Late Luteal Subphase Food Craving Is Enhanced in Women with Obesity and Premenstrual Dysphoric Disorder (PMDD)
Abstract
:1. Introduction
2. Materials and Method
2.1. Study Design
2.2. Food Cravings and Anthropometric Measures
2.3. Group Categorization
2.4. Statistical Analysis
3. Results
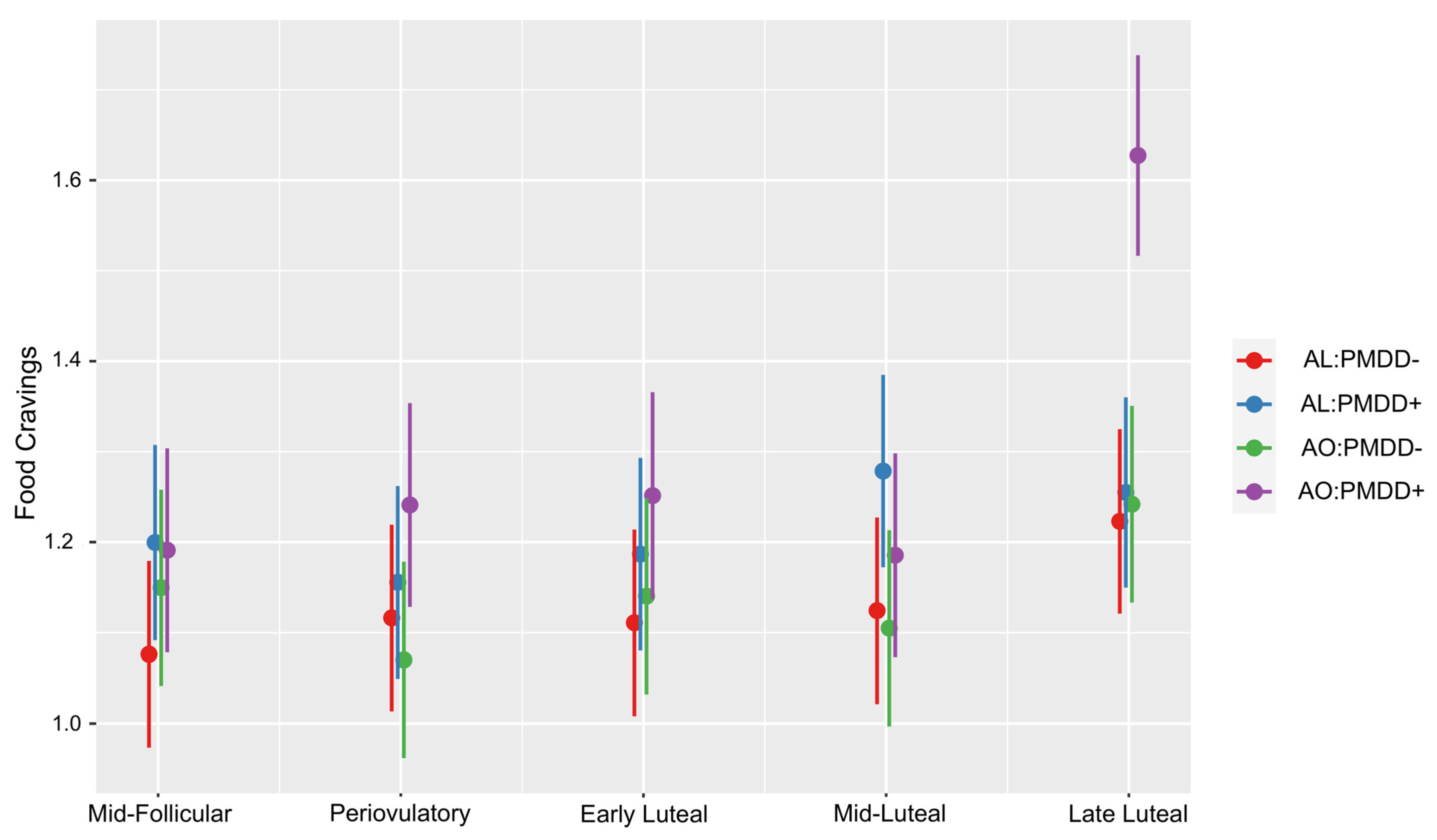
3.1. Food Cravings According to Subphase and Group
3.2. Food Cravings According to Group, Subphase and Affective Symptomatology
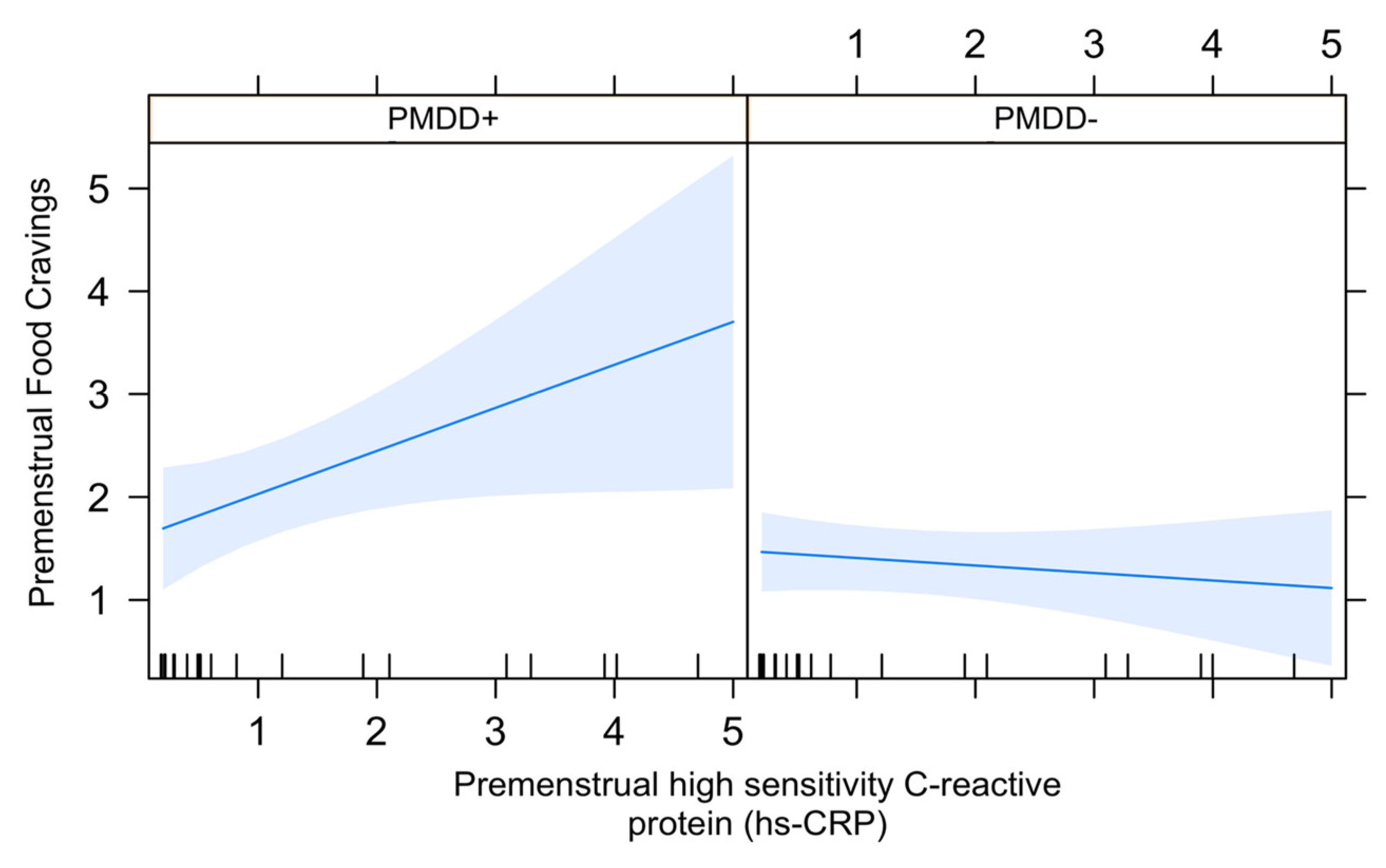
3.3. Association between Food Cravings and hs-crp in the LL Subphase in PMDD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hantsoo, L.; Epperson, C.N. Premenstrual Dysphoric Disorder: Epidemiology and Treatment. Curr. Psychiatry Rep. 2015, 17, 87. [Google Scholar] [CrossRef]
- Lu, D.; Aleknaviciute, J.; Kamperman, A.M.; Tamimi, R.M.; Ludvigsson, J.F.; Valdimarsdóttir, U.A.; Bertone-Johnson, E.R. Association Between Childhood Body Size and Premenstrual Disorders in Young Adulthood. JAMA Netw. Open 2022, 5, e221256. [Google Scholar] [CrossRef] [PubMed]
- Masho, S.W.; Adera, T.; South-Paul, J. Obesity as a risk factor for premenstrual syndrome. J. Psychosom. Obstet. Gynaecol. 2005, 26, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.J.; Hutchins, A.M.; Dawes, J.J. Effect of menstrual cycle on resting metabolism: A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236025. [Google Scholar] [CrossRef] [PubMed]
- Gorczyca, A.M.; Sjaarda, L.A.; Mitchell, E.M.; Perkins, N.J.; Schliep, K.C.; Wactawski-Wende, J.; Mumford, S.L. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur. J. Nutr. 2016, 55, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Hartlage, S.A.; Freels, S.; Gotman, N.; Yonkers, K. Criteria for premenstrual dysphoric disorder: Secondary analyses of relevant data sets. Arch. Gen. Psychiatry 2012, 69, 300–305. [Google Scholar] [CrossRef]
- Hamidovic, A.; Soumare, F.; Naveed, A.; Davis, J. Mid-Luteal Progesterone Is Inversely Associated with Premenstrual Food Cravings. Nutrients 2023, 15, 1097. [Google Scholar] [CrossRef]
- Kuczmarski, M.F.; Mason, M.A.; Allegro, D.; Zonderman, A.B.; Evans, M.K. Diet quality is inversely associated with C-reactive protein levels in urban, low-income African-American and white adults. J. Acad. Nutr. Diet. 2013, 113, 12. [Google Scholar] [CrossRef]
- Hamidovic, A.; Soumare, F.; Naveed, A.; Davis, J.; Sun, J.; Dang, N. Reduced Dehydroepiandrosterone-Sulfate Levels in the Mid-Luteal Subphase of the Menstrual Cycle: Implications to Women’s Health Research. Metabolites 2022, 12, 941. [Google Scholar] [CrossRef]
- Endicott, J.; Nee, J.; Harrison, W. Daily Record of Severity of Problems (DRSP): Reliability and validity. Arch. Womens Ment. Health 2006, 9, 41–49. [Google Scholar] [CrossRef]
- National Institutes of Health. The Practical Guide Identification, Evaluation, and Treatment of Overweight and Obesity in Adults; National Institutes of Health: Bethesda, MD, USA, 2000. [Google Scholar]
- Yonkers, K.A.; Kornstein, S.G.; Gueorguieva, R.; Merry, B.; Van Steenburgh, K.; Altemus, M. Symptom-Onset Dosing of Sertraline for the Treatment of Premenstrual Dysphoric Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2015, 72, 1037–1044. [Google Scholar] [CrossRef]
- Alberti KG, M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome--a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. J. Br. Diabet. Assoc. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar] [CrossRef]
- Hamidovic, A.; Davis, J.; Soumare, F.; Naveed, A.; Ghani, Y.; Semiz, S.; Khalil, D.; Wardle, M. Allopregnanolone Is Associated with a Stress-Induced Reduction of Heart Rate Variability in Premenstrual Dysphoric Disorder. J. Clin. Med. 2023, 12, 1553. [Google Scholar] [CrossRef] [PubMed]
- Abdella, H.M.; El Farssi, H.O.; Broom, D.R.; Hadden, D.A.; Dalton, C.F. Eating Behaviours and Food Cravings; Influence of Age, Sex, BMI and FTO Genotype. Nutrients 2019, 11, 377. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Aleknaviciute, J.; Bjarnason, R.; Tamimi, R.M.; Valdimarsdóttir, U.A.; Bertone-Johnson, E.R. Pubertal development and risk of premenstrual disorders in young adulthood. Hum. Reprod. Oxf. Engl. 2021, 36, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Boswell, R.G.; Kober, H. Food cue reactivity and craving predict eating and weight gain: A meta-analytic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016, 17, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Bertone-Johnson, E.R.; Hankinson, S.E.; Willett, W.C.; Johnson, S.R.; Manson, J.E. Adiposity and the development of premenstrual syndrome. J. Womens Health 2002 2010, 19, 1955–1962. [Google Scholar] [CrossRef]
- Mizgier, M.; Jarzabek-Bielecka, G.; Jakubek, E.; Kedzia, W. The relationship between body mass index, body composition and premenstrual syndrome prevalence in girls. Ginekol. Pol. 2019, 90, 256–261. [Google Scholar] [CrossRef]
- Thakur, H.; Pareek, P.; Sayyad, M.G.; Otiv, S. Association of Premenstrual Syndrome with Adiposity and Nutrient Intake Among Young Indian Women. Int. J. Womens Health 2022, 14, 665–675. [Google Scholar] [CrossRef]
- Buffenstein, R.; Poppitt, S.D.; McDevitt, R.M.; Prentice, A.M. Food intake and the menstrual cycle: A retrospective analysis, with implications for appetite research. Physiol. Behav. 1995, 58, 1067–1077. [Google Scholar] [CrossRef]
- Siep, N.; Roefs, A.; Roebroeck, A.; Havermans, R.; Bonte, M.L.; Jansen, A. Hunger is the best spice: An fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behav. Brain Res. 2009, 198, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Simmons, W.K.; Martin, A.; Barsalou, L.W. Pictures of appetizing foods activate gustatory cortices for taste and reward. Cereb. Cortex N. Y. N 1991 2005, 15, 1602–1608. [Google Scholar] [CrossRef] [PubMed]
- Stickel, S.; Wagels, L.; Wudarczyk, O.; Jaffee, S.; Habel, U.; Schneider, F.; Chechko, N. Neural correlates of depression in women across the reproductive lifespan—An fMRI review. J. Affect. Disord. 2019, 246, 556–570. [Google Scholar] [CrossRef]
- Bannbers, E.; Gingnell, M.; Engman, J.; Morell, A.; Comasco, E.; Kask, K.; Garavan, H.; Wikström, J.; Poromaa, I.S. The effect of premenstrual dysphoric disorder and menstrual cycle phase on brain activity during response inhibition. J. Affect. Disord. 2012, 142, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D. Interoception: The sense of the physiological condition of the body. Curr. Opin. Neurobiol. 2003, 13, 500–505. [Google Scholar] [CrossRef]
- Simmons, W.K.; Burrows, K.; Avery, J.A.; Kerr, K.L.; Bodurka, J.; Savage, C.R.; Drevets, W.C. Depression-Related Increases and Decreases in Appetite: Dissociable Patterns of Aberrant Activity in Reward and Interoceptive Neurocircuitry. Am. J. Psychiatry 2016, 173, 418–428. [Google Scholar] [CrossRef]
- Nord, C.L.; Lawson, R.P.; Dalgleish, T. Disrupted Dorsal Mid-Insula Activation During Interoception Across Psychiatric Disorders. Am. J. Psychiatry 2021, 178, 761–770. [Google Scholar] [CrossRef]
- Ali, M.M.; Hassan, C.; Masrur, M.; Bianco, F.M.; Naquiallah, D.; Mirza, I.; Frederick, P.; Fernandes, E.T.; Giulianotti, C.P.; Gangemi, A.; et al. Adipose Tissue Hypoxia Correlates with Adipokine Hypomethylation and Vascular Dysfunction. Biomedicines 2021, 9, 1034. [Google Scholar] [CrossRef]
- Gold, E.B.; Wells, C.; Rasor, M.O. The Association of Inflammation with Premenstrual Symptoms. J. Womens Health 2002 2016, 25, 865–874. [Google Scholar] [CrossRef]
- Myers, C.A.; Martin, C.K.; Apolzan, J.W. Food cravings and body weight: A conditioning response. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 298–302. [Google Scholar] [CrossRef]
| Demographic Variable | Category | Diagnostic Category | p Value | |||
|---|---|---|---|---|---|---|
| AL:PMDD− (n = 16) | AL:PMDD+ (n = 14) | AO:PMDD− (n = 15) | AO:PMDD+ (n = 13) | |||
| Age | 25.12 (4.68) | 26.57 (5.48) | 27.26 (5.22) | 25.07 (4.53) | 0.56 | |
| Race | White | 6 | 4 | 7 | 3 | 0.76 |
| Black or African American | 3 | 2 | 2 | 2 | ||
| American Indian or Alaska Native | 0 | 0 | 0 | 2 | ||
| Asian | 7 | 7 | 4 | 5 | ||
| Native Hawaiian or Other Pacific Islander | 0 | 0 | 0 | 0 | ||
| More than one race | 0 | 0 | 1 | 1 | ||
| Unknown/Do not want to specify | 0 | 1 | 1 | 0 | ||
| Ethnicity | Hispanic | 1 | 2 | 4 | 2 | 0.77 |
| Non-Hipanic | 14 | 11 | 10 | 11 | ||
| Unknown/Do not want to specify | 0 | 1 | 1 | 0 | ||
| Student Status | Yes | 5 | 7 | 5 | 8 | 0.33 |
| No | 11 | 7 | 10 | 5 | ||
| Marital Status | Single | 15 | 11 | 13 | 12 | 0.80 |
| Married | 1 | 2 | 2 | 1 | ||
| Divorced | 0 | 1 | 0 | 0 | ||
| Widowed | 0 | 0 | 0 | 0 | ||
| Income | Less than $20,000 | 10 | 6 | 5 | 6 | 0.51 |
| $20,000–$34,999 | 2 | 2 | 5 | 0 | ||
| $35,000–$49,999 | 1 | 3 | 1 | 3 | ||
| $50,000–$74,999 | 2 | 1 | 3 | 3 | ||
| 75,000 or more | 1 | 2 | 1 | 1 | ||
| Age of Menarche | 11.92 (1.40) | 12.30 (0.94) | 12.36 (1.56) | 11.83 (1.52) | 0.75 | |
| BMI | 21.47 (1.45) | 20.88 (2.32) | 27.80 (3.53) | 27.42 (3.98) | <0.001 | |
| Waist Circumference | 71.30 (3.89) | 72.37 (5.05) | 89.74 (6.31) | 91.63 (13.61) | <0.001 | |
| Contrast | Estimate | SE | df | t Ratio | p Value |
|---|---|---|---|---|---|
| Subphase = Mid-Follicular | |||||
| AL:PMDD− vs. AL:PMDD+ | −0.12325 | 0.0759 | 189 | −1.625 | 0.3673 |
| AL:PMDD− vs. AO:PMDD− | −0.07334 | 0.076 | 200 | −0.965 | 0.7695 |
| AL:PMDD− vs. AO:PMDD+ | −0.11472 | 0.0776 | 190 | −1.478 | 0.453 |
| AL:PMDD+ vs. AO:PMDD− | 0.04991 | 0.0778 | 194 | 0.642 | 0.9182 |
| AL:PMDD+ vs. AO:PMDD+ | 0.00853 | 0.0794 | 186 | 0.107 | 0.9996 |
| AO:PMDD− vs. AO:PMDD+ | −0.04138 | 0.0795 | 196 | −0.521 | 0.954 |
| Subphase = Periovulatory | |||||
| AL:PMDD− vs. AL:PMDD+ | −0.03927 | 0.0753 | 185 | −0.521 | 0.9539 |
| AL:PMDD− vs. AO:PMDD− | 0.0462 | 0.076 | 200 | 0.608 | 0.9295 |
| AL:PMDD− vs. AO:PMDD+ | −0.12472 | 0.0776 | 190 | −1.606 | 0.3775 |
| AL:PMDD+ vs. AO:PMDD− | 0.08547 | 0.0772 | 191 | 1.107 | 0.6857 |
| AL:PMDD+ vs. AO:PMDD+ | −0.08545 | 0.0788 | 182 | −1.084 | 0.6998 |
| AO:PMDD− vs. AO:PMDD+ | −0.17093 | 0.0795 | 196 | −2.151 | 0.141 |
| Subphase = Early Luteal | |||||
| AL:PMDD− vs. AL:PMDD+ | −0.07575 | 0.0753 | 185 | −1.006 | 0.7463 |
| AL:PMDD− vs. AO:PMDD− | −0.02933 | 0.076 | 200 | −0.386 | 0.9804 |
| AL:PMDD− vs. AO:PMDD+ | −0.14036 | 0.0783 | 195 | −1.792 | 0.2803 |
| AL:PMDD+ vs. AO:PMDD− | 0.04642 | 0.0772 | 191 | 0.601 | 0.9316 |
| AL:PMDD+ vs. AO:PMDD+ | −0.0646 | 0.0795 | 187 | −0.813 | 0.8485 |
| AO:PMDD− vs. AO:PMDD+ | −0.11103 | 0.0801 | 201 | −1.385 | 0.51 |
| Subphase = Mid-Luteal | |||||
| AL:PMDD− vs. AL:PMDD+ | −0.15419 | 0.0753 | 185 | −2.047 | 0.1748 |
| AL:PMDD− vs. AO:PMDD− | 0.01936 | 0.076 | 200 | 0.255 | 0.9942 |
| AL:PMDD− vs. AO:PMDD+ | −0.06117 | 0.0776 | 190 | −0.788 | 0.8599 |
| AL:PMDD+ vs. AO:PMDD− | 0.17354 | 0.0772 | 191 | 2.247 | 0.1144 |
| AL:PMDD+ vs. AO:PMDD+ | 0.09302 | 0.0788 | 182 | 1.18 | 0.6403 |
| AO:PMDD− vs. AO:PMDD+ | −0.08052 | 0.0795 | 196 | −1.013 | 0.742 |
| Subphase = Late Luteal | |||||
| AL:PMDD− vs. AL:PMDD+ | −0.03196 | 0.0746 | 177 | −0.429 | 0.9735 |
| AL:PMDD− vs. AO:PMDD− | −0.01896 | 0.0759 | 196 | −0.25 | 0.9945 |
| AL:PMDD− vs. AO:PMDD+ | −0.40446 | 0.0767 | 182 | −5.275 | <0.0001 |
| AL:PMDD+ vs. AO:PMDD− | 0.013 | 0.077 | 187 | 0.169 | 0.9983 |
| AL:PMDD+ vs. AO:PMDD+ | −0.3725 | 0.0778 | 174 | −4.789 | <.0001 |
| AO:PMDD− vs. AO:PMDD+ | −0.3855 | 0.079 | 191 | −4.878 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidovic, A.; Smadi, S.; Davis, J. Late Luteal Subphase Food Craving Is Enhanced in Women with Obesity and Premenstrual Dysphoric Disorder (PMDD). Nutrients 2023, 15, 5000. https://doi.org/10.3390/nu15235000
Hamidovic A, Smadi S, Davis J. Late Luteal Subphase Food Craving Is Enhanced in Women with Obesity and Premenstrual Dysphoric Disorder (PMDD). Nutrients. 2023; 15(23):5000. https://doi.org/10.3390/nu15235000
Chicago/Turabian StyleHamidovic, Ajna, Shahd Smadi, and John Davis. 2023. "Late Luteal Subphase Food Craving Is Enhanced in Women with Obesity and Premenstrual Dysphoric Disorder (PMDD)" Nutrients 15, no. 23: 5000. https://doi.org/10.3390/nu15235000
APA StyleHamidovic, A., Smadi, S., & Davis, J. (2023). Late Luteal Subphase Food Craving Is Enhanced in Women with Obesity and Premenstrual Dysphoric Disorder (PMDD). Nutrients, 15(23), 5000. https://doi.org/10.3390/nu15235000






