Association between Dietary Intake and Faecal Microbiota in Children with Cystic Fibrosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Nutritional Data Collection and Processing
2.3. Analysis of the Microbiota and Its Metabolic Activity
2.3.1. Microbiota Composition by 16S rRNA Amplicon Gene Sequencing
2.3.2. Short-Chain Fatty Acids (SCFA)
2.4. Statistical Analyses
3. Results
3.1. Clinical Characteristics of the Subjects
3.2. Dietary Assessment
3.3. Faecal Microbiota
3.4. Short-Chain Fatty Acids (SCFA)
3.5. Correlations between Dietary Components and Faecal Microbiota
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mariotti Zani, E.; Grandinetti, R.; Cunico, D.; Torelli, L.; Fainardi, V.; Pisi, G.; Esposito, S. Nutritional Care in Children with Cystic Fibrosis. Nutrients 2023, 15, 479. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Schwarzenberg, S.J. Pancreatic insufficiency in Cystic Fibrosis. J. Cyst. Fibros. 2017, 16 (Suppl. S2), S70–S78. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.; Katz, T.; Liu, V.; Quintano, J.; Brunner, R.; Tong, C.W.; Collins, C.E.; Ooi, C.Y. Dietary intake of energy-dense, nutrient-poor and nutrient-dense food sources in children with cystic fibrosis. J. Cyst. Fibros. 2018, 17, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Bonhoure, A.; Boudreau, V.; Litvin, M.; Colomba, J.; Bergeron, C.; Mailhot, M.; Tremblay, F.; Lavoie, A.; Rabasa-Lhoret, R. Overweight, obesity and significant weight gain in adult patients with cystic fibrosis association with lung function and cardiometabolic risk factors. Clin. Nutr. 2020, 39, 2910–2916. [Google Scholar] [CrossRef] [PubMed]
- Mckay, I.; Van Dorst, J.; Katz, T.; Doumit, M.; Prentice, B.; Owens, L.; Belessis, Y.; Chuang, S.; Jaffe, A.; Thomas, T.; et al. Diet and the gut-lung axis in cystic fibrosis-direct & indirect links. Gut Microbes 2023, 15, 2156254. [Google Scholar] [CrossRef] [PubMed]
- Habib, A.R.R.; Kajbafzadeh, M.; Desai, S.; Yang, C.L.; Skolnik, K.; Quon, B.S. A Systematic Review of the Clinical Efficacy and Safety of CFTR Modulators in Cystic Fibrosis. Sci. Rep. 2019, 9, 7234. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Rozga, M.; McDonald, C.M.; Bowser, E.K.; Farnham, K.; Mangus, M.; Padula, L.; Porco, K.; Alvarez, J.A. Effect of CFTR Modulators on Anthropometric Parameters in Individuals with Cystic Fibrosis: An Evidence Analysis Center Systematic Review. J. Acad. Nutr. Diet. 2021, 121, 1364–1378.e2. [Google Scholar] [CrossRef]
- Caley, L.R.; White, H.; de Goffau, M.C.; Floto, R.A.; Parkhill, J.; Marsland, B.; Peckham, D.G. Cystic Fibrosis-Related Gut Dysbiosis: A Systematic Review. Dig. Dis. Sci. 2023, 68, 1797–1814. [Google Scholar] [CrossRef]
- Thavamani, A.; Salem, I.; Sferra, T.J.; Sankararaman, S. Impact of Altered Gut Microbiota and Its Metabolites in Cystic Fibrosis. Metabolites 2021, 11, 123. [Google Scholar] [CrossRef]
- Burke, D.G.; Fouhy, F.; Harrison, M.J.; Rea, M.C.; Cotter, P.D.; O’Sullivan, O.; Stanton, C.; Hill, C.; Shanahan, F.; Plant, B.J.; et al. The altered gut microbiota in adults with cystic fibrosis. BMC Microbiol. 2017, 17, 58. [Google Scholar] [CrossRef]
- Coffey, M.J.; Nielsen, S.; Wemheuer, B.; Kaakoush, N.O.; Garg, M.; Needham, B.; Pickford, R.; Jaffe, A.; Thomas, T.; Ooi, C.Y. Gut Microbiota in Children with Cystic Fibrosis: A Taxonomic and Functional Dysbiosis. Sci. Rep. 2019, 9, 18593. [Google Scholar] [CrossRef] [PubMed]
- Manor, O.; Levy, R.; Pope, C.E.; Hayden, H.S.; Brittnacher, M.J.; Carr, R.; Radey, M.C.; Hager, K.R.; Heltshe, S.L.; Ramsey, B.W.; et al. Metagenomic evidence for taxonomic dysbiosis and functional imbalance in the gastrointestinal tracts of children with cystic fibrosis. Sci. Rep. 2016, 6, 22493. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.R.; Pope, C.E.; Hayden, H.S.; Heltshe, S.; Levy, R.; McNamara, S.; Jacobs, M.A.; Rohmer, L.; Radey, M.; Ramsey, B.W.; et al. Escherichia coli Dysbiosis Correlates with Gastrointestinal Dysfunction in Children with Cystic Fibrosis. Clin. Infect. Dis. 2014, 58, 396–399. [Google Scholar] [CrossRef] [PubMed]
- Antosca, K.M.; Chernikova, D.A.; Price, C.E.; Ruoff, K.L.; Li, K.; Guill, M.F.; Sontag, N.R.; Morrison, H.G.; Hao, S.; Drumm, M.L.; et al. Altered stool microbiota of infants with cystic fibrosis shows a reduction in genera associated with immune programming from birth. J. Bacteriol. 2019, 201, 16. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.I.; de Winter-De Groot, K.M.; Berkers, G.; Chu, M.L.J.N.; Arp, K.; Ghijsen, S.; Heijerman, H.G.M.; Arets, H.G.M.; Majoor, C.J.; Janssens, H.M.; et al. Development of the gut microbiota in early life: The impact of cystic fibrosis and antibiotic treatment. J. Cyst. Fibros. 2020, 19, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Miragoli, F.; Federici, S.; Ferrari, S.; Minuti, A.; Rebecchi, A.; Bruzzese, E.; Buccigrossi, V.; Guarino, A.; Callegari, M.L. Impact of cystic fibrosis disease on archaea and bacteria composition of gut microbiota. FEMS Microbiol. Ecol. 2017, 93, 230. [Google Scholar] [CrossRef] [PubMed]
- Ooi, C.Y.; Syed, S.A.; Rossi, L.; Garg, M.; Needham, B.; Avolio, J.; Young, K.; Surette, M.G.; Gonska, T. Impact of CFTR modulation with Ivacaftor on Gut Microbiota and Intestinal Inflammation. Sci. Rep. 2018, 8, 17834. [Google Scholar] [CrossRef] [PubMed]
- del Campo, R.; Garriga, M.; Pérez-Aragón, A.; Guallarte, P.; Lamas, A.; Máiz, L.; Bayón, C.; Roy, G.; Cantón, R.; Zamora, J.; et al. Improvement of digestive health and reduction in proteobacterial populations in the gut microbiota of cystic fibrosis patients using a Lactobacillus reuteri probiotic preparation: A double blind prospective study. J. Cyst. Fibros. 2014, 13, 716–722. [Google Scholar] [CrossRef]
- Sutherland, R.E.; Collins, C.; Brunner, R.; Ooi, C.Y.; Katz, T.E. 316 An historical perspective of dietary intake studies in children with CF. J. Cyst. Fibros. 2017, 16, S143. [Google Scholar] [CrossRef]
- He, Y.; Wang, B.; Wen, L.; Wang, F.; Yu, H.; Chen, D.; Su, X.; Zhang, C. Effects of dietary fiber on human health. Food Sci. Hum. Wellness 2022, 11, 1–10. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Hulst, J.; Boon, M.; Martins, T.; Ruperto, M.; Colombo, C.; Fornés-Ferrer, V.; Woodcock, S.; Claes, I.; Asseiceira, I.; et al. The Relative Contribution of Food Groups to Macronutrient Intake in Children with Cystic Fibrosis: A European Multicenter Assessment. J. Acad. Nutr. Diet. 2019, 119, 1305–1319. [Google Scholar] [CrossRef] [PubMed]
- Marlett, J.A.; Cheung, T.F. Database and quick methods of assessing typical dietary fiber intakes using data for 228 commonly consumed foods. J. Am. Diet. Assoc. 1997, 97, 1139–1151. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: The R Project for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 17 September 2023).
- McLaren, M.R.; Callahan, B.J. Silva 138.1 Prokaryotic SSU Taxonomic Training Data Formatted for DADA2; Zenodo: Geneva, Switzerland, 2021. [Google Scholar] [CrossRef]
- PMcMurdie, J.; Holmes, S. phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Adorno, M.A.T.; Hirasawa, J.S.; Varesche, M.B.A.; Adorno, M.A.T.; Hirasawa, J.S.; Varesche, M.B.A. Development and Validation of Two Methods to Quantify Volatile Acids (C2–C6) by GC/FID: Headspace (Automatic and Manual) and Liquid-Liquid Extraction (LLE). Am. J. Analyt. Chem. 2014, 5, 406–414. [Google Scholar] [CrossRef]
- Zou, H.; Hastie, T. Regularization and Variable Selection Via the Elastic Net. J. R. Stat. Soc. Ser. B Stat. Methodol. 2005, 67, 301–320. [Google Scholar] [CrossRef]
- Friedman, J.; Hastie, T.; Tibshirani, R. Regularization Paths for Generalized Linear Models via Coordinate Descent. J. Stat. Softw. 2010, 33, 1–22. [Google Scholar] [CrossRef]
- Turck, D.; Braegger, C.P.; Colombo, C.; Declercq, D.; Morton, A.; Pancheva, R.; Robberecht, E.; Stern, M.; Strandvik, B.; Wolfe, S.; et al. ESPEN-ESPGHAN-ECFS guidelines on nutrition care for infants, children, and adults with cystic fibrosis. Clin. Nutr. 2016, 35, 557–577. [Google Scholar] [CrossRef]
- FAO. Population Nutrient Intake Goals for Preventing Diet-Related Chronic Diseases. Available online: https://www.fao.org/3/AC911E/ac911e07.htm (accessed on 26 June 2023).
- WHO. WHO Updates Guidelines on Fats and Carbohydrates. Available online: https://www.who.int/news/item/17-07-2023-who-updates-guidelines-on-fats-and-carbohydrates (accessed on 3 November 2023).
- Calvo-Lerma, J.; Boon, M.; Hulst, J.; Colombo, C.; Asseiceira, I.; Garriga, M.; Masip, E.; Claes, I.; Bulfamante, A.; Janssens, H.M.; et al. Change in nutrient and dietary intake in european children with cystic fibrosis after a 6-month intervention with a self-management mhealth tool. Nutrients 2021, 13, 1801. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.A.; Xie, C.; Garcia, A.L. Dietary fibre and health in children and adolescents. Proc. Nutr. Soc. 2015, 74, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.; Overcash, F.; Belobrajdic, D.; Slavin, J. Perspective: Utilizing High Amylose Wheat Flour to Increase Dietary Fiber Intake of Children and Adolescents: A Health by Stealth Approach. Front. Public Health 2022, 10, 817967. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shao, D.; Han, C.; Huang, Q.; Zhao, W. Response of human gut microbiota under simulated microgravity. Appl. Microbiol. Biotechnol. 2022, 106, 5221–5231. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Del Chierico, F.; Russo, A.; Majo, F.; Rossitto, M.; Valerio, M.; Casadei, L.; La Storia, A.; De Filippis, F.; Rizzo, C.; et al. Gut microbiota signatures in cystic fibrosis: Loss of host CFTR function drives the microbiota enterophenotype. PLoS ONE 2018, 13, e0208171. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 2012, 57, 2096–2102. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.I.; de Winter-De Groot, K.M.; Berkers, G.; Chu, M.L.J.N.; Arp, K.; Ghijsen, S.; Heijerman, H.G.M.; Arets, H.G.M.; Majoor, C.J.; Janssens, H.M.; et al. Individual and group response of treatment with ivacaftor on airway and gut microbiota in people with cf and a s1251n mutation. J. Pers. Med. 2021, 11, 350. [Google Scholar] [CrossRef]
- Ooi, C.Y.; Durie, P.R. Cystic fibrosis from the gastroenterologist’s perspective. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 175–185. [Google Scholar] [CrossRef]
- Price, C.E.; O’Toole, G.A. The Gut-Lung Axis in Cystic Fibrosis. J. Bacteriol. 2021, 203, e0031121. [Google Scholar] [CrossRef]
- Bolte, L.A.; Vich Vila, A.; Imhann, F.; Collij, V.; Gacesa, R.; Peters, V.; Wijmenga, C.; Kurilshikov, A.; Campmans-Kuijpers, M.J.E.; Fu, J.; et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features of the gut microbiome. Gut 2021, 70, 1287–1298. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2013, 505, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Duncan, S.H.; Belenguer, A.; Holtrop, G.; Johnstone, A.M.; Flint, H.J.; Lobley, G.E. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl. Environ. Microbiol. 2007, 73, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Salonen, A.; Lahti, L.; Salojärvi, J.; Holtrop, G.; Korpela, K.; Duncan, S.H.; Date, P.; Farquharson, F.; Johnstone, A.M.; Lobley, G.E.; et al. Impact of diet and individual variation on intestinal microbiota composition and fermentation products in obese men. ISME J. 2014, 8, 2218–2230. [Google Scholar] [CrossRef] [PubMed]
- Blachier, F.; Beaumont, M.; Portune, K.J.; Steuer, N.; Lan, A.; Audebert, M.; Khodorova, N.; Andriamihaja, M.; Airinei, G.; Benamouzig, R.; et al. High-protein diets for weight management: Interactions with the intestinal microbiota and consequences for gut health. A position paper by the my new gut study group. Clin. Nutr. 2019, 38, 1012–1022. [Google Scholar] [CrossRef] [PubMed]
- Murphy, E.A.; Velazquez, K.T.; Herbert, K.M. Influence of high-fat diet on gut microbiota: A driving force for chronic disease risk. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 515–520. [Google Scholar] [CrossRef]
- Wang, Y.; Leong, L.E.X.; Keating, R.L.; Kanno, T.; Abell, G.C.J.; Mobegi, F.M.; Choo, J.M.; Wesselingh, S.L.; Mason, A.J.; Burr, L.D.; et al. Opportunistic bacteria confer the ability to ferment prebiotic starch in the adult cystic fibrosis gut. Gut Microbes 2019, 10, 367–381. [Google Scholar] [CrossRef]
- Bello-Perez, L.A.; Flores-Silva, P.C.; Agama-Acevedo, E.; Tovar, J. Starch digestibility: Past, present, and future. J. Sci. Food Agric. 2020, 100, 5009–5016. [Google Scholar] [CrossRef]
- Villarroel, P.; Gómez, C.; Vera, C.; Torres, J.; Villarroel, P.; Gómez, C.; Vera, C.; Torres, J. Almidón resistente: Características tecnológicas e intereses fisiológicos. Rev. Chil. Nutr. 2018, 45, 271–278. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Wong, J.M.W.; De Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Li, L.; Somerset, S. Digestive system dysfunction in cystic fibrosis: Challenges for nutrition therapy. Dig. Liver Dis. 2014, 46, 865–874. [Google Scholar] [CrossRef] [PubMed]
- Araújo, J.R.; Tomas, J.; Brenner, C.; Sansonetti, P.J. Impact of high-fat diet on the intestinal microbiota and small intestinal physiology before and after the onset of obesity. Biochimie 2017, 141, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.A.; Hoffmann, C.; Sherrill-Mix, S.A.; Keilbaugh, S.A.; Hamady, M.; Chen, Y.Y.; Knight, R.; Ahima, R.S.; Bushman, F.; Wu, G.D. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology 2009, 137, 1716–1724.e2. [Google Scholar] [CrossRef] [PubMed]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- De Weirdt, R.; Possemiers, S.; Vermeulen, G.; Moerdijk-Poortvliet, T.C.W.; Boschker, H.T.S.; Verstraete, W.; Van De Wiele, T. Human faecal microbiota display variable patterns of glycerol metabolism. FEMS Microbiol. Ecol. 2010, 74, 601–611. [Google Scholar] [CrossRef]
- Larriba, R.; Roca, M.; Masip, E.; Cañada-Martínez, A.; Ribes-Koninckx, C.; Calvo-Lerma, J. How macronutrients and pancreatic enzyme supplements dose variability affect fat, protein and starch absorption in children with cystic fibrosis. Dig. Liver Dis. 2023, 55, 513–518. [Google Scholar] [CrossRef]
- Tamargo, A.; Martin, D.; del Hierro, J.N.; Moreno-Arribas, M.V.; Muñoz, L.A. Intake of soluble fibre from chia seed reduces bioaccessibility of lipids, cholesterol and glucose in the dynamic gastrointestinal model simgi®. Food Res. Int. 2020, 137, 109364. [Google Scholar] [CrossRef]
- Jovel, J.; Patterson, J.; Wang, W.; Hotte, N.; O’Keefe, S.; Mitchel, T.; Perry, T.; Kao, D.; Mason, A.L.; Madsen, K.L.; et al. Characterization of the gut microbiome using 16S or shotgun metagenomics. Front. Microbiol. 2016, 7, 180723. [Google Scholar] [CrossRef]



| Name of the Group | Examples |
|---|---|
| Milk and dairy | Yoghurt, milk, cheese, etc. |
| Sugar-added dairy | Sweetened yoghurt, milkshakes, milk-based desserts, etc. |
| Fruit | All fresh fruit, dried fruit |
| Vegetables | All fresh vegetables |
| Legumes | All legumes |
| Nuts | All nuts |
| Whole-grain cereals | Bread, rice, pasta, etc. |
| Refined cereals | Bread, rice, pasta, boiled potato, etc. |
| Snacks and sweets | Chocolate, candies, chips, cocoa powder, sweeteners, others, for example, ultraprocessed foods |
| Meat | Fresh beef, chicken, pork, rabbit, etc. |
| Cold meats | Sausages, hamburgers, nuggets, ham, etc. |
| Fish | Seafood, white fish, blue fish, canned fish, etc. |
| Eggs | All eggs |
| Oils | Olive oil |
| Butter/margarine | Butter and margarine |
| Pastries | Regular cookies, chocolate cookies, breakfast cereals, doughnuts, cupcakes, etc. |
| Age (years) (mean (SD)) | 10.8 (4.9) |
| Sex (m/f) | 24/19 |
| Height (z-score) (mean (SD)) | −0.4 (1.6) |
| Weight (z-score) (mean (SD)) | −0.2 (0.9) |
| BMI (z-score) (mean (SD)) | −0.1 (1.1) |
| FEV1 (%)(mean (SD)) | 89.8 (20.0) |
| Pancreatic insufficiency (n) | 43 |
| PERT dose (LU/day*kg) (mean (SD)) | 10,145.6 (18,827.6) |
| CFTR modulator therapy (n) | 5 |
| Q1 | Median | Q3 | ||
|---|---|---|---|---|
| Energy (%) | Daily intake | 106.6 | 126.7 | 178.5 |
| Food groups (g/day) | Milk and dairy | 119.3 | 212.5 | 439.5 |
| Sugar-added dairy | 0.0 | 60.0 | 182.0 | |
| Fruit | 0.5 | 67.5 | 131.7 | |
| Vegetables | 28.3 | 52.5 | 120.3 | |
| Legumes | 0.0 | 10.0 | 49.3 | |
| Nuts | 0.0 | 0.0 | 0.0 | |
| Whole-grain cereals | 0.0 | 0.0 | 0.0 | |
| Refined cereals | 115.9 | 174.4 | 237.1 | |
| Snacks and sweets | 20.3 | 60.0 | 139.2 | |
| Meat | 44.0 | 83.3 | 135.0 | |
| Cold meats | 20.5 | 40.0 | 74.4 | |
| Fish | 0.0 | 26.7 | 50.0 | |
| Eggs | 0.0 | 0.0 | 13.9 | |
| Oil | 1.4 | 4.7 | 17.5 | |
| Butter/Margarine | 0.0 | 0.0 | 0.0 | |
| Pastries | 15.2 | 48.8 | 63.8 | |
| Macronutrients (% from daily energy intake) | Carbohydrates | 32.6 | 35.8 | 43.0 |
| Simple carbohydrates | 9.6 | 14.1 | 18.4 | |
| Complex carbohydrates | 15.5 | 19.6 | 22.8 | |
| Lipids | 40.7 | 43.9 | 50.9 | |
| SFA | 12.2 | 13.9 | 16.9 | |
| MUFA | 15.7 | 17.8 | 23.8 | |
| PUFA | 4.9 | 7.1 | 8.8 | |
| Protein | 10.8 | 14.5 | 16.6 | |
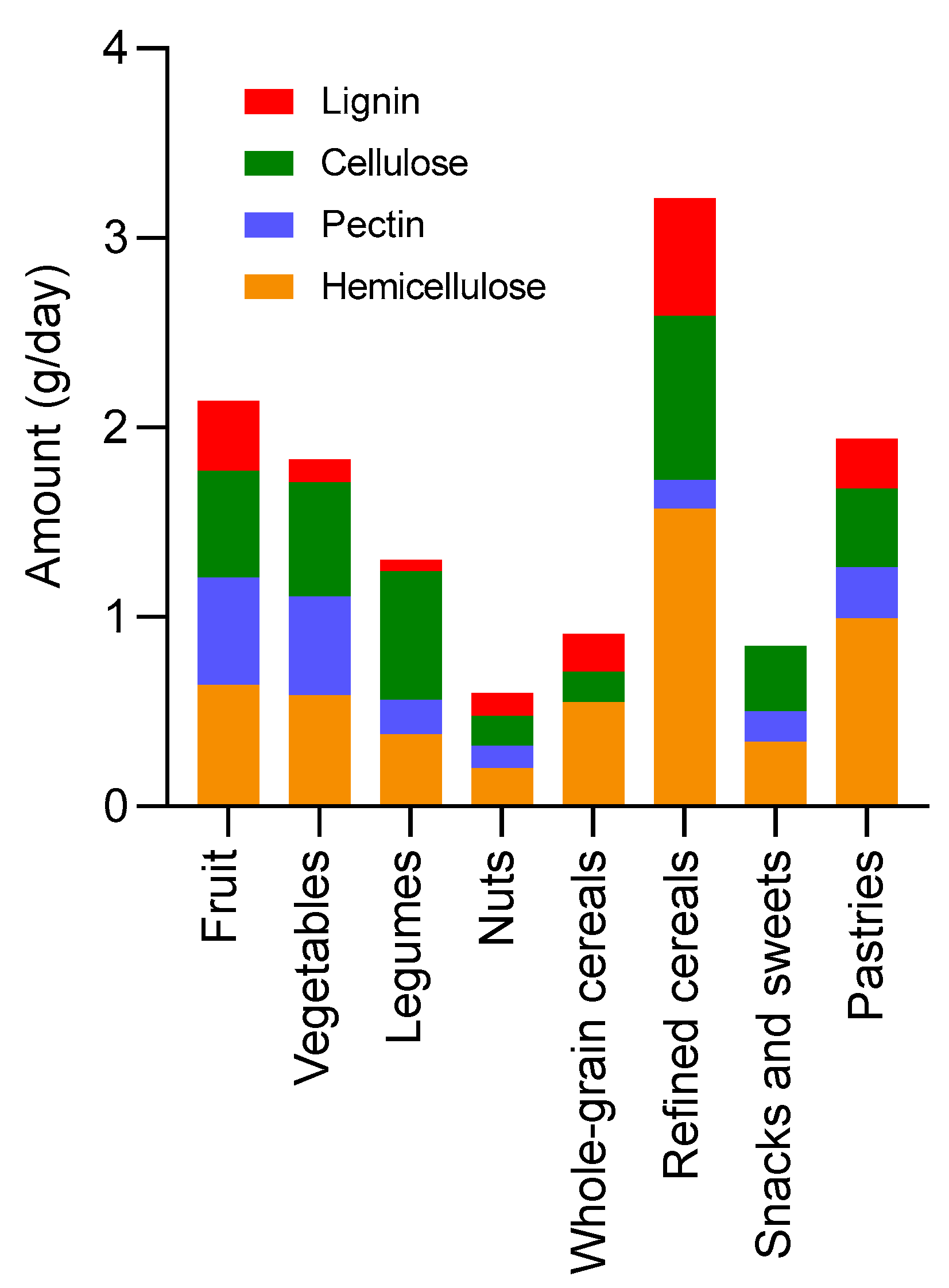
| Dietary fibre (g/day) | Total dietary fibre | 5.3 | 9.1 | 13.8 |
| Total insoluble fibre | 4.2 | 7.4 | 11.2 | |
| Insoluble cellulose | 1.4 | 2.2 | 3.4 | |
| Insoluble hemicellulose | 1.3 | 1.8 | 3.5 | |
| Insoluble pectin | 0.3 | 0.6 | 0.9 | |
| Insoluble lignin | 0.6 | 0.9 | 1.5 | |
| Total soluble fibre | 1.2 | 1.8 | 2.7 | |
| Soluble hemicellulose | 0.7 | 1.1 | 1.7 | |
| Soluble pectin | 0.2 | 0.4 | 0.5 |
| Metabolites | Concentration (mM) | ||
|---|---|---|---|
| Q1 | Median | Q3 | |
| Total SCFA | 19.6 | 25.1 | 34.2 |
| Acetic acid (AA) | 4.1 | 5.1 | 9.9 |
| Butyric acid (BA) | 6.5 | 10.4 | 12.5 |
| Propionic acid (PA) | 2.4 | 3.5 | 4.9 |
| Valeric acid (VA) | 0.1 | 0.2 | 0.7 |
| Total linear SCFA | 16.5 | 19.9 | 33.1 |
| Isobutyric acid (IBA) | 0.3 | 0.5 | 1.5 |
| Isovaleric acid (IVA) | 0.6 | 0.9 | 2.7 |
| Total branched SCFA | 0.9 | 1.5 | 4.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viteri-Echeverría, J.; Calvo-Lerma, J.; Ferriz-Jordán, M.; Garriga, M.; García-Hernández, J.; Heredia, A.; Ribes-Koninckx, C.; Andrés, A.; Asensio-Grau, A. Association between Dietary Intake and Faecal Microbiota in Children with Cystic Fibrosis. Nutrients 2023, 15, 5013. https://doi.org/10.3390/nu15245013
Viteri-Echeverría J, Calvo-Lerma J, Ferriz-Jordán M, Garriga M, García-Hernández J, Heredia A, Ribes-Koninckx C, Andrés A, Asensio-Grau A. Association between Dietary Intake and Faecal Microbiota in Children with Cystic Fibrosis. Nutrients. 2023; 15(24):5013. https://doi.org/10.3390/nu15245013
Chicago/Turabian StyleViteri-Echeverría, Jazmín, Joaquim Calvo-Lerma, Miguel Ferriz-Jordán, María Garriga, Jorge García-Hernández, Ana Heredia, Carmen Ribes-Koninckx, Ana Andrés, and Andrea Asensio-Grau. 2023. "Association between Dietary Intake and Faecal Microbiota in Children with Cystic Fibrosis" Nutrients 15, no. 24: 5013. https://doi.org/10.3390/nu15245013
APA StyleViteri-Echeverría, J., Calvo-Lerma, J., Ferriz-Jordán, M., Garriga, M., García-Hernández, J., Heredia, A., Ribes-Koninckx, C., Andrés, A., & Asensio-Grau, A. (2023). Association between Dietary Intake and Faecal Microbiota in Children with Cystic Fibrosis. Nutrients, 15(24), 5013. https://doi.org/10.3390/nu15245013






