Exercise and Caloric Restriction Exert Different Benefits on Skeletal Muscle Metabolism in Aging Condition
Abstract
:1. Introduction
2. Materials and Methods
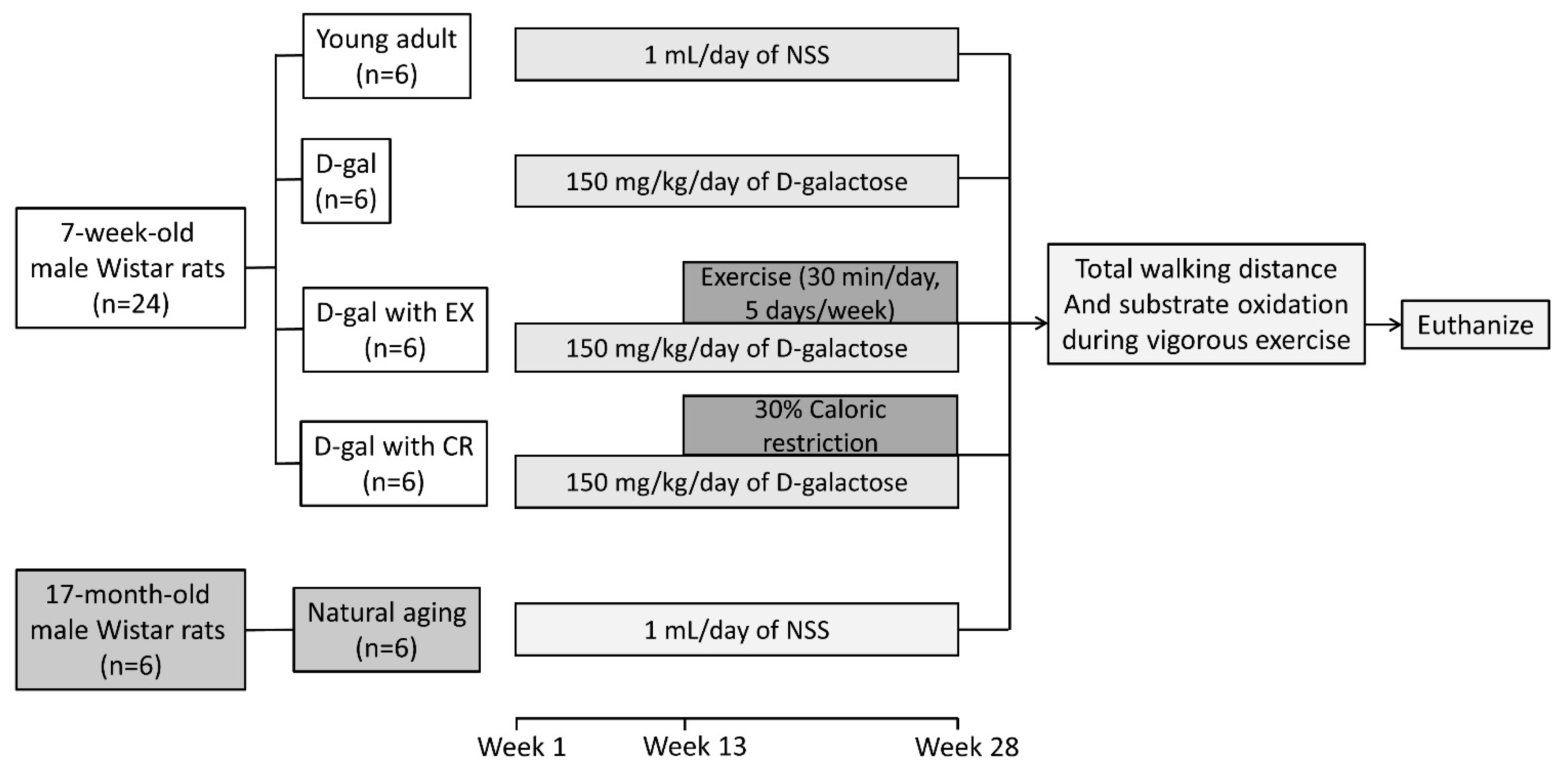
2.1. Experimental Protocol
2.2. Exercise Training Protocol
2.3. Blood Metabolic Parameters
2.4. Serum Testosterone Level
2.5. Walking Distance to Determine Locomotor Activity
2.6. Whole-Body Fatty Acid and Carbohydrate Oxidation during Physical Activity
2.7. Skeletal Muscle Metabolomics
2.8. Skeletal Muscle Protein Expression Analyses
2.9. Skeletal Muscle Reactive Oxygen Species (ROS) Level
2.10. Statistical Analyses
3. Results
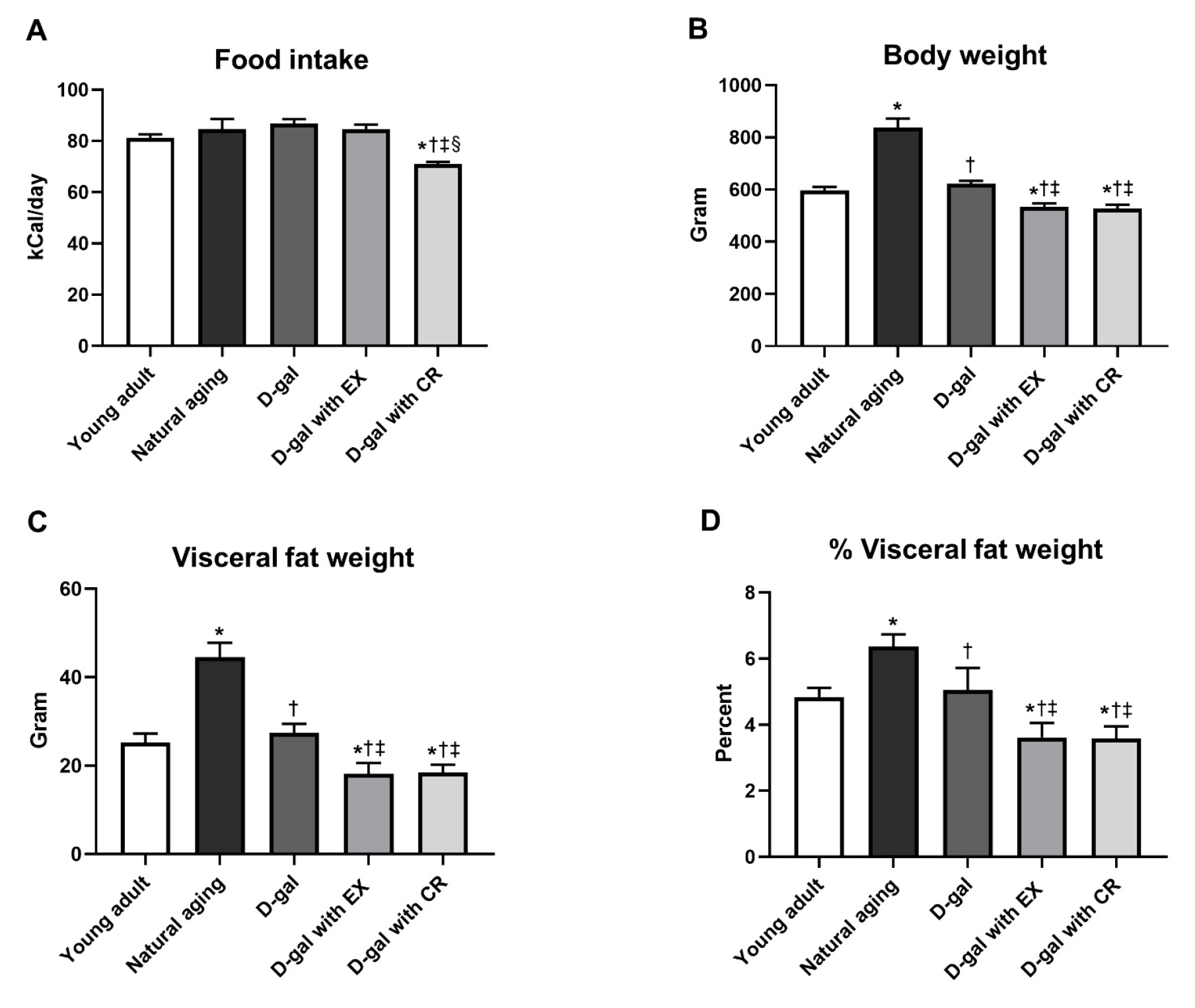
3.1. Exercise and Caloric Restriction Equally Decreased Body Weight and Visceral Fat Weight in D-Galactose-Induced Aged Rats
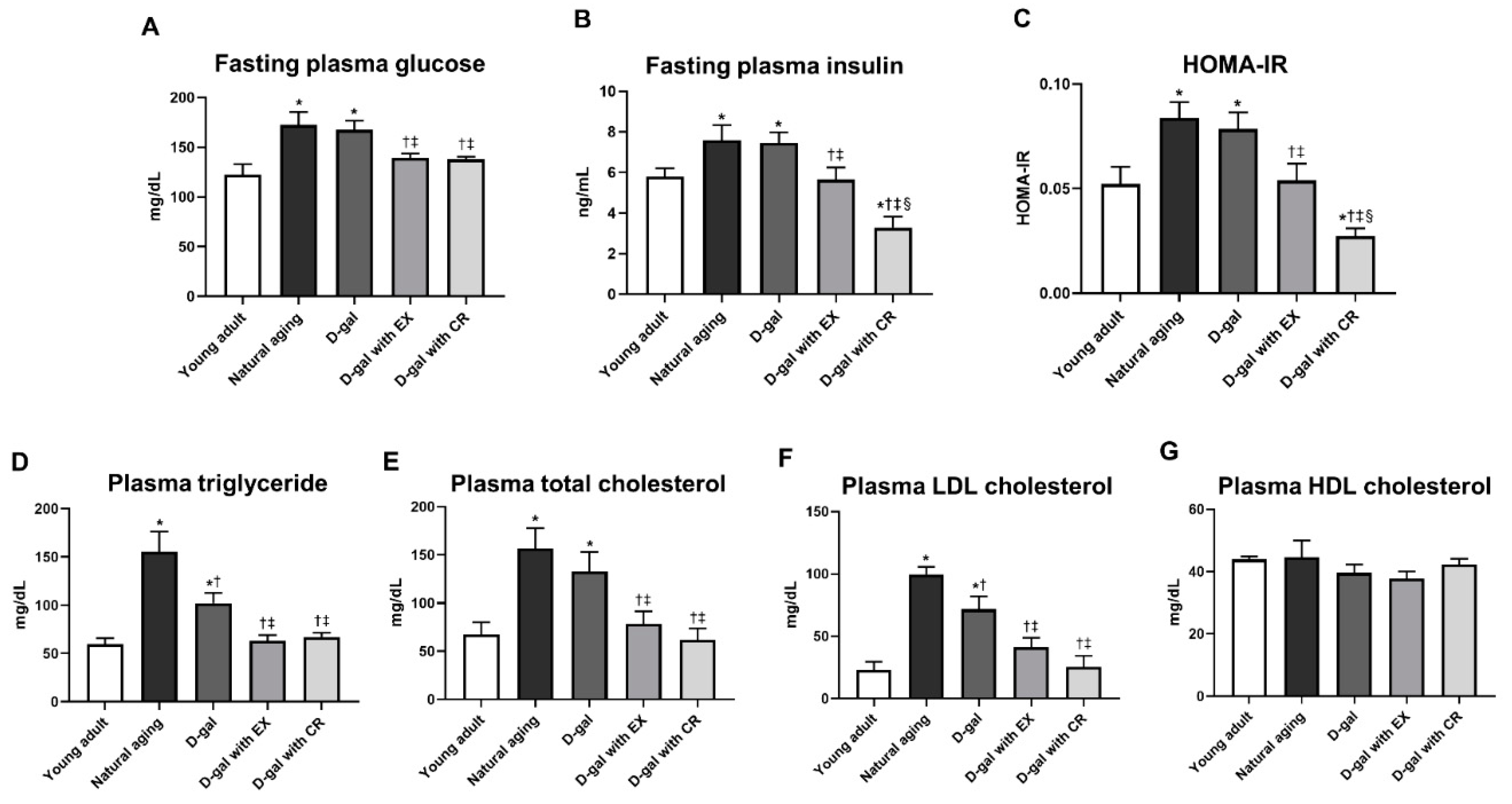
3.2. Caloric Restriction Is Superior to Exercise in Terms of Improving Systemic Insulin Sensitivity in D-Galactose-Induced Aged Rats
3.3. Exercise and Caloric Restriction Equally Improve the Blood Lipid Profile in D-Galactose-Induced Aged Rats
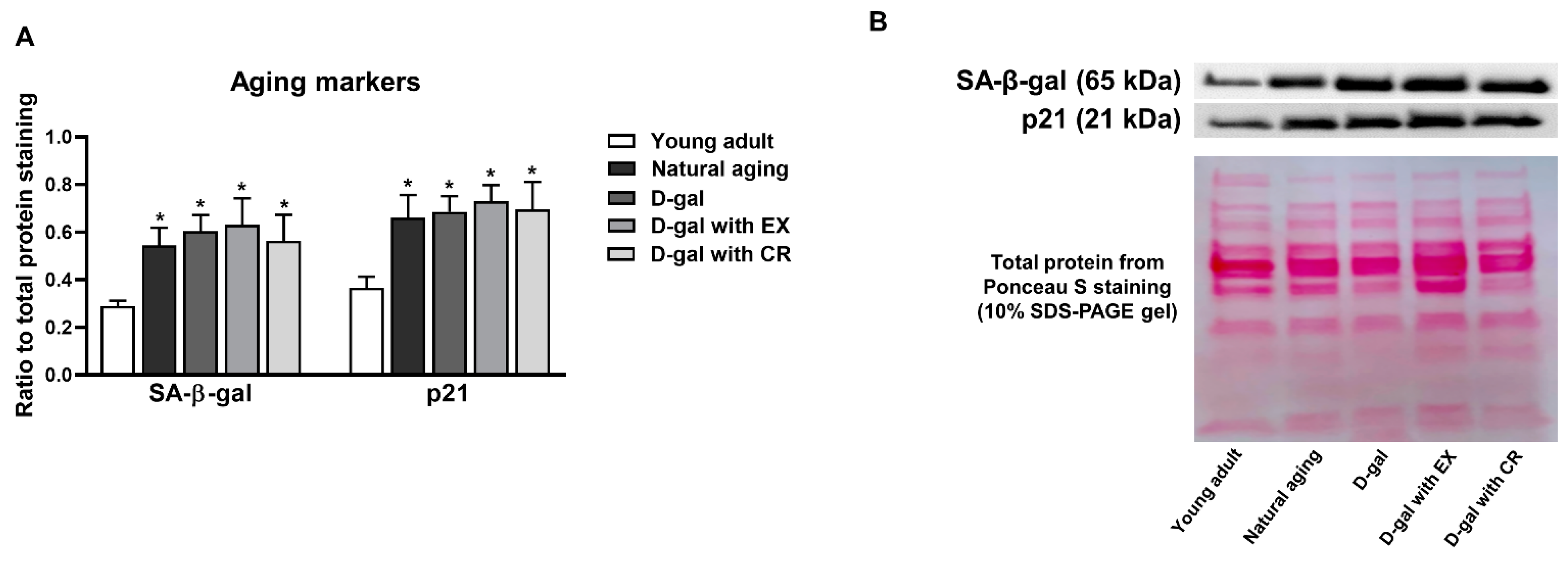
3.4. D-Galactose Administration Results in Cellular Senescence of Skeletal Muscle Which Cannot Be Attenuated by Either Exercise or Caloric Restriction
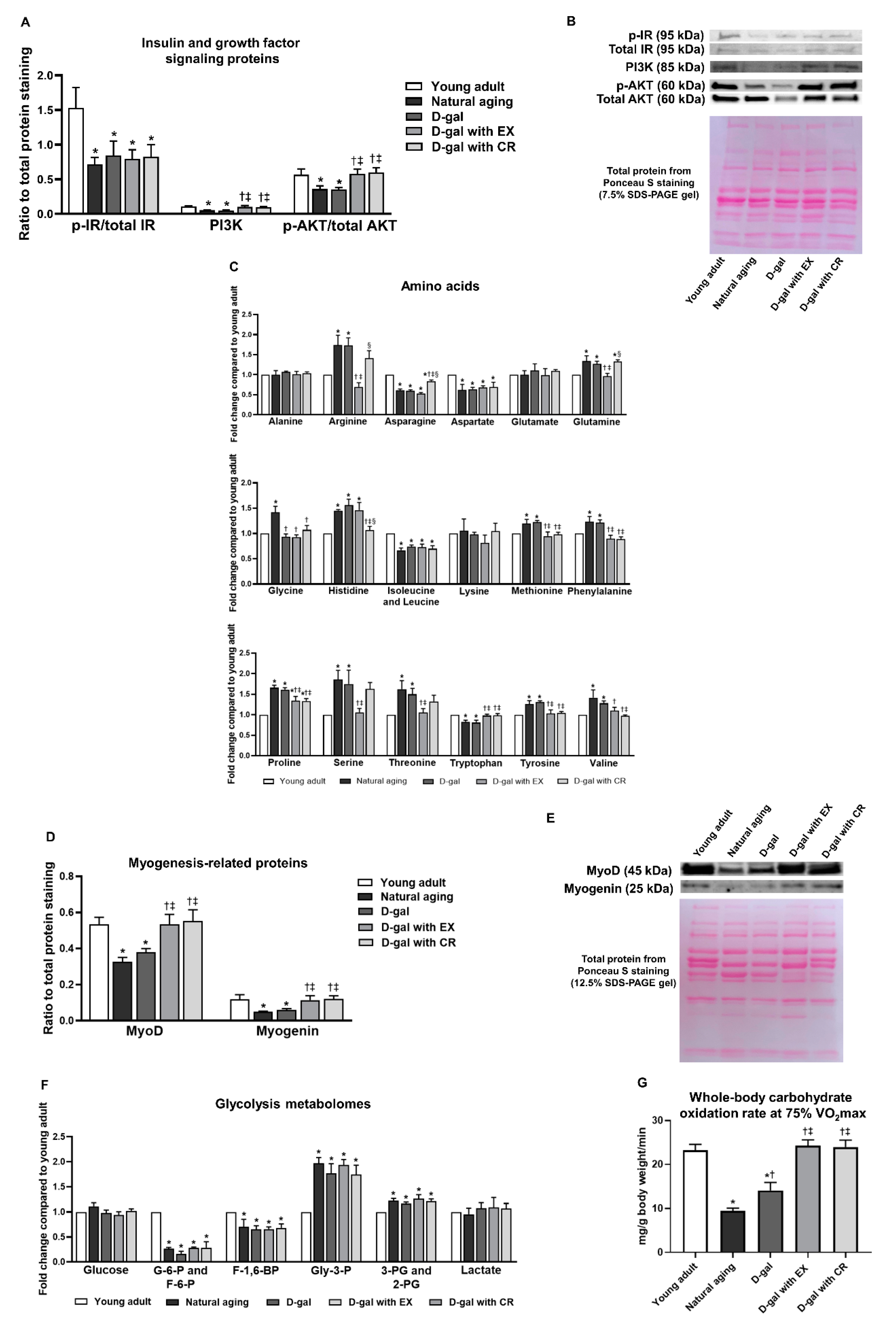
3.5. Exercise and Caloric Restriction Equally Alleviate the Impairment of Growth Factor Signaling in Skeletal Muscle and Improve Carbohydrate Oxidation during Physical Activity—However, These Interventions Fail to Attenuate Skeletal Muscle Insulin Sensitivity in the Sedentary State in the Aging Condition
3.6. Exercise Is More Effective than Caloric Restriction in Improving Skeletal Muscle Fatty Acid Oxidation in the Aging Condition
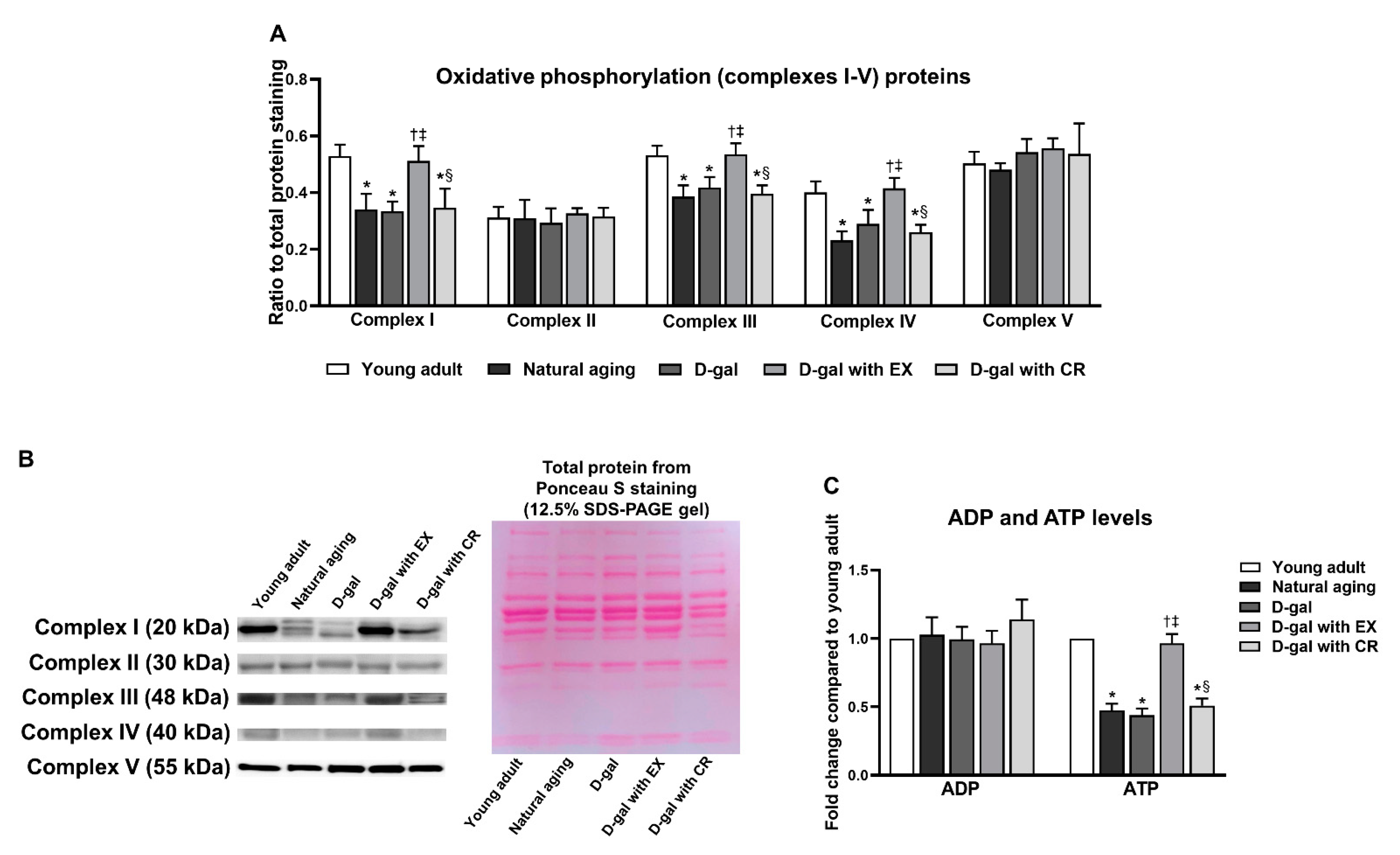
3.7. Exercise Improves Oxidative Phosphorylation and ATP Production of Skeletal Muscle in Aging Condition
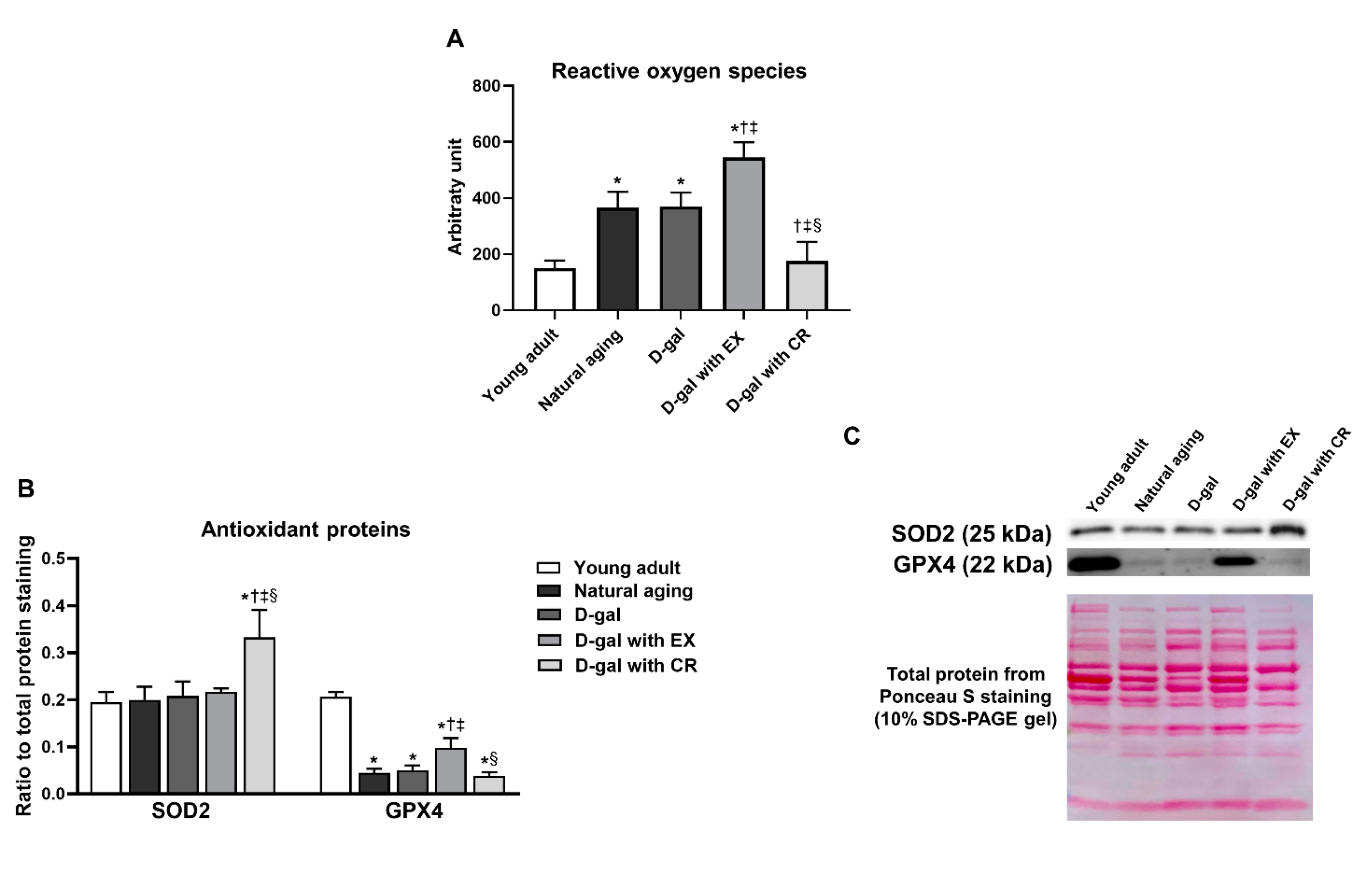
3.8. Caloric Restriction Decreases Aging-Induced Oxidative Stress in Skeletal Muscle, While Exercise Aggravates Aging-Induced Oxidative Stress in Skeletal Muscle
3.9. Caloric Restriction Is Superior to Exercise in Terms of Improving Antioxidative Capacity in Skeletal Muscle in the Aging Condition
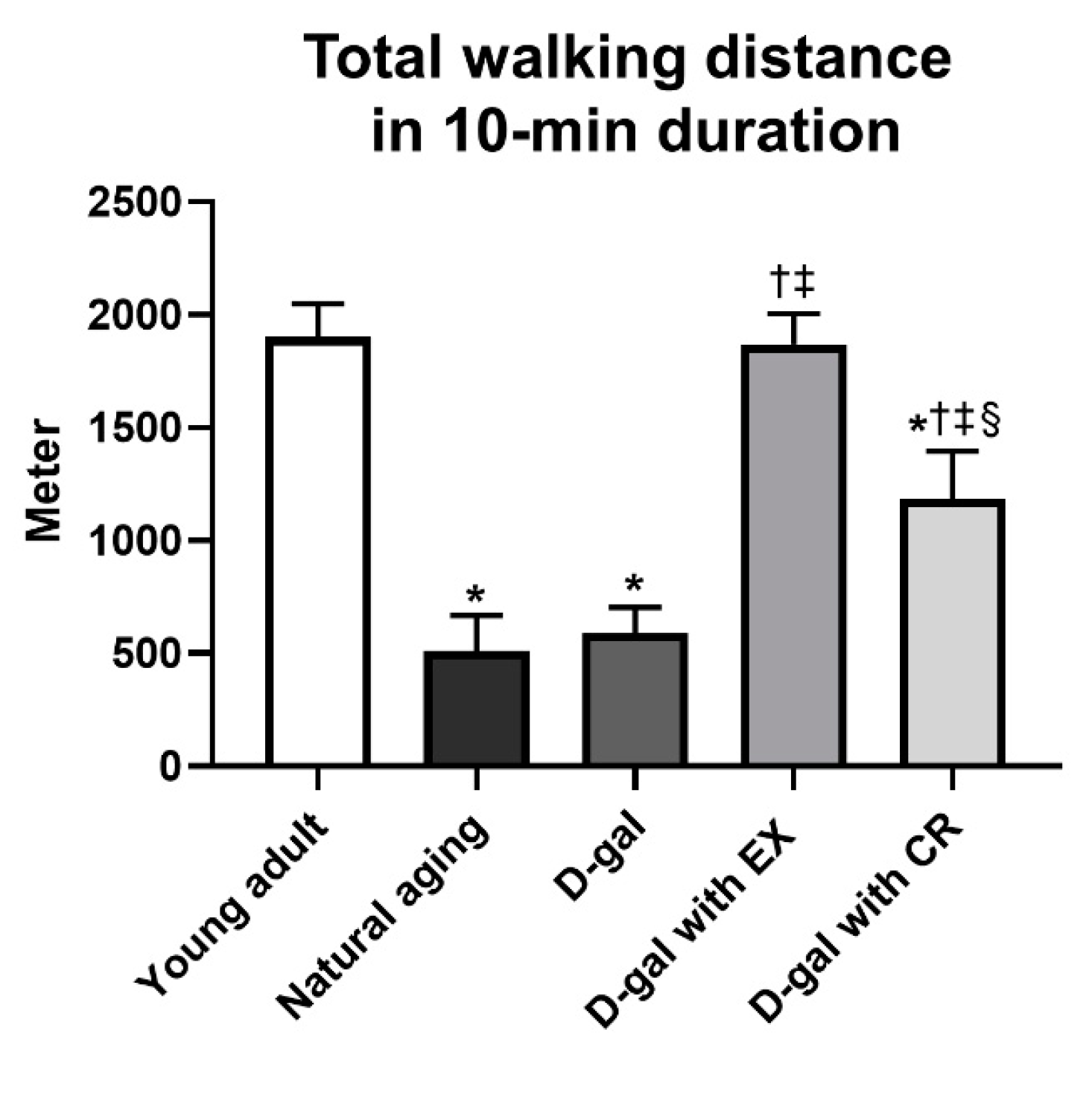
3.10. Exercise Is Superior to Caloric Restriction in Terms of Improving Locomotor Activity in Aging Condition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knickman, J.R.; Snell, E.K. The 2030 problem: Caring for aging baby boomers. Health Serv. Res. 2002, 37, 849–884. [Google Scholar] [CrossRef] [PubMed]
- Brody, J.A.; Grant, M.D. Age-associated diseases and conditions: Implications for decreasing late life morbidity. Aging 2001, 13, 64–67. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.; Vasilaki, A. Age-related changes in skeletal muscle: Changes to life-style as a therapy. Biogerontology 2018, 19, 519–536. [Google Scholar] [CrossRef] [PubMed]
- Frontera, W.R.; Ochala, J. Skeletal muscle: A brief review of structure and function. Calcif. Tissue Int. 2015, 96, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, W.K.; Wilkinson, D.J.; Phillips, B.E.; Lund, J.N.; Smith, K.; Atherton, P.J. Human Skeletal Muscle Protein Metabolism Responses to Amino Acid Nutrition. Adv. Nutr. 2016, 7, 828s–838s. [Google Scholar] [CrossRef]
- Blum, J.; Epstein, R.; Watts, S.; Thalacker-Mercer, A. Importance of Nutrient Availability and Metabolism for Skeletal Muscle Regeneration. Front. Physiol. 2021, 12, 696018. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Kraus, R.M.; Hickner, R.C.; Howell, A.W.; Wolfe, R.R.; Dohm, G.L. Impaired plasma fatty acid oxidation in extremely obese women. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E1076–E1081. [Google Scholar] [CrossRef]
- Stump, C.S.; Henriksen, E.J.; Wei, Y.; Sowers, J.R. The metabolic syndrome: Role of skeletal muscle metabolism. Ann. Med. 2006, 38, 389–402. [Google Scholar] [CrossRef]
- Rahman, F.A.; Quadrilatero, J. Mitochondrial network remodeling: An important feature of myogenesis and skeletal muscle regeneration. Cell. Mol. Life Sci. 2021, 78, 4653–4675. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building muscle: Molecular regulation of myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Husmann, I.; Soulet, L.; Gautron, J.; Martelly, I.; Barritault, D. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev. 1996, 7, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Arsic, N.; Zacchigna, S.; Zentilin, L.; Ramirez-Correa, G.; Pattarini, L.; Salvi, A.; Sinagra, G.; Giacca, M. Vascular endothelial growth factor stimulates skeletal muscle regeneration in vivo. Mol. Ther. 2004, 10, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 2010, 346, 267–278. [Google Scholar] [CrossRef]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, M.; Huertas, J.R.; Villalba, J.M.; Casuso, R.A. Mitochondrial adaptations to calorie restriction and bariatric surgery in human skeletal muscle: A systematic review with meta-analysis. Metabolism 2022, 138, 155336. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Dubé, J.J.; Coen, P.M.; Stefanovic-Racic, M.; Toledo, F.G.; Goodpaster, B.H. Physical inactivity and obesity underlie the insulin resistance of aging. Diabetes Care 2009, 32, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Amati, F.; Pennant, M.; Azuma, K.; Dubé, J.J.; Toledo, F.G.; Rossi, A.P.; Kelley, D.E.; Goodpaster, B.H. Lower thigh subcutaneous and higher visceral abdominal adipose tissue content both contribute to insulin resistance. Obesity 2012, 20, 1115–1117. [Google Scholar] [CrossRef]
- Safdar, A.; Hamadeh, M.J.; Kaczor, J.J.; Raha, S.; Debeer, J.; Tarnopolsky, M.A. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS ONE 2010, 5, e10778. [Google Scholar] [CrossRef]
- Lanza, I.R.; Short, D.K.; Short, K.R.; Raghavakaimal, S.; Basu, R.; Joyner, M.J.; McConnell, J.P.; Nair, K.S. Endurance exercise as a countermeasure for aging. Diabetes 2008, 57, 2933–2942. [Google Scholar] [CrossRef]
- Menshikova, E.V.; Ritov, V.B.; Dube, J.J.; Amati, F.; Stefanovic-Racic, M.; Toledo, F.G.S.; Coen, P.M.; Goodpaster, B.H. Calorie Restriction-induced Weight Loss and Exercise Have Differential Effects on Skeletal Muscle Mitochondria Despite Similar Effects on Insulin Sensitivity. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 73, 81–87. [Google Scholar] [CrossRef]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; van Kranenburg, J.; Nilwik, R.; van Loon, L.J. Elderly men and women benefit equally from prolonged resistance-type exercise training. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 769–779. [Google Scholar] [CrossRef]
- Bua, E.; McKiernan, S.H.; Aiken, J.M. Calorie restriction limits the generation but not the progression of mitochondrial abnormalities in aging skeletal muscle. FASEB J. 2004, 18, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Tomasiewicz, J.L.; Yang, S.E.; Miller, B.R.; Wakai, M.H.; Sherman, D.S.; Cummings, N.E.; Baar, E.L.; Brinkman, J.A.; Syed, F.A.; et al. Calorie-Restriction-Induced Insulin Sensitivity Is Mediated by Adipose mTORC2 and Not Required for Lifespan Extension. Cell Rep. 2019, 29, 236–248.e3. [Google Scholar] [CrossRef] [PubMed]
- Shwe, T.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp. Gerontol. 2018, 101, 13–36. [Google Scholar] [CrossRef] [PubMed]
- Bo-Htay, C.; Palee, S.; Apaijai, N.; Chattipakorn, S.C.; Chattipakorn, N. Effects of d-galactose-induced ageing on the heart and its potential interventions. J. Cell. Mol. Med. 2018, 22, 1392–1410. [Google Scholar] [CrossRef]
- Pantiya, P.; Thonusin, C.; Ongnok, B.; Chunchai, T.; Kongkaew, A.; Nawara, W.; Arunsak, B.; Chattipakorn, N.; Chattipakorn, S.C. Chronic D-galactose administration induces natural aging characteristics, in rat’s brain and heart. Toxicology 2023, 492, 153553. [Google Scholar] [CrossRef] [PubMed]
- Thonusin, C.; Pantiya, P.; Sumneang, N.; Chunchai, T.; Nawara, W.; Arunsak, B.; Siri-Angkul, N.; Sriwichaiin, S.; Chattipakorn, S.C.; Chattipakorn, N. Effectiveness of high cardiorespiratory fitness in cardiometabolic protection in prediabetic rats. Mol. Med. 2022, 28, 31. [Google Scholar] [CrossRef]
- Wang, R.; Tian, H.; Guo, D.; Tian, Q.; Yao, T.; Kong, X. Impacts of exercise intervention on various diseases in rats. J. Sport Health Sci. 2020, 9, 211–227. [Google Scholar] [CrossRef]
- Lee, J.; Cho, J.Y.; Kim, W.K. Anti-inflammation effect of Exercise and Korean red ginseng in aging model rats with diet-induced atherosclerosis. Nutr. Res. Pract. 2014, 8, 284–291. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Overmyer, K.A.; Evans, C.R.; Qi, N.R.; Minogue, C.E.; Carson, J.J.; Chermside-Scabbo, C.J.; Koch, L.G.; Britton, S.L.; Pagliarini, D.J.; Coon, J.J.; et al. Maximal oxidative capacity during exercise is associated with skeletal muscle fuel selection and dynamic changes in mitochondrial protein acetylation. Cell Metab. 2015, 21, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Farinatti, P.; Castinheiras Neto, A.G.; Amorim, P.R. Oxygen Consumption and Substrate Utilization During and After Resistance Exercises Performed with Different Muscle Mass. Int. J. Exerc. Sci. 2016, 9, 77–88. [Google Scholar] [PubMed]
- Gonzalez, J.T.; Green, B.P.; Campbell, M.D.; Rumbold, P.L.; Stevenson, E.J. The influence of calcium supplementation on substrate metabolism during exercise in humans: A randomized controlled trial. Eur. J. Clin. Nutr. 2014, 68, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Thonusin, C.; Nawara, W.; Khuanjing, T.; Prathumsup, N.; Arinno, A.; Ongnok, B.; Arunsak, B.; Sriwichaiin, S.; Chattipakorn, S.C.; Chattipakorn, N. Blood metabolomes as non-invasive biomarkers and targets of metabolic interventions for doxorubicin and trastuzumab-induced cardiotoxicity. Arch. Toxicol. 2022, 97, 603–618. [Google Scholar] [CrossRef]
- Thonusin, C.; IglayReger, H.B.; Soni, T.; Rothberg, A.E.; Burant, C.F.; Evans, C.R. Evaluation of intensity drift correction strategies using MetaboDrift, a normalization tool for multi-batch metabolomics data. J. Chromatogr. A 2017, 1523, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Thonusin, C.; Apaijai, N.; Jaiwongkam, T.; Kerdphoo, S.; Arunsak, B.; Amput, P.; Palee, S.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. The comparative effects of high dose atorvastatin and proprotein convertase subtilisin/kexin type 9 inhibitor on the mitochondria of oxidative muscle fibers in obese-insulin resistant female rats. Toxicol. Appl. Pharmacol. 2019, 382, 114741. [Google Scholar] [CrossRef] [PubMed]
- Thonusin, C.; Pantiya, P.; Jaiwongkam, T.; Kerdphoo, S.; Arunsak, B.; Amput, P.; Palee, S.; Pratchayasakul, W.; Chattipakorn, N.; Chattipakorn, S.C. A proprotein convertase subtilisin/kexin type 9 inhibitor provides comparable efficacy with lower detriment than statins on mitochondria of oxidative muscle of obese estrogen-deprived rats. Menopause 2020, 27, 1155–1166. [Google Scholar] [CrossRef]
- Papismadov, N.; Gal, H.; Krizhanovsky, V. The anti-aging promise of p21. Cell Cycle 2017, 16, 1997–1998. [Google Scholar] [CrossRef]
- Lee, B.Y.; Han, J.A.; Im, J.S.; Morrone, A.; Johung, K.; Goodwin, E.C.; Kleijer, W.J.; DiMaio, D.; Hwang, E.S. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell 2006, 5, 187–195. [Google Scholar] [CrossRef]
- Conte, F.; van Buuringen, N.; Voermans, N.C.; Lefeber, D.J. Galactose in human metabolism, glycosylation and congenital metabolic diseases: Time for a closer look. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129898. [Google Scholar] [CrossRef]
- Cabelof, D.C.; Yanamadala, S.; Raffoul, J.J.; Guo, Z.; Soofi, A.; Heydari, A.R. Caloric restriction promotes genomic stability by induction of base excision repair and reversal of its age-related decline. DNA Repair 2003, 2, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Werner, C.; Hanhoun, M.; Widmann, T.; Kazakov, A.; Semenov, A.; Pöss, J.; Bauersachs, J.; Thum, T.; Pfreundschuh, M.; Müller, P.; et al. Effects of physical exercise on myocardial telomere-regulating proteins, survival pathways, and apoptosis. J. Am. Coll. Cardiol. 2008, 52, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Kamei, Y.; Hatazawa, Y.; Uchitomi, R.; Yoshimura, R.; Miura, S. Regulation of Skeletal Muscle Function by Amino Acids. Nutrients 2020, 12, 261. [Google Scholar] [CrossRef] [PubMed]
- Syverud, B.C.; VanDusen, K.W.; Larkin, L.M. Growth Factors for Skeletal Muscle Tissue Engineering. Cells Tissues Organs 2016, 202, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.P.; Duarte, J.A. Protein Turnover in Skeletal Muscle: Looking at Molecular Regulation towards an Active Lifestyle. Int. J. Sports Med. 2023, 44, 763–777. [Google Scholar] [CrossRef]
- Gopalan, V.; Michael, N.; Ishino, S.; Lee, S.S.; Yang, A.Y.; Bhanu Prakash, K.N.; Yaligar, J.; Sadananthan, S.A.; Kaneko, M.; Zhou, Z.; et al. Effect of Exercise and Calorie Restriction on Tissue Acylcarnitines, Tissue Desaturase Indices, and Fat Accumulation in Diet-Induced Obese Rats. Sci. Rep. 2016, 6, 26445. [Google Scholar] [CrossRef]
- Nsiah-Sefaa, A.; McKenzie, M. Combined defects in oxidative phosphorylation and fatty acid β-oxidation in mitochondrial disease. Biosci. Rep. 2016, 36, e00313. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty Infiltration of Skeletal Muscle: Mechanisms and Comparisons with Bone Marrow Adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef]
- Stojković, B.M.; Đorđević, M. Interaction between mitochondrial and nuclear genomes: The role in life-history evolution. Biol. Serbica 2017, 39, 32–40. [Google Scholar]
- Henriksen, E.J.; Diamond-Stanic, M.K.; Marchionne, E.M. Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic. Biol. Med. 2011, 51, 993–999. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R.A.; Chang, Y.Z. Physical exercise: An inducer of positive oxidative stress in skeletal muscle aging. Life Sci. 2020, 252, 117630. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Valaei, K.; Arazi, H.; Suzuki, K. An Overview of Physical Exercise and Antioxidant Supplementation Influences on Skeletal Muscle Oxidative Stress. Antioxidants 2021, 10, 1528. [Google Scholar] [CrossRef] [PubMed]
- Antonioni, A.; Fantini, C.; Dimauro, I.; Caporossi, D. Redox homeostasis in sport: Do athletes really need antioxidant support? Res. Sports Med. 2019, 27, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Cartee, G.D.; Hepple, R.T.; Bamman, M.M.; Zierath, J.R. Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab. 2016, 23, 1034–1047. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Exercise Metabolism: Fuels for the Fire. Cold Spring Harb. Perspect. Med. 2018, 8, a029744. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, M.; Spriet, L.L. Skeletal muscle energy metabolism during exercise. Nat. Metab. 2020, 2, 817–828. [Google Scholar] [CrossRef] [PubMed]
- Korenblat, K.M.; Fabbrini, E.; Mohammed, B.S.; Klein, S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology 2008, 134, 1369–1375. [Google Scholar] [CrossRef]
- Lanza, I.R.; Zabielski, P.; Klaus, K.A.; Morse, D.M.; Heppelmann, C.J.; Bergen, H.R., 3rd; Dasari, S.; Walrand, S.; Short, K.R.; Johnson, M.L.; et al. Chronic caloric restriction preserves mitochondrial function in senescence without increasing mitochondrial biogenesis. Cell Metab. 2012, 16, 777–788. [Google Scholar] [CrossRef]
- Feuers, R.J.; Weindruch, R.; Hart, R.W. Caloric restriction, aging, and antioxidant enzymes. Mutat. Res. 1993, 295, 191–200. [Google Scholar] [CrossRef]
- Gredilla, R.; Barja, G. Minireview: The role of oxidative stress in relation to caloric restriction and longevity. Endocrinology 2005, 146, 3713–3717. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.E. Skeletal muscle fat oxidation: Timing and flexibility are everything. J. Clin. Investig. 2005, 115, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [PubMed]
- Bell, J.A.; Reed, M.A.; Consitt, L.A.; Martin, O.J.; Haynie, K.R.; Hulver, M.W.; Muoio, D.M.; Dohm, G.L. Lipid partitioning, incomplete fatty acid oxidation, and insulin signal transduction in primary human muscle cells: Effects of severe obesity, fatty acid incubation, and fatty acid translocase/CD36 overexpression. J. Clin. Endocrinol. Metab. 2010, 95, 3400–3410. [Google Scholar] [CrossRef] [PubMed]
- Kelly, D.M.; Jones, T.H. Testosterone and obesity. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2015, 16, 581–606. [Google Scholar] [CrossRef] [PubMed]
- You, J.S.; Barai, P.; Chen, J. Sex differences in skeletal muscle size, function, and myosin heavy chain isoform expression during post-injury regeneration in mice. Physiol. Rep. 2023, 11, e15791. [Google Scholar] [CrossRef] [PubMed]
- Haizlip, K.M.; Harrison, B.C.; Leinwand, L.A. Sex-based differences in skeletal muscle kinetics and fiber-type composition. Physiology 2015, 30, 30–39. [Google Scholar] [CrossRef]
- Lundsgaard, A.M.; Kiens, B. Gender differences in skeletal muscle substrate metabolism–molecular mechanisms and insulin sensitivity. Front. Endocrinol. 2014, 5, 195. [Google Scholar] [CrossRef]
- Alexander, S.E.; Pollock, A.C.; Lamon, S. The effect of sex hormones on skeletal muscle adaptation in females. Eur. J. Sport Sci. 2022, 22, 1035–1045. [Google Scholar] [CrossRef]
- Enns, D.L.; Tiidus, P.M. The influence of estrogen on skeletal muscle: Sex matters. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef]
- Herbst, K.L.; Bhasin, S. Testosterone action on skeletal muscle. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 271–277. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thonusin, C.; Pantiya, P.; Kongkaew, A.; Nawara, W.; Arunsak, B.; Sriwichaiin, S.; Chattipakorn, N.; Chattipakorn, S.C. Exercise and Caloric Restriction Exert Different Benefits on Skeletal Muscle Metabolism in Aging Condition. Nutrients 2023, 15, 5004. https://doi.org/10.3390/nu15235004
Thonusin C, Pantiya P, Kongkaew A, Nawara W, Arunsak B, Sriwichaiin S, Chattipakorn N, Chattipakorn SC. Exercise and Caloric Restriction Exert Different Benefits on Skeletal Muscle Metabolism in Aging Condition. Nutrients. 2023; 15(23):5004. https://doi.org/10.3390/nu15235004
Chicago/Turabian StyleThonusin, Chanisa, Patcharapong Pantiya, Aphisek Kongkaew, Wichwara Nawara, Busarin Arunsak, Sirawit Sriwichaiin, Nipon Chattipakorn, and Siriporn C. Chattipakorn. 2023. "Exercise and Caloric Restriction Exert Different Benefits on Skeletal Muscle Metabolism in Aging Condition" Nutrients 15, no. 23: 5004. https://doi.org/10.3390/nu15235004
APA StyleThonusin, C., Pantiya, P., Kongkaew, A., Nawara, W., Arunsak, B., Sriwichaiin, S., Chattipakorn, N., & Chattipakorn, S. C. (2023). Exercise and Caloric Restriction Exert Different Benefits on Skeletal Muscle Metabolism in Aging Condition. Nutrients, 15(23), 5004. https://doi.org/10.3390/nu15235004





