The Beneficial Effects of Dietary Interventions on Gut Microbiota—An Up-to-Date Critical Review and Future Perspectives
Abstract
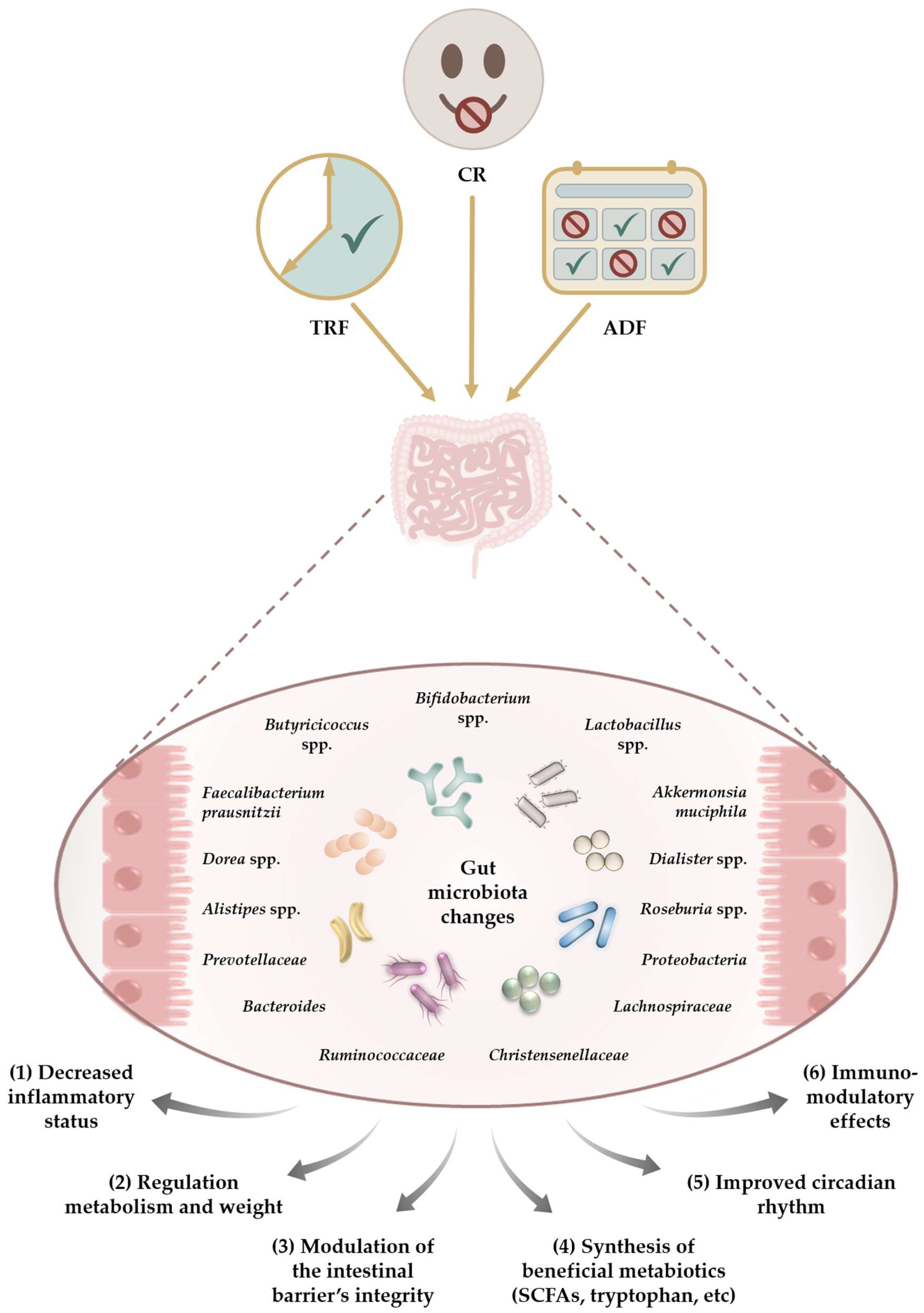
:1. Introduction
2. Updated Concept of Dietary Intervention and Gut Microbiome Links
3. Methodology
4. Effects of Dietary Intervention on the Animal Gut Microbiota
4.1. Studies Conducted on Healthy Animals
4.2. Studies Conducted on Different Animal Models
5. Effect of Dietary Interventions on the Human Gut Microbiota
5.1. Studies Conducted on Healthy Volunteers
5.1.1. CR Regimens
5.1.2. TRF Regimens
5.2. Studies Conducted on Obese Patients
5.2.1. CR Regimens
5.2.2. TRF Regimens
6. Comparability between Dietary Intervention Regimens
7. The Impact of CR Programs on the Gut Microbiota—Common Findings
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yassour, M.; Vatanen, T.; Siljander, H.; Hamalainen, A.M.; Harkonen, T.; Ryhanen, S.J.; Franzosa, E.A.; Vlamakis, H.; Huttenhower, C.; Gevers, D.; et al. Natural history of the infant gut microbiome and impact of antibiotic treatment on bacterial strain diversity and stability. Sci. Transl. Med. 2016, 8, 343ra381. [Google Scholar] [CrossRef] [PubMed]
- Spor, A.; Koren, O.; Ley, R. Unravelling the effects of the environment and host genotype on the gut microbiome. Nat. Rev. Microbiol. 2011, 9, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.J.; Lynch, D.B.; Murphy, K.; Ulaszewska, M.; Jeffery, I.B.; O’Shea, C.A.; Watkins, C.; Dempsey, E.; Mattivi, F.; Tuohy, K.; et al. Evolution of gut microbiota composition from birth to 24 weeks in the INFANTMET Cohort. Microbiome 2017, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Arboleya, S.; Sanchez, B.; Milani, C.; Duranti, S.; Solis, G.; Fernandez, N.; de los Reyes-Gavilan, C.G.; Ventura, M.; Margolles, A.; Gueimonde, M. Intestinal microbiota development in preterm neonates and effect of perinatal antibiotics. J. Pediatr. 2015, 166, 538–544. [Google Scholar] [CrossRef] [PubMed]
- Ottman, N.; Smidt, H.; de Vos, W.M.; Belzer, C. The function of our microbiota: Who is out there and what do they do? Front. Cell. Infect. Microbiol. 2012, 2, 104. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Duranti, S.; Bottacini, F.; Casey, E.; Turroni, F.; Mahony, J.; Belzer, C.; Delgado Palacio, S.; Arboleya Montes, S.; Mancabelli, L.; et al. The First Microbial Colonizers of the Human Gut: Composition, Activities, and Health Implications of the Infant Gut Microbiota. Microbiol. Mol. Biol. Rev. 2017, 81, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Zhang, X.; Ma, M.; Xie, Z.; Pan, Q.; Ma, Z.; Peppelenbosch, M.P. Remodeling of the gut microbiome during Ramadan-associated intermittent fasting. Am. J. Clin. Nutr. 2021, 113, 1332–1342. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Smith, A.H.; Sundset, M.A.; Mackie, R.I. The BaeSR two-component regulatory system mediates resistance to condensed tannins in Escherichia coli. Appl. Environ. Microbiol. 2008, 74, 535–539. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; de Vos, W.M. Effect of diet on the intestinal microbiota and its activity. Curr. Opin. Gastroenterol. 2014, 30, 189–195. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Smidt, H. Endothelial dysfunction: What is the role of the microbiota? Gut 2018, 67, 201–202. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Rajilic-Stojanovic, M.; de Vos, W.M. High-throughput diversity and functionality analysis of the gastrointestinal tract microbiota. Gut 2008, 57, 1605–1615. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; de Vos, W.M. The first 1000 cultured species of the human gastrointestinal microbiota. FEMS Microbiol. Rev. 2014, 38, 996–1047. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef] [PubMed]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef] [PubMed]
- Heiman, M.L.; Greenway, F.L. A healthy gastrointestinal microbiome is dependent on dietary diversity. Mol. Metab. 2016, 5, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108. [Google Scholar] [CrossRef]
- Purdel, C.; Ungurianu, A.; Adam-Dima, I.; Margina, D. Exploring the potential impact of probiotic use on drug metabolism and efficacy. Biomed. Pharmacother. 2023, 161, 114468. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Angoorani, P.; Hasani-Ranjbar, S.; Siadat, S.D.; Ghasemi, N.; Larijani, B.; Soroush, A.R. Adaptation of human gut microbiota to bariatric surgeries in morbidly obese patients: A systematic review. Microb. Pathog. 2018, 116, 13–21. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Soroush, A.R.; Angoorani, P.; Larijani, B.; Hasani-Ranjbar, S. Gut Microbiota as a Target in the Pathogenesis of Metabolic Disorders: A New Approach to Novel Therapeutic Agents. Horm. Metab. Res. 2016, 48, 349–358. [Google Scholar] [CrossRef]
- Ejtahed, H.S.; Hasani-Ranjbar, S.; Larijani, B. Human Microbiome as an Approach to Personalized Medicine. Altern. Ther. Health Med. 2017, 23, 8–9. [Google Scholar]
- Ejtahed, H.S.; Angoorani, P.; Soroush, A.R.; Siadat, S.D.; Shirzad, N.; Hasani-Ranjbar, S.; Larijani, B. Our Little Friends with Big Roles: Alterations of the Gut Microbiota in Thyroid Disorders. Endocr. Metab. Immune Disord. Drug Targets 2020, 20, 344–350. [Google Scholar] [CrossRef]
- Ozkul, C.; Yalinay, M.; Karakan, T. Islamic fasting leads to an increased abundance of Akkermansia muciniphila and Bacteroides fragilis group: A preliminary study on intermittent fasting. Turk. J. Gastroenterol. 2019, 30, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Angoorani, P.; Ejtahed, H.S.; Hasani-Ranjbar, S.; Siadat, S.D.; Soroush, A.R.; Larijani, B. Gut microbiota modulation as a possible mediating mechanism for fasting-induced alleviation of metabolic complications: A systematic review. Nutr. Metab. 2021, 18, 105. [Google Scholar] [CrossRef]
- Ruiz, L.; Margolles, A.; Sanchez, B. Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front. Microbiol. 2013, 4, 396. [Google Scholar] [CrossRef]
- Li, J.; Zhao, F.; Wang, Y.; Chen, J.; Tao, J.; Tian, G.; Wu, S.; Liu, W.; Cui, Q.; Geng, B.; et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 2017, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhou, J.; Wang, L. Role and Mechanism of Gut Microbiota in Human Disease. Front. Cell. Infect. Microbiol. 2021, 11, 625913. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Jordan, B.F. Gut microbiota-mediated inflammation in obesity: A link with gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 671–682. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.M.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1469–1476. [Google Scholar] [CrossRef]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef]
- Wang, X.; Gibson, G.R. Effects of the in vitro fermentation of oligofructose and inulin by bacteria growing in the human large intestine. J. Appl. Bacteriol. 1993, 75, 373–380. [Google Scholar] [CrossRef]
- Wang, T.; Rijnaarts, I.; Hermes, G.D.A.; de Roos, N.M.; Witteman, B.J.M.; de Wit, N.J.W.; Govers, C.; Smidt, H.; Zoetendal, E.G. Fecal Microbiota Signatures Are Not Consistently Related to Symptom Severity in Irritable Bowel Syndrome. Dig. Dis. Sci. 2022, 67, 5137–5148. [Google Scholar] [CrossRef]
- Wang, T.; van Dijk, L.; Rijnaarts, I.; Hermes, G.D.A.; de Roos, N.M.; Witteman, B.J.M.; de Wit, N.J.W.; Govers, C.; Smidt, H.; Zoetendal, E.G. Methanogen Levels Are Significantly Associated with Fecal Microbiota Composition and Alpha Diversity in Healthy Adults and Irritable Bowel Syndrome Patients. Microbiol. Spectr. 2022, 10, e0165322. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.A.; Kenney, L.S.; Goulet, B.; Abdel-Aalel, S. Dietary starch type affects body weight and glycemic control in freely fed but not energy-restricted obese rats. J. Nutr. 2009, 139, 1881–1889. [Google Scholar] [CrossRef] [PubMed]
- Keenan, M.J.; Zhou, J.; McCutcheon, K.L.; Raggio, A.M.; Bateman, H.G.; Todd, E.; Jones, C.K.; Tulley, R.T.; Melton, S.; Martin, R.J.; et al. Effects of resistant starch, a non-digestible fermentable fiber, on reducing body fat. Obesity 2006, 14, 1523–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef]
- Schoeler, M.; Caesar, R. Dietary lipids, gut microbiota and lipid metabolism. Rev. Endocr. Metab. Disord. 2019, 20, 461–472. [Google Scholar] [CrossRef]
- Flach, J.; van der Waal, M.B.; Kardinaal, A.F.M.; Schloesser, J.; Ruijschop, R.M.A.J.; Claassen, E. Probiotic research priorities for the healthy adult population: A review on the health benefits of Lactobacillus rhamnosus GG and Bifidobacterium animalis subspecies lactis BB-12. Cogent Food Agric. 2018, 4, 1452839. [Google Scholar] [CrossRef]
- Caesar, R.; Tremaroli, V.; Kovatcheva-Datchary, P.; Cani, P.D.; Backhed, F. Crosstalk between Gut Microbiota and Dietary Lipids Aggravates WAT Inflammation through TLR Signaling. Cell Metab. 2015, 22, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Pifferi, F.; Terrien, J.; Perret, M.; Epelbaum, J.; Blanc, S.; Picq, J.L.; Dhenain, M.; Aujard, F. Promoting healthspan and lifespan with caloric restriction in primates. Commun. Biol. 2019, 2, 107. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.E.; Gumpricht, E.; Sears, D.D.; Sweazea, K.L. Recent advances and health implications of dietary fasting regimens on the gut microbiome. Am. J. Physiol. Gastrointest. Liver Physiol. 2021, 320, G847–G863. [Google Scholar] [CrossRef]
- Attinà, A.; Leggeri, C.; Paroni, R.; Pivari, F.; Dei Cas, M.; Mingione, A.; Dri, M.; Marchetti, M.; Di Renzo, L. Fasting: How to Guide. Nutrients 2021, 13, 1570. [Google Scholar] [CrossRef] [PubMed]
- Forslund, S.K. Fasting intervention and its clinical effects on the human host and microbiome. J. Intern. Med. 2023, 293, 166–183. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, N.S.; Lorentz, A. Dietary restrictions modulate the gut microbiota: Implications for health and disease. Nutr. Res. 2021, 89, 10–22. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic Effects of Intermittent Fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [PubMed]
- Stockman, M.C.; Thomas, D.; Burke, J.; Apovian, C.M. Intermittent Fasting: Is the Wait Worth the Weight? Curr. Obes. Rep. 2018, 7, 172–185. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef] [PubMed]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, S.; Li, Y.; Zhao, M.; Kuang, J.; Liang, D.; Wang, J.; Wei, M.; Rajani, C.; Ma, X.; et al. Gut microbiota-bile acid crosstalk contributes to the rebound weight gain after calorie restriction in mice. Nat. Commun. 2022, 13, 2060. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, H.; Wang, L.; Hu, C.; Gao, A.; Wu, Q.; Wang, Q.; Lin, H.; Chen, B.; Wang, X.; et al. Fasting and refeeding triggers specific changes in bile acid profiles and gut microbiota. J. Diabetes 2023, 15, 165–180. [Google Scholar] [CrossRef]
- Hernandez, A.R.; Kemp, K.M.; Burke, S.N.; Buford, T.W.; Carter, C.S. Influence of Aging, Macronutrient Composition and Time-Restricted Feeding on the Fischer344 x Brown Norway Rat Gut Microbiota. Nutrients 2022, 14, 1758. [Google Scholar] [CrossRef]
- Messina, M.; Iacumin, L.; Pascon, G.; Tulli, F.; Tibaldi, E.; Cardinaletti, G. Effect of feed restriction and refeeding on body condition, digestive functionality and intestinal microbiota in rainbow trout (Oncorhynchus mykiss). Fish. Physiol. Biochem. 2023, 49, 169–189. [Google Scholar] [CrossRef]
- Song, X.; Zhai, Y.; Song, J.; Zhang, J.; Li, X. The structural discrepancy between the small and large gut microbiota of Asiatic toad (Bufo gargarizans) during hibernation. Folia Microbiol. 2023, 68, 537–546. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, X.; Loh, Y.J.; Yang, X.; Zhang, C. The effect of calorie intake, fasting, and dietary composition on metabolic health and gut microbiota in mice. BMC Biol. 2021, 19, 51. [Google Scholar] [CrossRef]
- Ma, R.X.; Hu, J.Q.; Fu, W.; Zhong, J.; Cao, C.; Wang, C.C.; Qi, S.Q.; Zhang, X.L.; Liu, G.H.; Gao, Y.D. Intermittent fasting protects against food allergy in a murine model. Front. Immunol. 2023, 14, 1167562. [Google Scholar] [CrossRef]
- Xia, J.; Guo, W.; Hu, M.; Jin, X.; Zhang, S.; Liu, B.; Qiu, H.; Wang, K.; Zhuge, A.; Li, S.; et al. Resynchronized rhythmic oscillations of gut microbiota drive time-restricted feeding induced nonalcoholic steatohepatitis alleviation. Gut Microbes 2023, 15, 2221450. [Google Scholar] [CrossRef]
- Liang, Y.; Yin, W.; Luo, C.; Sun, L.; Feng, T.; Zhang, Y.; Yin, Y.; Zhang, W. Maternal intermittent fasting in mice disrupts the intestinal barrier leading to metabolic disorder in adult offspring. Commun. Biol. 2023, 6, 30. [Google Scholar] [CrossRef]
- Wu, J.; Man, D.; Shi, D.; Wu, W.; Wang, S.; Wang, K.; Li, Y.; Yang, L.; Bian, X.; Wang, Q.; et al. Intermittent Fasting Alleviates Risk Markers in a Murine Model of Ulcerative Colitis by Modulating the Gut Microbiome and Metabolome. Nutrients 2022, 14, 5311. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Sun, J.; Huang, Y.; Liu, T.; Ye, Z.; Hu, J.; Zhang, G.; Chen, H.; He, Y.; et al. Calorie restriction ameliorates hyperglycemia, modulates the disordered gut microbiota, and mitigates metabolic endotoxemia and inflammation in type 2 diabetic rats. J. Endocrinol. Investig. 2023, 46, 699–711. [Google Scholar] [CrossRef]
- Huang, W.; Yan, Y.; Wu, M.; Hu, J.; Zhao, J.; Chen, X.; Liu, W.; Liu, K.; Li, C. Preoperative fasting confers protection against intestinal ischaemia/reperfusion injury by modulating gut microbiota and their metabolites in a mouse model. Br. J. Anaesth. 2022, 128, 501–512. [Google Scholar] [CrossRef]
- Teker, H.T.; Ceylani, T. Intermittent fasting supports the balance of the gut microbiota composition. Int. Microbiol. 2023, 26, 51–57. [Google Scholar] [CrossRef]
- Yang, H.; Li, C.; Che, M.; Li, Y.; Feng, R.; Sun, C. Gut microbiota mediates the anti-obesity effect of intermittent fasting by inhibiting intestinal lipid absorption. J. Nutr. Biochem. 2023, 116, 109318. [Google Scholar] [CrossRef] [PubMed]
- Prisco, S.Z.; Eklund, M.; Moutsoglou, D.M.; Prisco, A.R.; Khoruts, A.; Weir, E.K.; Thenappan, T.; Prins, K.W. Intermittent Fasting Enhances Right Ventricular Function in Preclinical Pulmonary Arterial Hypertension. J. Am. Heart Assoc. 2021, 10, e022722. [Google Scholar] [CrossRef] [PubMed]
- Soares, N.L.; Dorand, V.A.M.; Cavalcante, H.C.; Batista, K.S.; de Souza, D.M.; Lima, M.D.S.; Salvadori, M.G.D.S.; Magnani, M.; Alves, A.F.; Aquino, J.S. Does intermittent fasting associated with aerobic training influence parameters related to the gut-brain axis of Wistar rats? J. Affect. Disord. 2021, 293, 176–185. [Google Scholar] [CrossRef]
- Graef, F.A.; Celiberto, L.S.; Allaire, J.M.; Kuan, M.T.Y.; Bosman, E.S.; Crowley, S.M.; Yang, H.; Chan, J.H.; Stahl, M.; Yu, H.; et al. Fasting increases microbiome-based colonization resistance and reduces host inflammatory responses during an enteric bacterial infection. PLoS Pathog. 2021, 17, e1009719. [Google Scholar] [CrossRef] [PubMed]
- Ménard, A.; Smet, A. Review: Other Helicobacter species. Helicobacter 2019, 24 (Suppl. S1), e12645. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Xie, G. Bile acid-microbiota crosstalk in gastrointestinal inflammation and carcinogenesis. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Heeney, D.D.; Gareau, M.G.; Marco, M.L. Intestinal Lactobacillus in health and disease, a driver or just along for the ride? Curr. Opin. Biotechnol. 2018, 49, 140–147. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Pan, F.; Zhang, L.; Li, M.; Hu, Y.; Zeng, B.; Yuan, H.; Zhao, L.; Zhang, C. Predominant gut Lactobacillus murinus strain mediates anti-inflammaging effects in calorie-restricted mice. Microbiome 2018, 6, 54. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Yang, L.; Huang, P.; Li, W.; Wang, S.; Zhao, G.; Zhang, M.; Pang, X.; Yan, Z.; et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat. Commun. 2013, 4, 2163. [Google Scholar] [CrossRef]
- Luu, M.; Monning, H.; Visekruna, A. Exploring the Molecular Mechanisms Underlying the Protective Effects of Microbial SCFAs on Intestinal Tolerance and Food Allergy. Front. Immunol. 2020, 11, 1225. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Osadchiy, V.; Labus, J.S.; Gupta, A.; Jacobs, J.; Ashe-McNalley, C.; Hsiao, E.Y.; Mayer, E.A. Correlation of tryptophan metabolites with connectivity of extended central reward network in healthy subjects. PLoS ONE 2018, 13, e0201772. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, A.R.; Watson, C.; Federico, Q.P.; Fletcher, R.; Brotgandel, A.; Buford, T.W.; Carter, C.S.; Burke, S.N. Twelve Months of Time-Restricted Feeding Improves Cognition and Alters Microbiome Composition Independent of Macronutrient Composition. Nutrients 2022, 14, 3977. [Google Scholar] [CrossRef]
- Janssen, A.W.F.; Katiraei, S.; Bartosinska, B.; Eberhard, D.; Willems van Dijk, K.; Kersten, S. Loss of angiopoietin-like 4 (ANGPTL4) in mice with diet-induced obesity uncouples visceral obesity from glucose intolerance partly via the gut microbiota. Diabetologia 2018, 61, 1447–1458. [Google Scholar] [CrossRef]
- Li, S.; Qi, Y.; Ren, D.; Qu, D.; Sun, Y. The Structure Features and Improving Effects of Polysaccharide from. Antibiotics 2019, 9, 8. [Google Scholar] [CrossRef]
- Tooley, K.L. Effects of the Human Gut Microbiota on Cognitive Performance, Brain Structure and Function: A Narrative Review. Nutrients 2020, 12, 3009. [Google Scholar] [CrossRef]
- Li, G.; Xie, C.; Lu, S.; Nichols, R.G.; Tian, Y.; Li, L.; Patel, D.; Ma, Y.; Brocker, C.N.; Yan, T.; et al. Intermittent Fasting Promotes White Adipose Browning and Decreases Obesity by Shaping the Gut Microbiota. Cell Metab. 2017, 26, 672–685.e674. [Google Scholar] [CrossRef]
- Lilja, S.; Bäck, H.; Duszka, K.; Hippe, B.; Suarez, L.; Höfinger, I.; Debebe, T.; König, J.; Haslberger, A. Fasting and fasting mimetic supplementation address sirtuin expression, miRNA and microbiota composition. Funct. Foods Health Dis. 2020, 10, 439–455. [Google Scholar] [CrossRef]
- Lilja, S.; Stoll, C.; Krammer, U.; Hippe, B.; Duszka, K.; Debebe, T.; Höfinger, I.; König, J.; Pointner, A.; Haslberger, A. Five Days Periodic Fasting Elevates Levels of Longevity Related. Int. J. Mol. Sci. 2021, 22, 2331. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yin, J.; Lei, J.; Liu, F.; Zheng, H.; Wang, S.; Wu, S.; Sheng, H.; McGovern, E.; Zhou, H. Fasting challenges human gut microbiome resilience and reduces Fusobacterium. Med. Microecol. 2019, 1–2, 100003. [Google Scholar] [CrossRef]
- Mesnage, R.; Grundler, F.; Schwiertz, A.; Le Maho, Y.; Wilhelmi de Toledo, F. Changes in human gut microbiota composition are linked to the energy metabolic switch during 10 d of Buchinger fasting. J. Nutr. Sci. 2019, 8, e36. [Google Scholar] [CrossRef]
- Ozkul, C.; Yalinay, M.; Karakan, T. Structural changes in gut microbiome after Ramadan fasting: A pilot study. Benef. Microbes 2020, 11, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Liu, K.; Long, D.; Faisal, S.; Hilal, M.G.; Huang, X.; Long, R. Ramadan Fasting Leads to Shifts in Human Gut Microbiota Structured by Dietary Composition. Front. Microbiol. 2021, 12, 642999. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, A.; Roshanravan, N.; Mesri Alamdari, N.; Safaiyan, A.; Mosharkesh, E.; Hadi, A.; Barati, M.; Ostadrahimi, A. The interplay between fasting, gut microbiota, and lipid profile. Int. J. Clin. Pract. 2021, 75, e14591. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Ali, I.; Li, X.; Long, D.; Zhang, Y.; Long, R.; Huang, X. Shifts in Fecal Metabolite Profiles Associated With Ramadan Fasting Among Chinese and Pakistani Individuals. Front. Nutr. 2022, 9, 845086. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Chen, A.; Xu, C.; Jianglei, R.; Feng, Q.; Li, M. Time-restricted feeding is associated with changes in human gut microbiota related to nutrient intake. Nutrition 2020, 78, 110797. [Google Scholar] [CrossRef] [PubMed]
- Remely, M.; Hippe, B.; Geretschlaeger, I.; Stegmayer, S.; Hoefinger, I.; Haslberger, A. Increased gut microbiota diversity and abundance of Faecalibacterium prausnitzii and Akkermansia after fasting: A pilot study. Wien. Klin. Wochenschr. 2015, 127, 394–398. [Google Scholar] [CrossRef]
- Maifeld, A.; Bartolomaeus, H.; Löber, U.; Avery, E.G.; Steckhan, N.; Markó, L.; Wilck, N.; Hamad, I.; Šušnjar, U.; Mähler, A.; et al. Fasting alters the gut microbiome reducing blood pressure and body weight in metabolic syndrome patients. Nat. Commun. 2021, 12, 1970. [Google Scholar] [CrossRef]
- Guo, Y.; Luo, S.; Ye, Y.; Yin, S.; Fan, J.; Xia, M. Intermittent Fasting Improves Cardiometabolic Risk Factors and Alters Gut Microbiota in Metabolic Syndrome Patients. J. Clin. Endocrinol. Metab. 2021, 106, 64–79. [Google Scholar] [CrossRef]
- Khan, M.N.; Khan, S.I.; Rana, M.I.; Ayyaz, A.; Khan, M.Y.; Imran, M. Intermittent fasting positively modulates human gut microbial diversity and ameliorates blood lipid profile. Front. Microbiol. 2022, 13, 922727. [Google Scholar] [CrossRef]
- Alemán, J.O.; Bokulich, N.A.; Swann, J.R.; Walker, J.M.; De Rosa, J.C.; Battaglia, T.; Costabile, A.; Pechlivanis, A.; Liang, Y.; Breslow, J.L.; et al. Fecal microbiota and bile acid interactions with systemic and adipose tissue metabolism in diet-induced weight loss of obese postmenopausal women. J. Transl. Med. 2018, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Dao, M.C.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Frost, F.; Storck, L.J.; Kacprowski, T.; Gärtner, S.; Rühlemann, M.; Bang, C.; Franke, A.; Völker, U.; Aghdassi, A.A.; Steveling, A.; et al. A structured weight loss program increases gut microbiota phylogenetic diversity and reduces levels of Collinsella in obese type 2 diabetics: A pilot study. PLoS ONE 2019, 14, e0219489. [Google Scholar] [CrossRef]
- Simões, C.D.; Maukonen, J.; Scott, K.P.; Virtanen, K.A.; Pietiläinen, K.H.; Saarela, M. Impact of a very low-energy diet on the fecal microbiota of obese individuals. Eur. J. Nutr. 2014, 53, 1421–1429. [Google Scholar] [CrossRef]
- Ruiz, A.; Cerdó, T.; Jáuregui, R.; Pieper, D.H.; Marcos, A.; Clemente, A.; García, F.; Margolles, A.; Ferrer, M.; Campoy, C.; et al. One-year calorie restriction impacts gut microbial composition but not its metabolic performance in obese adolescents. Environ. Microbiol. 2017, 19, 1536–1551. [Google Scholar] [CrossRef] [PubMed]
- Jian, C.; Silvestre, M.P.; Middleton, D.; Korpela, K.; Jalo, E.; Broderick, D.; de Vos, W.M.; Fogelholm, M.; Taylor, M.W.; Raben, A.; et al. Gut microbiota predicts body fat change following a low-energy diet: A PREVIEW intervention study. Genome Med. 2022, 14, 54. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Zanfirescu, A.; Margina, D. Sirtuins, resveratrol and the intertwining cellular pathways connecting them. Ageing Res. Rev. 2023, 88, 101936. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Margina, D. Regulation of Gene Expression through Food-Curcumin as a Sirtuin Activity Modulator. Plants 2022, 11, 1741. [Google Scholar] [CrossRef]
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes. Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef]
- Nakagawa, T.; Guarente, L. Sirtuins at a glance. J. Cell Sci. 2011, 124, 833–838. [Google Scholar] [CrossRef]
- Mindikoglu, A.L.; Abdulsada, M.M.; Jain, A.; Choi, J.M.; Jalal, P.K.; Devaraj, S.; Mezzari, M.P.; Petrosino, J.F.; Opekun, A.R.; Jung, S.Y. Intermittent fasting from dawn to sunset for 30 consecutive days is associated with anticancer proteomic signature and upregulates key regulatory proteins of glucose and lipid metabolism, circadian clock, DNA repair, cytoskeleton remodeling, immune system and cognitive function in healthy subjects. J. Proteomics 2020, 217, 103645. [Google Scholar] [CrossRef]
- Zeb, F.; Wu, X.; Chen, L.; Fatima, S.; Haq, I.U.; Chen, A.; Majeed, F.; Feng, Q.; Li, M. Effect of time-restricted feeding on metabolic risk and circadian rhythm associated with gut microbiome in healthy males. Br. J. Nutr. 2020, 123, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Comte, J.; Fauteux, L.; Del Giorgio, P.A. Links between metabolic plasticity and functional redundancy in freshwater bacterioplankton communities. Front. Microbiol. 2013, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Marcell, J.; Cares, K.; Kalam, F.; Cienfuegos, S.; Ezpeleta, M.; Varady, K.A. Effect of time restricted feeding on the gut microbiome in adults with obesity: A pilot study. Nutr. Health 2020, 26, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Heinsen, F.A.; Fangmann, D.; Müller, N.; Schulte, D.M.; Rühlemann, M.C.; Türk, K.; Settgast, U.; Lieb, W.; Baines, J.F.; Schreiber, S.; et al. Beneficial Effects of a Dietary Weight Loss Intervention on Human Gut Microbiome Diversity and Metabolism Are Not Sustained during Weight Maintenance. Obes. Facts 2016, 9, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Stanislawski, M.A.; Frank, D.N.; Borengasser, S.J.; Ostendorf, D.M.; Ir, D.; Jambal, P.; Bing, K.; Wayland, L.; Siebert, J.C.; Bessesen, D.H.; et al. The Gut Microbiota during a Behavioral Weight Loss Intervention. Nutrients 2021, 13, 3248. [Google Scholar] [CrossRef] [PubMed]
- Ferrocino, I.; Pellegrini, M.; D’Eusebio, C.; Goitre, I.; Ponzo, V.; Fadda, M.; Rosato, R.; Mengozzi, G.; Beccuti, G.; Merlo, F.D.; et al. The Effects of Time-Restricted Eating on Metabolism and Gut Microbiota: A Real-Life Study. Nutrients 2022, 14, 2569. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Repiso, C.; Molina-Vega, M.; Bernal-López, M.R.; Garrido-Sánchez, L.; García-Almeida, J.M.; Sajoux, I.; Moreno-Indias, I.; Tinahones, F.J. Different Weight Loss Intervention Approaches Reveal a Lack of a Common Pattern of Gut Microbiota Changes. J. Pers. Med. 2021, 11, 109. [Google Scholar] [CrossRef]
- Siebert, J.C.; Stanislawski, M.A.; Zaman, A.; Ostendorf, D.M.; Konigsberg, I.R.; Jambal, P.; Ir, D.; Bing, K.; Wayland, L.; Scorsone, J.J.; et al. Multiomic Predictors of Short-Term Weight Loss and Clinical Outcomes During a Behavioral-Based Weight Loss Intervention. Obesity 2021, 29, 859–869. [Google Scholar] [CrossRef]
- Sowah, S.A.; Milanese, A.; Schübel, R.; Wirbel, J.; Kartal, E.; Johnson, T.S.; Hirche, F.; Grafetstätter, M.; Nonnenmacher, T.; Kirsten, R.; et al. Calorie restriction improves metabolic state independently of gut microbiome composition: A randomized dietary intervention trial. Genome Med. 2022, 14, 30. [Google Scholar] [CrossRef]
- Gutiérrez-Repiso, C.; Hernández-García, C.; García-Almeida, J.M.; Bellido, D.; Martín-Núñez, G.M.; Sánchez-Alcoholado, L.; Alcaide-Torres, J.; Sajoux, I.; Tinahones, F.J.; Moreno-Indias, I. Effect of Synbiotic Supplementation in a Very-Low-Calorie Ketogenic Diet on Weight Loss Achievement and Gut Microbiota: A Randomized Controlled Pilot Study. Mol. Nutr. Food Res. 2019, 63, e1900167. [Google Scholar] [CrossRef]
- Cignarella, F.; Cantoni, C.; Ghezzi, L.; Salter, A.; Dorsett, Y.; Chen, L.; Phillips, D.; Weinstock, G.M.; Fontana, L.; Cross, A.H.; et al. Intermittent Fasting Confers Protection in CNS Autoimmunity by Altering the Gut Microbiota. Cell Metab. 2018, 27, 1222–1235.e1226. [Google Scholar] [CrossRef] [PubMed]
- Bronzini, M.; Maglione, A.; Rosso, R.; Matta, M.; Masuzzo, F.; Rolla, S.; Clerico, M. Feeding the gut microbiome: Impact on multiple sclerosis. Front. Immunol. 2023, 14, 1176016. [Google Scholar] [CrossRef] [PubMed]
- Leylabadlo, H.E.; Ghotaslou, R.; Feizabadi, M.M.; Farajnia, S.; Moaddab, S.Y.; Ganbarov, K.; Khodadadi, E.; Tanomand, A.; Sheykhsaran, E.; Yousefi, B.; et al. The critical role of Faecalibacterium prausnitzii in human health: An overview. Microb. Pathog. 2020, 149, 104344. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Delzenne, N.M. Benefits of bariatric surgery: An issue of microbial-host metabolism interactions? Gut 2011, 60, 1166–1167. [Google Scholar] [CrossRef]
- Seganfredo, F.B.; Blume, C.A.; Moehlecke, M.; Giongo, A.; Casagrande, D.S.; Spolidoro, J.V.N.; Padoin, A.V.; Schaan, B.D.; Mottin, C.C. Weight-loss interventions and gut microbiota changes in overweight and obese patients: A systematic review. Obes. Rev. 2017, 18, 832–851. [Google Scholar] [CrossRef] [PubMed]
- Haro, C.; Garcia-Carpintero, S.; Alcala-Diaz, J.F.; Gomez-Delgado, F.; Delgado-Lista, J.; Perez-Martinez, P.; Rangel Zuñiga, O.A.; Quintana-Navarro, G.M.; Landa, B.B.; Clemente, J.C.; et al. The gut microbial community in metabolic syndrome patients is modified by diet. J. Nutr. Biochem. 2016, 27, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Santacruz, A.; Collado, M.C.; García-Valdés, L.; Segura, M.T.; Martín-Lagos, J.A.; Anjos, T.; Martí-Romero, M.; Lopez, R.M.; Florido, J.; Campoy, C.; et al. Gut microbiota composition is associated with body weight, weight gain and biochemical parameters in pregnant women. Br. J. Nutr. 2010, 104, 83–92. [Google Scholar] [CrossRef]
- Cao, M.Z.; Wei, C.H.; Wen, M.C.; Song, Y.; Srivastava, K.; Yang, N.; Shi, Y.M.; Miao, M.; Chung, D.; Li, X.M. Clinical efficacy of weight loss herbal intervention therapy and lifestyle modifications on obesity and its association with distinct gut microbiome: A randomized double-blind phase 2 study. Front. Endocrinol. 2023, 14, 1054674. [Google Scholar] [CrossRef]
- Hill, E.B.; Konigsberg, I.R.; Ir, D.; Frank, D.N.; Jambal, P.; Litkowski, E.M.; Lange, E.M.; Lange, L.A.; Ostendorf, D.M.; Scorsone, J.J.; et al. The Microbiome, Epigenome, and Diet in Adults with Obesity during Behavioral Weight Loss. Nutrients 2023, 15, 3588. [Google Scholar] [CrossRef] [PubMed]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A Dietary Fiber-Deprived Gut Microbiota Degrades the Colonic Mucus Barrier and Enhances Pathogen Susceptibility. Cell 2016, 167, 1339–1353.e1321. [Google Scholar] [CrossRef] [PubMed]
- Ke, H.; Li, F.; Deng, W.; Li, Z.; Wang, S.; Lv, P.; Chen, Y. Metformin Exerts Anti-inflammatory and Mucus Barrier Protective Effects by Enriching Akkermansia muciniphila in Mice With Ulcerative Colitis. Front. Pharmacol. 2021, 12, 726707. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The Genus. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef]
- Huang, Y.J.; Pai, Y.C.; Yu, L.C. Host-Microbiota Interaction and Intestinal Epithelial Functions under Circadian Control: Implications in Colitis and Metabolic Disorders. Chin. J. Physiol. 2018, 61, 325–340. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Wang, Y.; Liu, C.; Wang, W.; Wang, J.; et al. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235.e225. [Google Scholar] [CrossRef] [PubMed]
- Lew, L.C.; Hor, Y.Y.; Yusoff, N.A.A.; Choi, S.B.; Yusoff, M.S.B.; Roslan, N.S.; Ahmad, A.; Mohammad, J.A.M.; Abdullah, M.F.I.L.; Zakaria, N.; et al. Probiotic Lactobacillus plantarum P8 alleviated stress and anxiety while enhancing memory and cognition in stressed adults: A randomised, double-blind, placebo-controlled study. Clin. Nutr. 2019, 38, 2053–2064. [Google Scholar] [CrossRef]
- Hiippala, K.; Jouhten, H.; Ronkainen, A.; Hartikainen, A.; Kainulainen, V.; Jalanka, J.; Satokari, R. The Potential of Gut Commensals in Reinforcing Intestinal Barrier Function and Alleviating Inflammation. Nutrients 2018, 10, 988. [Google Scholar] [CrossRef]
- Martin, A.M.; Sun, E.W.; Rogers, G.B.; Keating, D.J. The Influence of the Gut Microbiome on Host Metabolism Through the Regulation of Gut Hormone Release. Front. Physiol. 2019, 10, 428. [Google Scholar] [CrossRef]
- Du, Y.; Gao, Y.; Zeng, B.; Fan, X.; Yang, D.; Yang, M. Effects of anti-aging interventions on intestinal microbiota. Gut Microbes 2021, 13, 1994835. [Google Scholar] [CrossRef]
- Jackson, M.A.; Jeffery, I.B.; Beaumont, M.; Bell, J.T.; Clark, A.G.; Ley, R.E.; O’Toole, P.W.; Spector, T.D.; Steves, C.J. Signatures of early frailty in the gut microbiota. Genome Med. 2016, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Biagi, E.; Franceschi, C.; Rampelli, S.; Severgnini, M.; Ostan, R.; Turroni, S.; Consolandi, C.; Quercia, S.; Scurti, M.; Monti, D.; et al. Gut Microbiota and Extreme Longevity. Curr. Biol. 2016, 26, 1480–1485. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Hua, Y.; Zeng, B.; Ning, R.; Li, Y.; Zhao, J. Gut microbiota signatures of longevity. Curr. Biol. 2016, 26, R832–R833. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.W.; Adams, G.B.; Perin, L.; Wei, M.; Zhou, X.; Lam, B.S.; Da Sacco, S.; Mirisola, M.; Quinn, D.I.; Dorff, T.B.; et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell 2014, 14, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Di Tano, M.; Mattson, M.P.; Guidi, N. Intermittent and periodic fasting, longevity and disease. Nat. Aging 2021, 1, 47–59. [Google Scholar] [CrossRef]
- Yilmaz, Ö.H.; Katajisto, P.; Lamming, D.W.; Gültekin, Y.; Bauer-Rowe, K.E.; Sengupta, S.; Birsoy, K.; Dursun, A.; Yilmaz, V.O.; Selig, M.; et al. mTORC1 in the Paneth cell niche couples intestinal stem-cell function to calorie intake. Nature 2012, 486, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Wang, Y.; Zhang, Q.; Zou, R.; Guo, M.; Zheng, H. Characteristics of gut microbiota in people with obesity. PLoS ONE 2021, 16, e0255446. [Google Scholar] [CrossRef]
- Pinart, M.; Dötsch, A.; Schlicht, K.; Laudes, M.; Bouwman, J.; Forslund, S.K.; Pischon, T.; Nimptsch, K. Gut Microbiome Composition in Obese and Non-Obese Persons: A Systematic Review and Meta-Analysis. Nutrients 2021, 14, 12. [Google Scholar] [CrossRef]
- Sanmiguel, C.; Gupta, A.; Mayer, E.A. Gut Microbiome and Obesity: A Plausible Explanation for Obesity. Curr. Obes. Rep. 2015, 4, 250–261. [Google Scholar] [CrossRef]
- Wilson, I.D.; Nicholson, J.K. Gut microbiome interactions with drug metabolism, efficacy, and toxicity. Transl. Res. 2017, 179, 204–222. [Google Scholar] [CrossRef]
- Weersma, R.K.; Zhernakova, A.; Fu, J. Interaction between drugs and the gut microbiome. Gut 2020, 69, 1510–1519. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.; Hahn, S.L.; Bakke, J. Intermittent fasting: Consider the risks of disordered eating for your patient. Clin. Diabetes Endocrinol. 2023, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, S.; Corapi, S.; Gabel, K.; Ezpeleta, M.; Kalam, F.; Lin, S.; Pavlou, V.; Varady, K.A. Effect of Intermittent Fasting on Reproductive Hormone Levels in Females and Males: A Review of Human Trials. Nutrients 2022, 14, 2343. [Google Scholar] [CrossRef] [PubMed]
| Animal Model | Intervention | Biospecimen | Microbiota Variations Assessment Method | Main Outcome on Microbiota | References |
|---|---|---|---|---|---|
| C57BL/6J mice, 6-week-old | 12 weeks Control: food and water ad libitum(n = 8) HF(n = 8) CR (n = 8) | cecum | whole-genome, shotgun metagenomic sequencing | ↑ Firmicutes, ↑ Actinobacteria, ↑ Firmicutes: Bacteroidetes ratio (in HF and CR) ↓ Bacteroidetes; ↓ Parabacteroides (in HF and CR) ↑ Bifidobacteriaceae,↑ Lactobacillus johnsonii, ↑ Bifidobacterium pseudolongum, ↑ Faecalibaculum (in CR) | [56] |
| Male C57BL/6 mice, 6-week-old | (a) Control (b) mice starved for 24 h (c) mice starved for 24 h and then fed ad libitum for 24 h | faeces | 16S rRNA gene sequencing | In (b) and (c): ↑ Akkermansia, ↑ Parabacteroides ↑ Muribaculum, ↑ Muribaculaceae ↑ Eubacterium coprostanoligenes ↓ Lactobacillus, ↓ Bifidobacterium | [57] |
| Male Fisher 344 x Brown Norway hybrid F1 rats | Control: food and water ad libitum (n = 10) TRF Keto (n = 11) TRF Control (n = 12) | feces | 16S rRNA gene sequencing | ↓ Actinobacteria, ↓ Patescibacteria in TRF Keto and TRF Control (young and old) ↑ Verrucomicrobia in TRF Keto and TRF Control (young and old) | [58] |
| Asian toads | Hibernating Asian toads from 2 cities (TJ, n = 22; XZ, n = 23) [59] | small and/or large gut | 16S rRNA gene amplicon sequencing | ↑ Proteobacteria in the small intestine ↑ Bacteroidetes and Firmicutes in the large intestine | [60] |
| Rainbow trout | 5 weeks Control: food and water ad libitum (n = 32) CR for 3 weeks, followed by 2 weeks of feeding (n = 32) IF for 3 weeks followed by 2 weeks of feeding (n = 32) | proximal intestine | 16S rRNA gene sequencing (V3 region) | In CR and IF groups: ↑ Helicobacter, ↑ Bacteroidetes, ↑ Firmicutes ↓ Actinobacteria | [59] |
| C57BL/6 male mice, 7-week-old | 11 weeks n = 6–7/group; (1) control (2) CR group (30% calorie restriction) (3) IF: 2-day fasting + 5-day ad libitum (4) IFCtrl: 2-day fasting + 5-day feeding, with normal amounts of food | faeces | 16S rRNA gene V3-V4 region | Lactobacillus murinus was the predominant bacterium in the CR, IF, and IFCtrl groups but barely present after fasting | [61] |
| Female Balb/c mice with allergies 6–8 weeks old | 56 days Control: food and water ad libitum (n = 10) 16:8 IF (n = 10) 24: 24 IF (n = 10) | faeces | 16s rRNA sequencing | 24:24 group: ↑ Alistipes, ↑ Eubacterium, ↑ Rikenellaceae-RC9-gut-group, ↑ Alloprevotella 16:8 group: ↑ Lachnospiraceae-UCG-006 ↓ Firmicutes in both IF groups | [62] |
| NASH male C57BL/6 mice, 6-week-old | 16 weeks WDAL: western diet ad libitum (n = 10) WDTRF: western diet time-restricted feeding (16:8) (n = 10) NDAL: normal chow diet ad libitum (n = 10) NDTRF: normal chow diet time-restricted feeding (16:8) (n= 10) | faeces | 16S rRNA gene sequencing | WD treated mice (WDAL and WDTRF): ↓ Bacteroidota, ↓ Proteobacteria ↓ Cyanobacteria ↑ Verrucomicrobia in WDTRF vs WDAL NDTRF group: ↑ Lactobacillus ↑ Muribaculaceae ↑ Dubosiella, ↑ Clostridia ↑ Faecalibacterium, ↓ Helicobacter, ↓ Mucispirillum | [63] |
| C57BL/6 mice, 4-week-old | M-IF female mice (n = 10), 12 weeks Control ad libitum (n = 10) O-IF ND: offspring from M-IF feed with a normal diet (n = 10) O-AL ND: offspring from ad libitum feed with a normal diet (n = 10) | ileum | 16S rRNA gene sequencing | ↓ Lactobacillus intestinalis in O-IF ND and O-AL ND | [64] |
| C57BL/6 mice with induced colitis | SIF, 2 weeks (n = 8) LIF, 20 weeks (n = 8) IF = fed every other day (n = 8) SAL (n = 8) LAL (n = 8) | feces | 16S rRNA sequencing | SIF vs. SAL: ↓ Firmicutes, ↑ Bacteroidetes ↓ Verrucomicrobia in both SIF and LIF At family level (SIF vs. SAL): ↑ Muribaculaceae,↑ Akkermansiaceae, ↓ Lachnospiraceae,↓ Ruminococcaceae At genus level (SIF vs. SAL): ↑ Bacteroides, ↑ Muribaculum, ↑ Akkermansia, ↓ Ruminiclostridium LIF vs. LAD: ↓ Akkermansiaceae, ↑ Lactobacillaceae | [65] |
| Sprague–Dawley rats, 6–8 weeks old | T2DM group: fed ad libitum with HFD (n = 5) T2DM + CR (30% calories of the HFD) (n = 5) Control: food and water ad libitum (n = 5) | feces | 16S rRNA gene sequencing | In T2DM + CR vs. T2DM: ↑ Alistipes, ↑Allobacum ↑ Lachnospiraceae_NK4A136_group, ↑ Ruminococcaceae_9, ↓ Bacteroides, ↓ Lachnoclostridium, ↓ Bifidobacterium | [66] |
| Male C57BL/6 mice -intestinal ischemia/reperfusion model 6–8 weeks old | Preoperative fasting for 6, 12 or 24 h Control: food and water ad libitum (n= not mentioned) | faeces | 16S rDNA sequencing, metabolomic analysis | Fasting group: ↓ Helicobacter ↑ Ruminococcaceae-UCG-014, ↑ Akkermansia, ↑ Parabacteroides, ↑ Desulfovibrio,↑ Oceanisphaera, ↑ Psychrobacter | [67] |
| Male Wistar rats, 12-month-old, high-fat diet-induced obesity | 5 weeks IF(18/6F) (n = 7); Control: food and water ad libitum (n = 7) | cecum | Genomic DNA isolation | ↓ Firmicutes: Bacteroidetes ratio, ↓ Bacillus velezensis, ↑ Lachnospiraceae, ↑ Lactobacillaceae (in IF group) | [68] |
| Male C57BL/6J mice 6-week-old | HF (60% fat) (n = 6) IF- fed every other day, 12 weeks (n = 6), Control: food and water ad libitum (n = 6) | ileum | 16S rRNA gene V4 amplicon | ↑ Lactobacillus in IF ↑ Akkermansia muciniphila | [69] |
| Male Sprague-Dawley rats (7–8 weeks) monocrotaline model of pulmonary arterial hypertension | 24 days Control (n = 10) Monocrotaline-ad libitum feeding (n = 10) Monocrotaline-IF (every other day feeding) (n = 10) | faeces | 16S ribosomal RNA gene V4-amplicon | in monocrotaline-IF: ↑ Lactobacillus | [70] |
| Male Wistar rats, 20 weeks old | 4 weeks SC (n = 10) TC (n = 10) SIF (n = 10) TIF (n = 10) | feces | Incubation of colonies | ↓ Bifidobacterium, ↓ Lactobacillus, ↓ Enterococcus (in TIF group) ↑ Bifidobacterium, ↑ Enterococcus (in SIF group) | [71] |
| C57BL/6N female mice, 8–10-week-old | SPF: female mice in specific pathogen-free conditions, 48 fasting or not (n = 5) GF: Germfree mice, 24 h fasting or not (n = 5) + oral infections with S. Typhimurium in SPF or GF mice | cecum | 16S rRNA gene sequencing | ↑ Akkermansia, ↓ Bacilli, ↓ Erysipelotrichia in the fasted mice | [72] |
| Healthy Mice [56] | Healthy Mice [57] | Healthy Mice [64] | Mice with Induced-Colitis (Short-Term IF) [65] | Mice with Induced- Colitis (Long-Term IF) [65] | Mice Ischemia/Reperfusion Model [67] | NASH Mice [63] | Mice Infected with S. Typhimurium [72] | Mice with Allergy (16:8 IF) [62] | Mice with Allergy (24:24 IF) [62] | Asian Toads [60] | Rainbow Trouts [59] | Healthy Rats [58] | Rats with HTA [70] | Trained Rats [71] | Obese Rats [68] | Obese Rats [69] | T2DM Model Rats [66] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | ▲ | ▼ | ▼ | |||||||||||||||
| Akkermansia | ▲ | ▲ | ▼ | ▲ | ▲ | ▲ | ||||||||||||
| Alistipes | ▲ | ▲ | ||||||||||||||||
| Allobaculum | ▼ | ▲ | ||||||||||||||||
| Alloprevotella | ▲ | |||||||||||||||||
| Bacilli | ▼ | ▲ | ▼ | |||||||||||||||
| Bacteroides | ▼ | ▲ | ▼ | |||||||||||||||
| Bacteroidetes | ▼ | ▲ | ▲ | ▲ | ||||||||||||||
| Bifidobacterium | ▼ | ▼ | ▼ | |||||||||||||||
| Butyricicoccus | ▼ | |||||||||||||||||
| Clostridium spp. | ▲ | ▲ | ||||||||||||||||
| Dubosiella | ▲ | |||||||||||||||||
| Enterococcus | ▲ | ▼ | ||||||||||||||||
| Eubacterium spp. | ▲ | ▲ | ▼ | |||||||||||||||
| Faecalibacterium | ▲ | |||||||||||||||||
| Firmicutes | ▲ | ▼ | ▼ | ▼ | ▼ | ▲ | ▲ | |||||||||||
| Helicobacter | ▼ | ▼ | ▲ | |||||||||||||||
| Lachnospiraceae | ▼ | ▲ | ▲ | ▲ | ||||||||||||||
| Lactobacillus | ▼ | ▼ | ▲ | ▲ | ▲ | ▼ | ▲ | ▲ | ||||||||||
| Ligactobacillus | ▲ | |||||||||||||||||
| Muribaculaceae | ▲ | ▲ | ▲ | |||||||||||||||
| Parabacteroides | ▼ | ▲ | ▲ | ▼ | ||||||||||||||
| Prevotella | ▼ | |||||||||||||||||
| Proteobacteria | ▲ | ▲ | ||||||||||||||||
| Rhudospirillacea | ▼ | |||||||||||||||||
| Rikenellaceae | ▲ | |||||||||||||||||
| Ruminococcaceae | ▼ | ▲ | ▲ | |||||||||||||||
| Verrucomicrobia | ▲ | ▼ | ▼ | ▲ | ||||||||||||||
| Lachnoclostridium | ▼ |
| Design | Study Population(s) | Dietary Intervention | Main Outcome on Microbiota | References |
|---|---|---|---|---|
| A randomised, controlled, single-blinded study | 151 healthy volunteers | 5 days, Buchinger fasting program (n = 20) Fasting mimetic (n = 100) Control (n = 31) | Buchinger fasting: ↑ Proteobacteria ↓ Firmicutes/Bacteroides ratio Sirtfood intervention: ↑ Actinobacteria | [88] |
| A randomised, controlled, single-blinded trial | 51 healthy volunteers | 5 days, Buchinger fasting program (n = 20) Control (n = 31) | ↓ Firmicutes/Bacteroides ratio ↑ Christensenellaceae species | [89] |
| Intervention pre-and post-design | 16 healthy volunteers | water-only fast (n = 6) juice fast (n = 10) 7 days | The water-only fasting: ↓ Fusobacterium ↑ homogenous gut microbiota | [90] |
| Longitudinal physiologic data in 2 cohorts sampled in 2 different years | 67 young and middle age healthy volunteers | Ramadan IF young (n = 30) Ramadan IF middle age (n = 27) Control (n = 10) 30 days | ↑ alpha diversity only in the younger group ↑ Lachnospiraceae, ↑ Ruminococcaceae ↓ Bacteroidales (Prevotellaceae) | [7] |
| Pilot trial | 15 healthy volunteers | 10 days, Buchinger fasting programme, and subsequent 3-month refeeding | ↓ Firmicutes (Lachnospiraceae and Ruminococcaceae) ↑ Bacteroides, Proteobacteria (Escherichia coli, Bilophila wadsworthia) | [91] |
| Pilot trial | 9 volunteers | Ramadan IF, 29 days | ↑ Faecalibacterium prausnitzii, Roseburia, Eubacterium, and Akkermansia, Bacteroides ↑ Butyricicoccus pullicaecorum | [92] |
| Pilot trial | 9 volunteers | Ramadan IF, 29 days | ↑ A. muciniphila and Bacteroides fragilis | [23] |
| Cohort trial | 34 volunteers (16 Chineseand 18 Pakistaniadults) | Ramadan IF, 29 days | ↓ Coprococcus, Clostridium_XlV spp., Lachnospiracea (both groups) ↑ Dorea, Klebsiella, Faecalibacterium (Chinese group) ↑ Sutterella, Parabacteroides, Alistipes, Bacteroides (Pakistani group) | [93] |
| Cross-sectional study | 30 healthy subjects | Ramadan IF, 29 days | ↑ Bacteroides (both sexes) ↑ Firmicutes (only women) ↑ serum levels of butyrate | [94] |
| Cohort trial | 34 volunteers (16 Chinese; 18 Pakistanis) | Ramadan IF, 29 days | ↑ L-histidine, cordycepin, lycofawcine (Chinese group) ↑ brucine (Pakistani group) | [95] |
| Cohort trial | 30 healthy men | TRF (n = 15) non-TRF (n = 15). | ↑ Bacteroidetes phylum (Prevotella_9, Faecalibacterium, Dialister) in TRF group | [96] |
| Pilot trial | 13 obese patients | 7 days Buchinger fasting program and laxative followed by 6 week probiotic formula | ↑ Faecalibacterium prausnitzii, ↑ Akkermanis, ↑Bifidobacteria | [97] |
| A randomised controlled trial | 35 hypertensive patients with metabolic syndrome | 5-days Buchinger fasting program, followed by a Mediteran-like diet for 3 months | ↓ Bifidobacterium, Coprococcus comes, Roseburia ↑ Faecalibacterium prausnitzii, Bacteroides, Anaerotruncus ↑ propionate production capacity and mucin degradation gene modules | [98] |
| A randomised controlled trial | 39 patients with metabolic syndrome | IF group (n = 21) Control group (n = 18) 8 weeks | ↑ SCFA levels ↑ Ruminococcaceae, Roseburia, and Clostridium ↓ lipopolysaccharides | [99] |
| Intervention pre-and post-design | 71 underweight, normal, or obese volunteers | Women (n = 40) Men (n = 31) TRF regimen (16:8) for 16 days. | ↑ alpha diversity ↑ Bifidobacteria, Lactobacillus (all groups) ↓ Firmicutes, (obese women) ↑ Proteobacteria, Bacteroidetes, Actinobacteria (all women groups) ↑ Firmicutes (normal/underweight female groups) ↓ Firmicutes, Actinobacteria (male normal group) ↑ Proteobacteria, Bacteroidetes (male normal group) ↑ Firmicutes, Bacteroidetes, and Actinobacteria (underweight group) ↓ Proteobacteria (underweight group) | [100] |
| Prospective cohort study | 10 obese postmenopausal women | VLCD (800 kcal/day) for 46 days | ↓ Faecalibacterium prausnitzii, Roseburia genus ↑ Christensenellaceae | [101] |
| Cohort trial | 49 overweight and obese adults | 6-week caloric restriction and 6-week weight stabilisation diet | ↓ Akkermansia muciniphila (Akk HI group) | [102] |
| Pilot trial | 12 obese type 2 diabetics | VLCD, 6 weeks 15 week follow up | ↑ alpha diversity ↓ Collinsella genus, Roseburia, Lachnospiraceae spp. changes observed had reverted until the end of the follow-up | [103] |
| Randomised trial | 16 obese patients | 6-week VLCD+ 12 months follow-up period | ↓ Bacteroides spp., Lactobacillus, but the change was transient. | [104] |
| Cross-sectional study | obese/overweight adolescent patients (n = 13) Lean (n = 8) | Long-term CR (1700 kcal/day), 12 months | ↓ Actinobacteria, ↓ Firmicutes:Bacteroidetes ratio ↑ Bacteroides, Roseburia, Faecalibacterium and Clostridium XIVa | [105] |
| Multicentre trial | 211 obese/pre-diabetic patients | CR (800–1200 kcal/day), 8 weeks | ↑ Akkermansia and Christensenellaceae R-7 group ↓ Blautia, Bifidobacterium spp. | [106] |
| Design | Population | Dietary Regimens | Main Findings | References |
|---|---|---|---|---|
| Randomised trial | 59 overweight or obese adults | CR (n = 25) IF (n = 34) 3 months |
| [116] |
| Real-life study | 49 obese patients | TRF (n = 25) CR(n = 24) 12 weeks |
| [117] |
| Controlled parallel design trial | 61 obese patients | VLCKD (n = 18), 2 months BS (n = 22) MetDiet (n = 21) 6 months |
| [118] |
| Randomised trial | 62 overweight or obese patients | CCR (n = 27) IF (n = 35) 12 months |
| [119] |
| A parallel-arm randomised controlled study | 147 overweight or obese adults | IF 5:2 (n = 47) CCR (n = 46) Control (n = 51) 50 weeks |
| [120] |
| Healthy Chinese; Ramadan [86] | Healthy Pakistani; Ramadan [86] | Healthy (Both Groups); Ramadan [86] | Healthy; Buchinger [83] | Healthy Volunteers; IF(16:8) [90] | Healthy Volunteers; Buchinger [80] | Healthy; Ramadan [88] | Healthy Volunteers; Ramadan [23] | Healthy Volunteers; Ramadan [85] | Healthy Volunteers; Ramadan [7] | Obese Women; VLCD [95] | Obese Patients; TRF [106] | Obese Patients; VLCD [102] | Obese Patients; VLCKD [107] | Metabolic Syndrome; IF(5:2) [94] | Obese/Pre-Diabetic; CR [104] | Obese Patients; IF(16:8) [99] | HTA/metab. syn; Buchinger [93] | Obese Patiemts; Buchinger [92] | Obese Adolescents; CR [97] | Overweight Patients; CR [105] | Obese Patients; IF(5:2) [109] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinobacteria | ▼ | ▲ | ▼ | |||||||||||||||||||
| Actinomyces | ▲ | |||||||||||||||||||||
| Akkermansia | ▲ | ▲ | ▲ | ▲ | ▲ | |||||||||||||||||
| Alistipes | ▲ | ▲ | ▲ | ▲ | ||||||||||||||||||
| Alpha diversity | ▲ | ▲ | ▲ | ▲ | ▲ | |||||||||||||||||
| Anaerotruncus | ▲ | |||||||||||||||||||||
| Bacteroides | ▼ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | ▲ | |||||||||||||
| Bifidobacterium | ▼ | ▲ | ▼ | ▲ | ||||||||||||||||||
| Blautia | ▼ | ▼ | ||||||||||||||||||||
| Butyricoccus | ▲ | ▲ | ||||||||||||||||||||
| Christensellaceae spp. | ▲ | ▲ | ▲ | ▲ | ||||||||||||||||||
| Clostridium XIV spp. | ▼ | ▲ | ▲ | |||||||||||||||||||
| Collinsella | ▼ | ▼ | ||||||||||||||||||||
| Coprococcus | ▼ | ▼ | ▼ | |||||||||||||||||||
| Dialister | ▼ | ▲ | ||||||||||||||||||||
| Dorea | ▲ | ▲ | ||||||||||||||||||||
| Eggerthella | ▲ | |||||||||||||||||||||
| Eubacterium | ▼ | ▲ | ▲ | |||||||||||||||||||
| Faecalibacterium | ▲ | ▼ | ▲ | ▲ | ▲ | ▼ | ▲ | |||||||||||||||
| Lachnospiraceae spp. | ▼ | ▲ | ▲ | ▼ | ▼ | |||||||||||||||||
| Lactobacillus | ▲ | ▲ | ||||||||||||||||||||
| Oscillibacter | ▼ | |||||||||||||||||||||
| Parabacteroides | ▲ | ▲ | ▲ | |||||||||||||||||||
| Prevotella | ▲ | ▼ | ||||||||||||||||||||
| Pseudoflavonifractor | ▲ | |||||||||||||||||||||
| Romboutsia | ▼ | ▼ | ▲ | ▲ | ||||||||||||||||||
| Roseburia | ▼ | ▲ | ▼ | ▼ | ▼ | ▲ | ▲ | |||||||||||||||
| Ruminococcaceae spp. | ▲ | |||||||||||||||||||||
| Streptococcus | ▼ | ▼ | ||||||||||||||||||||
| Subdoligranulum | ▲ | ▼ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Purdel, C.; Margină, D.; Adam-Dima, I.; Ungurianu, A. The Beneficial Effects of Dietary Interventions on Gut Microbiota—An Up-to-Date Critical Review and Future Perspectives. Nutrients 2023, 15, 5005. https://doi.org/10.3390/nu15235005
Purdel C, Margină D, Adam-Dima I, Ungurianu A. The Beneficial Effects of Dietary Interventions on Gut Microbiota—An Up-to-Date Critical Review and Future Perspectives. Nutrients. 2023; 15(23):5005. https://doi.org/10.3390/nu15235005
Chicago/Turabian StylePurdel, Carmen, Denisa Margină, Ines Adam-Dima, and Anca Ungurianu. 2023. "The Beneficial Effects of Dietary Interventions on Gut Microbiota—An Up-to-Date Critical Review and Future Perspectives" Nutrients 15, no. 23: 5005. https://doi.org/10.3390/nu15235005
APA StylePurdel, C., Margină, D., Adam-Dima, I., & Ungurianu, A. (2023). The Beneficial Effects of Dietary Interventions on Gut Microbiota—An Up-to-Date Critical Review and Future Perspectives. Nutrients, 15(23), 5005. https://doi.org/10.3390/nu15235005







