D3 and extracellular calcium promote mineral deposition via NPP1 activity in a mature osteoblast cell line MLO-A5. Mol. Cell. Endocrinol. 2015, 412, 140–147. [Google Scholar] [CrossRef]
- Yajima, A.; Tsuchiya, K.; Burr, D.B.; Wallace, J.M.; Damrath, J.D.; Inaba, M.; Tominaga, Y.; Satoh, S.; Nakayama, T.; Tanizawa, T.; et al. The Importance of Biologically Active Vitamin D for Mineralization by Osteocytes After Parathyroidectomy for Renal Hyperparathyroidism. JBMR Plus 2019, 3, e10234. [Google Scholar] [CrossRef]
- Lieben, L.; Masuyama, R.; Torrekens, S.; Van Looveren, R.; Schrooten, J.; Baatsen, P.; Lafage-Proust, M.-H.; Dresselaers, T.; Feng, J.Q.; Bonewald, L.F.; et al. Normocalcemia is maintained in mice under conditions of calcium malabsorption by vitamin D–induced inhibition of bone mineralization. J. Clin. Investig. 2012, 122, 1803–1815. [Google Scholar] [CrossRef]
- Woeckel, V.J.; van der Eerden, B.C.; Schreuders-Koedam, M.; Eijken, M.; Van Leeuwen, J.P. 1α,25-dihydroxyvitamin D3stimulates activin A production to fine-tune osteoblast-induced mineralization. J. Cell. Physiol. 2013, 228, 2167–2174. [Google Scholar] [CrossRef]
- Kitazawa, S.; Kajimoto, K.; Kondo, T.; Kitazawa, R. Vitamin D3 supports osteoclastogenesis via functional vitamin D response element of human RANKL gene promoter. J. Cell. Biochem. 2003, 89, 771–777. [Google Scholar] [CrossRef]
- Shymanskyi, I.; Lisakovska, O.; Mazanova, A.; Labudzynskyi, D.; Veliky, M. Vitamin D3 Modulates Impaired Crosstalk Between RANK and Glucocorticoid Receptor Signaling in Bone Marrow Cells After Chronic Prednisolone Administration. Front. Endocrinol. 2018, 9, 303. [Google Scholar] [CrossRef]
- Khalaf, R.M.; Almudhi, A.A. The effect of vitamin D deficiency on the RANKL/OPG ratio in rats. J. Oral Biol. Craniofacial Res. 2022, 12, 228–232. [Google Scholar] [CrossRef]
- Kim, S.; Yamazaki, M.; Zella, L.A.; Shevde, N.K.; Pike, J.W. Activation of Receptor Activator of NF-κB Ligand Gene Expression by 1,25-Dihydroxyvitamin D3 Is Mediated through Multiple Long-Range Enhancers. Mol. Cell. Biol. 2006, 26, 6469–6486. [Google Scholar] [CrossRef]
- Bouillon, R.; Carmeliet, G. Vitamin D and the skeleton. Curr. Opin. Endocr. Metab. Res. 2018, 3, 68–73. [Google Scholar] [CrossRef]
- Carlberg, C.; Muñoz, A. An update on vitamin D signaling and cancer. Semin. Cancer Biol. 2020, 79, 217–230. [Google Scholar] [CrossRef]
- Carlberg, C. Vitamin D and Its Target Genes. Nutrients 2022, 14, 1354. [Google Scholar] [CrossRef]
- Haussler, M.R.; Livingston, S.; Sabir, Z.L.; Haussler, C.A.; Jurutka, P.W. Vitamin D Receptor Mediates a Myriad of Biological Actions Dependent on Its 1,25-Dihydroxyvitamin D Ligand: Distinct Regulatory Themes Revealed by Induction of Klotho and Fibroblast Growth Factor-23. JBMR Plus 2021, 5, e10432. [Google Scholar] [CrossRef]
- Haussler, M.R.; Whitfield, G.K.; Kaneko, I.; Haussler, C.A.; Hsieh, D.; Hsieh, J.-C.; Jurutka, P.W. Molecular Mechanisms of Vitamin D Action. Calcif. Tissue Int. 2013, 92, 77–98. [Google Scholar] [CrossRef]
- Owen, T.A.; Aronow, M.S.; Barone, L.M.; Bettencourt, B.; Stein, G.S.; Lian, J.B. Pleiotropic Effects of Vitamin D on Osteoblast Gene Expression Are Related to the Proliferative and Differentiated State of the Bone Cell Phenotype: Dependency upon Basal Levels of Gene Expression, Duration of Exposure, and Bone Matrix Competency in Normal Rat Osteoblast Cultures. Endocrinology 1991, 128, 1496–1504. [Google Scholar] [CrossRef]
- Saji, F.; Shigematsu, T.; Sakaguchi, T.; Ohya, M.; Orita, H.; Maeda, Y.; Ooura, M.; Mima, T.; Negi, S. Fibroblast growth factor 23 production in bone is directly regulated by 1α,25-dihydroxyvitamin D, but not PTH. Am. J. Physiol. Physiol. 2010, 299, F1212–F1217. [Google Scholar] [CrossRef]
- Yamamoto, R.; Minamizaki, T.; Yoshiko, Y.; Yoshioka, H.; Tanne, K.; Aubin, J.E.; Maeda, N. 1,25-dihydroxyvitamin D3 acts predominately in mature osteoblasts under conditions of high extracellular phosphate to increase fibroblast growth factor 23 production in vitro. J. Endocrinol. 2010, 206, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Quarles, L.D. Skeletal secretion of FGF-23 regulates phosphate and vitamin D metabolism. Nat. Rev. Endocrinol. 2012, 8, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Interactions between FGF23 and vitamin D. Endocr. Connect. 2022, 11, e220239. [Google Scholar] [CrossRef] [PubMed]
- Lanske, B.; Densmore, M.J.; Erben, R.G. Vitamin D endocrine system and osteocytes. BoneKEy Rep. 2014, 3, 494. [Google Scholar] [CrossRef]
- Hines, E.R.; Kolek, O.I.; Jones, M.D.; Serey, S.H.; Sirjani, N.B.; Kiela, P.R.; Jurutka, P.W.; Haussler, M.R.; Collins, J.F.; Ghishan, F.K. 1,25-Dihydroxyvitamin D3 Down-regulation of PHEX Gene Expression Is Mediated by Apparent Repression of a 110 kDa Transfactor That Binds to a Polyadenine Element in the Promoter. J. Biol. Chem. 2004, 279, 46406–46414. [Google Scholar] [CrossRef]
- Turner, R.T.; Puzas, J.E.; Forte, M.D.; Lester, G.E.; Gray, T.K.; Howard, G.A.; Baylink, D.J. In vitro synthesis of 1 alpha,25-dihydroxycholecalciferol and 24,25-dihydroxycholecalciferol by isolated calvarial cells. Proc. Natl. Acad. Sci. USA 1980, 77, 5720–5724. [Google Scholar] [CrossRef]
- Howard, G.A.; Turner, R.T.; Sherrard, D.J.; Baylink, D.J. Human bone cells in culture metabolize 25-hydroxyvitamin D3 to 1,25-dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3. J. Biol. Chem. 1981, 256, 7738–7740. [Google Scholar] [CrossRef]
- Geng, S.; Zhou, S.; Glowacki, J. Effects of 25-hydroxyvitamin D3 on proliferation and osteoblast differentiation of human marrow stromal cells require CYP27B1/1α-hydroxylase. J. Bone Miner. Res. 2011, 26, 1145–1153. [Google Scholar] [CrossRef]
- Lou, Y.-R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D3 induces osteogenic differentiation of human mesenchymal stem cells. Sci. Rep. 2017, 7, srep42816. [Google Scholar] [CrossRef]
- Geng, S.; Zhou, S.; Glowacki, J. Age-related decline in osteoblastogenesis and 1α-hydroxylase/CYP27B1 in human mesenchymal stem cells: Stimulation by parathyroid hormone. Aging Cell 2011, 10, 962–971. [Google Scholar] [CrossRef]
- Bikle, D.D.; Patzek, S.; Wang, Y. Physiologic and pathophysiologic roles of extra renal CYP27b1: Case report and review. Bone Rep. 2018, 8, 255–267. [Google Scholar] [CrossRef]
- Anderson, P.H.; Lam, N.N.; Turner, A.G.; Davey, R.A.; Kogawa, M.; Atkins, G.J.; Morris, H.A. The pleiotropic effects of vitamin D in bone. J. Steroid Biochem. Mol. Biol. 2013, 136, 190–194. [Google Scholar] [CrossRef]
- Hewison, M.; Zehnder, D.; Chakraverty, R.; Adams, J.S. Vitamin D and barrier function: A novel role for extra-renal 1α-hydroxylase. Mol. Cell. Endocrinol. 2004, 215, 31–38. [Google Scholar] [CrossRef]
- Yang, D.; Anderson, P.H.; Turner, A.G.; Morris, H.A.; Atkins, G.J. Comparison of the biological effects of exogenous and endogenous 1,25-dihydroxyvitamin D3 on the mature osteoblast cell line MLO-A5. J. Steroid Biochem. Mol. Biol. 2016, 164, 374–378. [Google Scholar] [CrossRef]
- Meyer, M.B.; Pike, J.W. Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of Cyp27b1 and Cyp24a1 expression. J. Steroid Biochem. Mol. Biol. 2020, 196, 105500. [Google Scholar] [CrossRef]
- Zhou, S.; LeBoff, M.S.; Glowacki, J. Vitamin D Metabolism and Action in Human Bone Marrow Stromal Cells. Endocrinology 2010, 151, 14–22. [Google Scholar] [CrossRef]
- St-Arnaud, R.; Jones, G. Chapter 6—CYP24A1: Structure, Function, and Physiological Role. In Vitamin D, 4th ed.; Feldman, D., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 81–95. [Google Scholar]
- Van Leeuwen, J.P.T.M.; Van Den Bemd, G.J.C.M.; Van Driel, M.; Buurman, C.J.; Pols, H.A.P. 24,25-Dihydroxyvitamin D3 and bone metabolism. Steroids 2001, 66, 375–380. [Google Scholar] [CrossRef]
- Väisänen, S.; Dunlop, T.W.; Sinkkonen, L.; Frank, C.; Carlberg, C. Spatio-temporal Activation of Chromatin on the Human CYP24 Gene Promoter in the Presence of 1α,25-Dihydroxyvitamin D3. J. Mol. Biol. 2005, 350, 65–77. [Google Scholar] [CrossRef]
- Henry, H.L. The 25(OH)D3/1α,25(OH)2D3-24R-hydroxylase: A catabolic or biosynthetic enzyme? Steroids 2001, 66, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Moena, D.; Nardocci, G.; Acevedo, E.; Lian, J.; Stein, G.; Stein, J.; Montecino, M. Ezh2-dependent H3K27me3 modification dynamically regulates vitamin D3-dependent epigenetic control of CYP24A1 gene expression in osteoblastic cells. J. Cell. Physiol. 2020, 235, 5404–5412. [Google Scholar] [CrossRef]
- Pols, H.A.; Birkenhager, J.C.; Schilte, J.P.; Visser, T.J. Evidence that the self-induced metabolism of 1,25-dihydroxyvitamin D-3 limits the homologous up-regulation of its receptor in rat osteosarcoma cells. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 1988, 970, 122–129. [Google Scholar] [CrossRef]
- Staal, A.; vandenBemd, G.; Birkenhager, J.; Pols, H.; van Leeuwen, J. Consequences of vitamin D receptor regulation for the 1,25-dihydroxyvitamin D3-induced 24-hydroxylase activity in osteoblast-like cells: Initiation of the C24-oxidation pathway. Bone 1997, 20, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Henry, H.L.; Norman, A.W. Vitamin D: Two Dihydroxylated Metabolites Are Required for Normal Chicken Egg Hatchability. Science 1978, 201, 835–837. [Google Scholar] [CrossRef]
- Norman, A.W.; Henry, H.L.; Malluche, H.H. 24R,25-dihydroxyvitamin D3 and 1α,25-dihydroxyvitamin D3 are both indispensable for calcium and phosphorus homeostasis. Life Sci. 1980, 27, 229–237. [Google Scholar] [CrossRef]
- Endo, H.; Kiyoki, M.; Kawashima, K.; Naruchi, T.; Hashimoto, Y. Vitamin D3 metabolites and PTH synergistically stimulate bone formation of chick embryonic femur in vitro. Nature 1980, 286, 262–264. [Google Scholar] [CrossRef]
- Galus, K.; Szymendera, J.; Zaleski, A.; Schreyer, K. Effects of 1α-hydroxyvitamin D3 and 24R,25-dihydroxyvitamin D3 on bone remodeling. Calcif. Tissue Int. 1980, 31, 209–213. [Google Scholar] [CrossRef]
- Erben, R.G.; Weiser, H.; Sinowatz, F.; Rambeck, W.A.; Zucker, H. Vitamin D metabolites prevent vertebral osteopenia in ovariectomized rats. Calcif. Tissue Int. 1992, 50, 228–236. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ezawa, I.; Morita, K.; Kawanobe, Y.; Ogata, E. Effect of Vitamin D Metabolites on Bone Metabolism in a Rat Model of Postmenopausal Osteoporosis. J. Nutr. Sci. Vitaminol. 1985, 31, S61–S65. [Google Scholar] [CrossRef]
- Kato, A.; Seo, E.G.; Einhorn, T.A.; Bishop, J.E.; Norman, A.W. Studies on 24R,25-dihydroxyvitamin D3: Evidence for a nonnuclear membrane receptor in the chick tibial fracture-healing callus. Bone 1998, 23, 141–146. [Google Scholar] [CrossRef]
- Seo, E.-G.; Einhorn, T.A.; Norman, A.W. 24R,25-Dihydroxyvitamin D3: An Essential Vitamin D3 Metabolite for Both Normal Bone Integrity and Healing of Tibial Fracture in Chicks. Endocrinology 1997, 138, 3864–3872. [Google Scholar] [CrossRef]
- Martineau, C.; Kaufmann, M.; Arabian, A.; Jones, G.; St-Arnaud, R. Preclinical safety and efficacy of 24R,25-dihydroxyvitamin D3 or lactosylceramide treatment to enhance fracture repair. J. Orthop. Transl. 2020, 23, 77–88. [Google Scholar] [CrossRef] [PubMed]
- St-Arnaud, R. CYP24A1-deficient mice as a tool to uncover a biological activity for vitamin D metabolites hydroxylated at position 24. J. Steroid Biochem. Mol. Biol. 2010, 121, 254–256. [Google Scholar] [CrossRef]
- Weisman, Y.; Salama, R.; Harell, A.; Edelstein, S. Serum 24,25-dihydroxyvitamin D and 25-hydroxyvitamin D concentrations in femoral neck fracture. BMJ 1978, 2, 1196–1197. [Google Scholar] [CrossRef]
- Birkenhager-Frenkel, D.H.; Pols, H.A.; Zeelenberg, J.; Eijgelsheim, J.J.; Schot, R.; Nigg, A.L.; Weimar, W.; Mulder, P.G.; Birkenhager, J.C. Effects of 24r,25-dihydroxyvitamin D3 in combination with 1α-hydroxyvitamin D3 in predialysis renal insufficiency: Biochemistry and histomorphometry of cancellous bone. J. Bone Miner. Res. 1995, 10, 197–204. [Google Scholar] [CrossRef]
| Condition | # of Genes UP | # of Genes DOWN |
|---|---|---|
| Pre-mineralization phase | 155 | 164 |
| Mineralization phase | 166 | 236 |
| In both phases | 10 | 8 |
| Upregulated | Downregulated |
|---|---|
| ABCC3 | AGAP10 |
| CYP24A1 | CCL20 |
| MAGEE1 | DDIT3 |
| RARRES2 | GRK4 |
| RICH2 | LOC727869 |
| SLC25A45 | NFE2L2 |
| SULT1C2 | ODF1 |
| THBD | TSC22D2 |
| TMEM180 | |
| TOX3 |
| Condition | # of Genes UP | # of Genes DOWN |
|---|---|---|
| Pre-mineralization phase | 65 | 66 |
| Mineralization phase | 77 | 100 |
| Pre-Mineralization Phase | Mineralization Phase | ||||||
|---|---|---|---|---|---|---|---|
| Upregulated | Downregulated | Upregulated | Downregulated | ||||
| AQR | RAB9BP1 | ADAM22 | RARA | ABCD4 | MYH11 | AASDH | MOSPD1 |
| ARHGEF7 | RLTPR | ADORA1 | RBM | AKAP13 | NFIX | ABCD3 | MRPS23 |
| ATAT1 | SARDH | ATF7IP2 | RIMKLB | ANKRD11 | ORC5L | ABT1 | MS4A1 |
| ATG16L1 | SHISA8 | BAGE | SLC19A1 | APIP | PCDHB3 | ACTR3C | MTUS2 |
| ATP1A4 | SLC38A11 | BRS3 | SLC26A7 | ARHGDIB | PDLIM5 | ANUBL1 | NCRNA00188 |
| BCL11A | SZT2 | BRWD1 | SLC3A1 | ASH1L | PDZRN4 | AP5S1 | NDRG2 |
| BMF | TEX9 | BST2 | SNRPN | ATM | PGAP1 | B4GALNT2 | NDUFB7 |
| BMP15 | TMEM120B | C1orf68 | TBK1 | BNC2 | PLEKHG2 | C11orf65 | NRAP |
| C15orf48 | TMEM33 | CACNA1A | TFAP4 | BPTF | PPP4R4 | C14orf156 | NUDT14 |
| C2orf27A | UBE2G2 | CCDC144C | THPO | BRD4 | PRPF18 | C14orf2 | OGFR |
| C3orf20 | UBXN10 | CSF2RA | TMPRSS15 | CAP1 | PTGES | C17orf104 | PANK2 |
| C8orf34 | UNC13C | CTNS | TRIB3 | CCDC67 | PTGS1 | C4orf36 | PAPPA |
| CCDC124 | ZC3H12A-DT | DEFB132 | TRMT2A | CCDC76 | RAB3IP | CCL5 | PAX8 |
| COL24A1 | ZNF668 | EDA | TTBK2 | CD14 | RASAL2 | CCT2 | PIP5K1A |
| CTU2 | ZNF703 | ERCC6L2 | ZNF396 | CLCN4 | RG9MTD2 | CNOT2 | PLCH1 |
| DCTN2 | FAM219A | ZNF93 | CROCCL1 | SERTAD4 | COX7C | PMCH | |
| DOCK6 | FCGR2C | DCLK3 | SMARCA4 | CSRP2BP | PML | ||
| DST | FLJ10213 | DPP4 | SRGAP1 | DAZL | POLE4 | ||
| DUSP28 | FSD1L | EGFR | SRRM2 | DBI | POLR2K | ||
| EPG5 | GAS2 | EP300 | SULF1 | DCUN1D1 | PTPRA | ||
| EYA2 | GLIPR1 | FAM102A | TBC1D13 | DNAH1 | RHEB | ||
| GABRB3 | GPR155 | FAM186A | TBL1X | DUSP16 | RPAIN | ||
| GNRHR | HM13 | FAM20C | UGGT2 | EEF1D | RPL13 | ||
| HCRTR2 | ICA1 | FGF7 | VCAN | EGFL8 | RPL14 | ||
| HIST1H4C | KLHL36 | FLJ11292 | ZNF397 | EHD1 | RPL34 | ||
| HSPB7 | KLK7 | FLJ13773 | ZNF462 | ELP6 | RPS11 | ||
| IL1RN | LEKR1 | FOXP2 | ZSWIM1 | ESPNL | SEMA6D | ||
| KCNJ15 | LELP1 | GABRA5 | EXOG | SHLD1 | |||
| LOC100131283 | LIN28B | H2AFY | EXOSC2 | SLC10A7 | |||
| LOC148987 | LOC100286895 | HMCN1 | FABP4 | SLC9A5 | |||
| LOC149351 | LOC100287114 | HOXA6 | FAM126A | SNAP23 | |||
| LOC285205 | LOC283854 | HSPA12A | FAM27A | SNCAIP | |||
| LOC645591 | LOC285692 | IL17C | FAXC | SNTG1 | |||
| LOC728903 | LOC390595 | INTS4 | FUT7 | STEEP1 | |||
| LOC780529 | LOC440944 | KCNAB1 | GOSR1 | STK32A | |||
| LRRC46 | MAN1A2 | KCNG3 | GPR39 | STMN3 | |||
| LYZL6 | MAPRE3 | KRTAP3-3 | GSN | SUPT16H | |||
| MGC42157 | MGC12916 | LOC100127980 | HCG4P6 | TAL1 | |||
| MRS2 | MRPL19 | LOC100128640 | IRGQ | TBC1D8 | |||
| NCOR2 | MSR1 | LOC100131993 | KCNIP3 | TEN1 | |||
| NOX4 | MYO10 | LOC283682 | KY | TLK1 | |||
| NTRK2 | NR2E3 | LOC285500 | LOC100133109 | TWF1 | |||
| OR1J4 | NUP210L | LOC388210 | LOC100287911 | TXNIP | |||
| PDE1A | OTX2 | LOC441461 | LOC100289246 | UHRF1BP1L | |||
| PENK | PCLO | MAGEB18 | LOC338862 | UQCRB | |||
| PGM2L1 | PKP2 | MARK2 | LOC643749 | UQCRQ | |||
| PHC3 | PLXNA2 | MEGF10 | LPAR5 | VMA21 | |||
| POU2F1 | POU2F2 | MGAT5B | MATR3 | WFDC21P | |||
| PRRG2 | PRLR | MLXIP | MMP16 | XAF1 | |||
| PTCD3 | RAD54L2 | MS4A6A | MMP17 | ZNF880 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Driel, M.; van Leeuwen, J.P.T.M. Vitamin D and Bone: A Story of Endocrine and Auto/Paracrine Action in Osteoblasts. Nutrients 2023, 15, 480. https://doi.org/10.3390/nu15030480
van Driel M, van Leeuwen JPTM. Vitamin D and Bone: A Story of Endocrine and Auto/Paracrine Action in Osteoblasts. Nutrients. 2023; 15(3):480. https://doi.org/10.3390/nu15030480
Chicago/Turabian Stylevan Driel, Marjolein, and Johannes P. T. M. van Leeuwen. 2023. "Vitamin D and Bone: A Story of Endocrine and Auto/Paracrine Action in Osteoblasts" Nutrients 15, no. 3: 480. https://doi.org/10.3390/nu15030480
APA Stylevan Driel, M., & van Leeuwen, J. P. T. M. (2023). Vitamin D and Bone: A Story of Endocrine and Auto/Paracrine Action in Osteoblasts. Nutrients, 15(3), 480. https://doi.org/10.3390/nu15030480






