1. Introduction
Iron deficiency (ID) and iron deficiency anemia (IDA) are highly prevalent in South Asia [
1], with estimated rates for mild to moderate IDA in children in Bangladesh exceeding 55% [
2], a level well above global estimates [
3]. Rural areas in Bangladesh have higher rates of IDA than do urban areas [
4]. Although supplementation has been used as a strategy for addressing ID in countries like Bangladesh, this strategy has faced a number of challenges, including poor compliance [
5]. Additionally, contaminated water, poor hygiene, and lack of bioavailability make supplementation a less sustainable option [
6].
A more sustainable alternative to supplementation is iron fortification, and there are many benefits of fortifying staple foods such as lentils with iron. For example, fortification improves the availability of iron-rich food sources in locations which previously have had little access. It also increases the bioavailability of iron to be absorbed [
7,
8]. Using crops that are regionally available and culturally relevant to the target population, as lentil is to Bangladesh, is beneficial when implementing iron fortification. Many studies have used beans, pearl millet, and lentils, depending on the geographic location and cultural food staples of the target population. By using culturally appropriate staple food crops, the residents of an area are more likely to sustainably integrate the iron-fortified crops into their diet. All of this suggests that the provision of iron-fortified lentils in Bangladesh may be an effective way to address the iron needs of female adolescents, as well as others at risk of ID.
The benefits of addressing ID with either fortified or biofortified foods go beyond improvements in systemic iron levels to include improvements in perception, cognition, and brain function. For example, we [
9] showed that the consumption of a salt doubly fortified with potassium iodate and microencapsulated ferrous fumarate by women of reproductive age led to improvements in visual perception, attentional control, and memory. Similar improvements have been found using other iron biofortified staples (as reviewed in [
10]). In addition, there is evidence that improvements in the behavioral measures of attention and memory are accompanied by improvements in brain function, as measured using electroencephalography [
11,
12].
In the present study, an analysis of a subsample from a larger trial [
13], our primary concern was to determine whether changes in levels of systemic iron due to the consumption of fortified lentils would be accompanied by changes in cognitive performance. This question was motivated by the findings just discussed, i.e., that improvements in iron status by way of consuming fortified or biofortified components of the diet also produced improvements in both behavioral measures of cognitive function and neural measures of brain function. Changes in systemic levels of iron were documented in the larger trial and those results were consistent with studies documenting improvements in iron status using staple components of diet by way of both fortification and biofortification.
2. Materials and Methods
2.1. Participants
The current study was an analysis of a sub-sample from a larger double-blind, cluster-randomized community trial [
13], which can be referred to for additional methodological details.
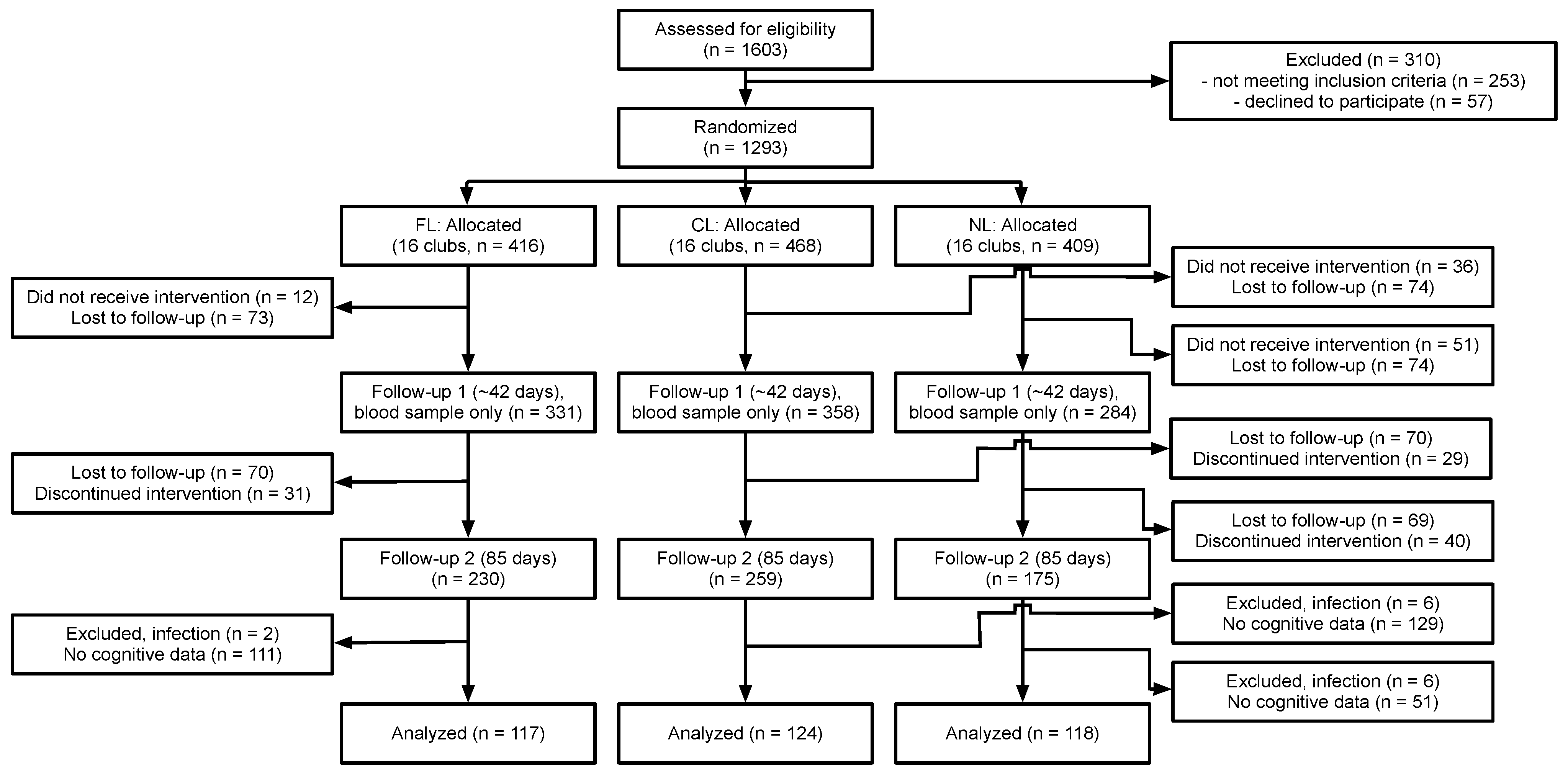
Figure 1 summarizes the flow of participants through the study and how we arrived at our subsample. Participants for the larger study were recruited from four upazilas (sub-districts) in Bangladesh, including Muktagacha, Mymensingh Sadar, Bhaluka, and Gaffargaon in the Mymensingh district. The study was conducted at the Bangladesh Rural Advancement Committee (BRAC) clubs within the four upazilas. This program provides scholarly education, health education, social education, poverty education, etc., for both young women and men regardless of socioeconomic status and education.
Two upazilas (Gaffargaon and Bhaluka) were selected for the recruitment of participants for the cognitive testing. A total of 21 BRAC clubs in these two upazilas were selected to cover all three intervention conditions. The chance to participate in the cognitive testing was offered to all girls and enrollment continued until the desired sample size (>120 participants per treatment condition, assuming 50% attrition) was reached or exceeded. Sample sizes were determined using an online calculator for clustered designs (
http://www.sample-size.net/means-sample-sizeclustered/; accessed on 11 August 2018), assuming α = 0.05, 1 − β = 0.90 and an effect size of 0.70 (based on results in [
14]). The final sample for the cognitive tests consisted of 359 adolescent girls aged 10–17 years. Participants were generally healthy and were excluded if they were either pregnant or breastfeeding. A total of 118 girls were included in the no supplied lentil (NL) condition, 124 in the non-fortified control lentil (CL) condition, and 117 in the fortified lentil (FL) condition. The menstrual status of the girls was assessed using a yes/no question at both BL and EL, and the proportion of the girls who reported having reached menarche at both time points as a function of age is presented in
Supplementary Figure S2.
2.2. Iron-Fortified Lentils
The fortified lentils used in the present study were small red lentils grown in Saskatchewan fortified with a solution of sodium ferric ethylenediaminetetraacetate (NaFeEDTA), with approximately 13–14 mg of iron per 100 g of lentils. An initial feasibility study was conducted to determine the correct amount and preparation procedure of the lentils, regionally called daal, for adolescent girls in Bangladesh. It was determined that 37.5 g (~200 g cooked) of a thick preparation, which provided 6.9 g of iron, or 86% of the recommended dietary allowance for the younger girls (9–13 years old) and 46% for the older girls (14–18 years old) [
15,
16] would be the most effective way to add the iron-fortified lentils into the diet of Bangladeshi adolescent girls with a high compliance rate during a 4-month-long feeding trial. The lentils served in the FL condition were fortified with 1600 ppm of iron, while the lentils served in the CL condition contained approximately 75–90 ppm. In the FL and CL conditions, 37.5 g of raw lentils (~200 g cooked) were served five days per week for a total of 85 feeding days. All lentils were served with one cup of cooked rice.
2.3. Laboratory Measures
At baseline (BL, prior to the start of the trial) and endline (EL, after 85 days), participants provided self-report measures of demographics and completed a food frequency questionnaire (FFQ) and anthropometrics. In addition, a 6 mL sample of venous blood was taken to assess the iron biomarkers. A blood sample was also taken at approximately day 42; those data were not used in the analyses reported here as there were no cognitive measures taken at that time.
Venous blood samples were collected by a trained phlebotomist using lithium heparinized vacutainers following an aseptic procedure and using a disposable syringe and needle. Vacutainers were transported within 12 h of collection to the International Center for Diarrhoeal Disease Research, Bangladesh (ICDDRB), based in Dhaka. Serum samples were separated and stored at a temperature of 2–8 °C until they were analyzed. All BL blood samples were analyzed at the same time using the same procedures. Later, all EL samples were analyzed at the same time (at the end of the trial) using the same procedures. Blood measures included a complete blood count (CBC), which included measures of hemoglobin (Hb), hematocrit (HCT), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), mean corpuscular volume (MCV), red blood cell count (RBC), and white blood cell count (WBC). In addition, assays were performed for serum ferritin (sFt), soluble transferrin receptor (sTfR), and C-reactive protein (CRP). Values of sFt were adjusted when there was evidence for inflammation (CRP > 5 and WBC > 11.5) [
17,
18]. Total body iron (TBI) was calculated from sFt and sTfR values using Cook’s equation [
19]:
Additional details of the laboratory assays are reported in [
13].
2.4. Cognitive Measures
A total of five tasks were used to measure cognitive performance, including the simple reaction time task (SRT), go/no-go task (GNG), attentional network task (ANT), Sternberg memory search task (SMS), and cued recognition task (CRT). We have used these tasks in previous field studies on the effects of iron repletion [
9,
11]. The tasks were computer-based (programmed by MJW) and presented on laptop computers using DMDX [
20], which allowed for the precise control of the presentation of stimuli and millisecond accuracy of measured reaction times (RTs). The SRT is a simple measure of RT that requires no decision-making or discrimination. The GNG assesses sustained attention and inhibitory control. The ANT assesses three levels of attention: low-level attentional capture, mid-level spatial selective attention, and high-level attentional control [
21]. The SMS assesses the speed with which a person can search their short-duration memory [
22]. The CRT is a standard recognition memory paradigm in which the participant is presented with pictures of common, nameable objects to study and then tested with both studied (old) and unstudied (new) items.
2.5. Ethics
Ethical approvals were received from the University of Saskatchewan, Canada (Bio#17–177), Marywood University, USA (IRB#1139116–2) and the Bangladesh Medical Research Council (BMRC/NREC/2016–2019/455), per their respective protocols. Informed written consent and assent were obtained from each participant and their respective parents, and a copy of the signed assent and consent form was given to the participants and parents.
2.6. Statistical Analyses
Condition differences at BL in the frequency of occurrence of characteristics such as anemia and ID were assessed using χ2 tests. Condition differences in the values of the blood and behavioral variables at BL were assessed using a one-way analysis of covariance (ANCOVA) with conditions (FL, CL, NL) as a between-participants factor and age as a covariate, using Tukey’s HSD tests for post hoc comparisons. Condition differences in the values of the EL blood and behavioral variables were assessed using a one-way ANCOVA with condition as a between-participants factor and age and BL value of the variables as covariates, using Tukey’s HSD tests for post hoc comparisons. Tests of the plausibility that changes from BL to EL in the iron markers were responsible for changes in the behavioral variables were assessed by regressing each of the behavioral change variables onto three sets of predictors: (a) Hb, sFt, and sTfR; (b) Hb, log10(sFt), and sTfR; and (c) Hb and TBI. For each set of predictors, a “best” model was determined using stepwise model selection to maximize the variance accounted for (R2) with the smallest number of predictors. The final model was selected from among the “best” models based on a non-zero estimate of the slope(s) for the predictor(s) and the highest R2 value.
The final analyses assessed differences in the change of the behavioral variables as a function of the extent to which individuals responded to the intervention. Three response conditions were defined using the biological daily coefficients of variation (CV) for Hb, sFt, and TBI reported in [
23]: (a) those whose blood variables decreased by more than the biological daily CV; (b) those whose blood variables did not change (either declining or improving) more than the biological daily CV; and (c) those whose blood variables increased by more than the biological daily CV. Differences in the number of individuals in each response category across the three conditions were assessed using χ
2 tests. Changes in each of the behavioral variables as a function of response status were assessed using one-way analyses of variance (ANOVAs) with response status (decrease, no change, increase) as a between-participants factor and no co-variates and Tukey HSD tests for post hoc comparisons, with this being undertaken separately for Hb, sFt, and TBI response. All analyses were performed using SAS 9.4 for Linux.
Prior to analyses, the distribution of sFt was checked and found to be non-normal using the Kolmogorov–Smirnov test (
D = 0.085,
p < 0.010). When the values were log10 transformed, the distribution was still found to be non-normal (
D = 0.087,
p < 0.010). Consequently, we applied the Box–Cox transformation [
24]
with λ = 0.5 (as estimated using transformation regression). This transformation also failed to produce a normal distribution (
D = 0.178,
p < 0.010). We then checked all our analyses involving sFt and found that there were no qualitative differences between the raw and either of the transformed values. Consequently, we report all results excepting those of the plausibility analyses using the raw values of sFt.
4. Discussion
The primary objective of the present study was to determine whether the consumption of an iron-fortified lentil would produce positive outcomes with respect to cognitive performance in a sample of adolescent girls in Bangladesh. Our results indicate that this was the case. Participants in the FL condition were generally faster than those in the CL and NL conditions at EL, with this advantage being present in three of the five tasks. The analyses examining the plausibility of attributing change in the cognitive variables to changes in the blood iron biomarkers were mixed in their results. While there were significant relationships found for many of the cognitive variables (the RTs), these relationships were reasonably weak, accounting for only small amounts of the total variance. The reason for this may be found in the analyses performed to examine the relationship between responses to the intervention in the blood variables and change in the cognitive variables. These analyses revealed that, while most participants showed either negative responses or no response to the intervention (a decline or lack of change in Hb, sFt, and TBI), there were still several participants who showed positive responses (increases in Hb, sFt, and TBI), and the likelihood of showing an increase was related to BL levels, with lower BL levels associated with increases from BL to EL. The majority of those having a positive response to the intervention were in the FL condition, and those who showed a positive response, specifically with respect to sFt and TBI, showed greater improvements (reductions in RTs and an increase in capacity in recognition memory) relative to those who either did not respond or showed a negative response. This was true for all the cognitive variables except RTs in the SRT, and RTs in the zero cue and center cue conditions of the ANT.
Age proved to be a significant covariate in the analyses of cognitive performance at both BL and EL. This is likely due to the fact that, across the age range for this sample (10–17 y), there are significant increases in processing speed, measured as decreases in RTs. It is also the case that this age range includes the mean menarcheal age (15.8 y) for girls in Bangladesh, with there being evidence that there are significant variations in RTs across the phases of the menstrual cycle [
25].
An unexpected finding from this study was the overall lack of improvement in any of the iron status biomarkers from BL to EL, which contrasts with the results obtained in the larger study. Indeed, there were decreases in sFt and TBI while there was no change in Hb and sTfR. This also contrasts with the results of prior work with fortified and biofortified foods, in which improvements in iron status have been obtained. This outcome should be interpreted with caution, as the larger study involved a much larger sample, with that sample size determined based on detecting improvements in iron status. That is, the present analysis of the subset of participants may be under-powered to detect improvements in iron status. In addition, at least one important difference between the present study and the previous work is that participants at BL in those studies had either clinically defined ID or IDA, while only a minority of the participants in this analysis had clinically defined ID (4%) or IDA (7%) at BL. Further, it should be noted that the decreases in sFt and TBI were the smallest for the FL condition and that, at EL, the levels of Hb, sFt, and TBI were significantly higher than those in the CL or NL conditions, and that the level of sTfR in the FL condition was significantly lower than those in the CL or NL conditions. It should also be acknowledged that there was an inverse relationship between the BL levels of the biomarkers and the amount of change from BL to EL. Finally, it was suggested that participants’ samples from BL and EL should be re-analyzed at the same time in order to confirm that the changes from BL and EL were reliable and were not confounded with any variations due to measurement time. This is because BL and EL samples were analyzed at different time points (although all BL samples were analyzed at the same time, following BL data collection, and all EL samples were analyzed at the same time, following EL data collection), emphasizing that we used well-validated measures. As re-analysis was not feasible, it must be acknowledged as a potential weakness. A final weakness to be acknowledged is that we did not have information on the phase of participants’ menstrual cycles at the time that blood iron levels were measured. It is unclear as to how this might have influenced the observed outcomes. Thus, whatever the reason for the overall lack of change or decline in iron status from BL to EL, the consumption of the fortified lentils can be seen as having a protective effect against declining iron stores over time, which is in agreement with the findings from the larger trial.
Thus, we conclude that the provision of iron-fortified lentils offered protective or beneficial effects with respect to both cognitive performance and iron status. The preliminary work for this study demonstrated that the daily provision of lentils was acceptable to participants, with a high rate of compliance with the feeding regimen. This suggests that the protective benefits of iron-fortified lentils can be obtained and sustained by integrating these lentils into the daily diet.
A strength of the current study was the reliance on the BRAC clubs within the Upazilas, which allowed for broad and consistent access to the study participants. An additional strength was the use of validated cognitive tests that have been used in a set of studies examining the effects of consuming iron-fortified and biofortified foods on cognitive performance. These studies have shown that the addition of dietary sources of iron resulted in improvements in these cognitive measures, which was the case for those in the present study who showed positive responses to the intervention. An additional strength is that we observed lower levels of inflammation in this sample of relatively healthy young women than has been the case in other studies. The unexpected result of the present study is that levels of sFt and TBI decreased from BL to EL and that Hb levels did not change. Possible explanations for this include not controlling for the age of onset of menarche, resulting in the inclusion of young women who may not have been meeting their increased iron requirements, or regression to the mean.
In sum, the present results suggest that the addition of fortified lentils to the low-iron Bangladeshi dietary pattern may prevent a decline in iron status, thus conferring benefits to cognitive performance. These results offer further evidence that the addition of fortified staple foods is an effective alternative to iron supplementation in environments in which dietary insufficiencies persist.