Carrot Juice Intake Affects the Cytokine and Chemokine Response in Human Blood after Ex Vivo Lipopolysaccharide-Induced Inflammation
Abstract
1. Introduction
2. Materials and Methods
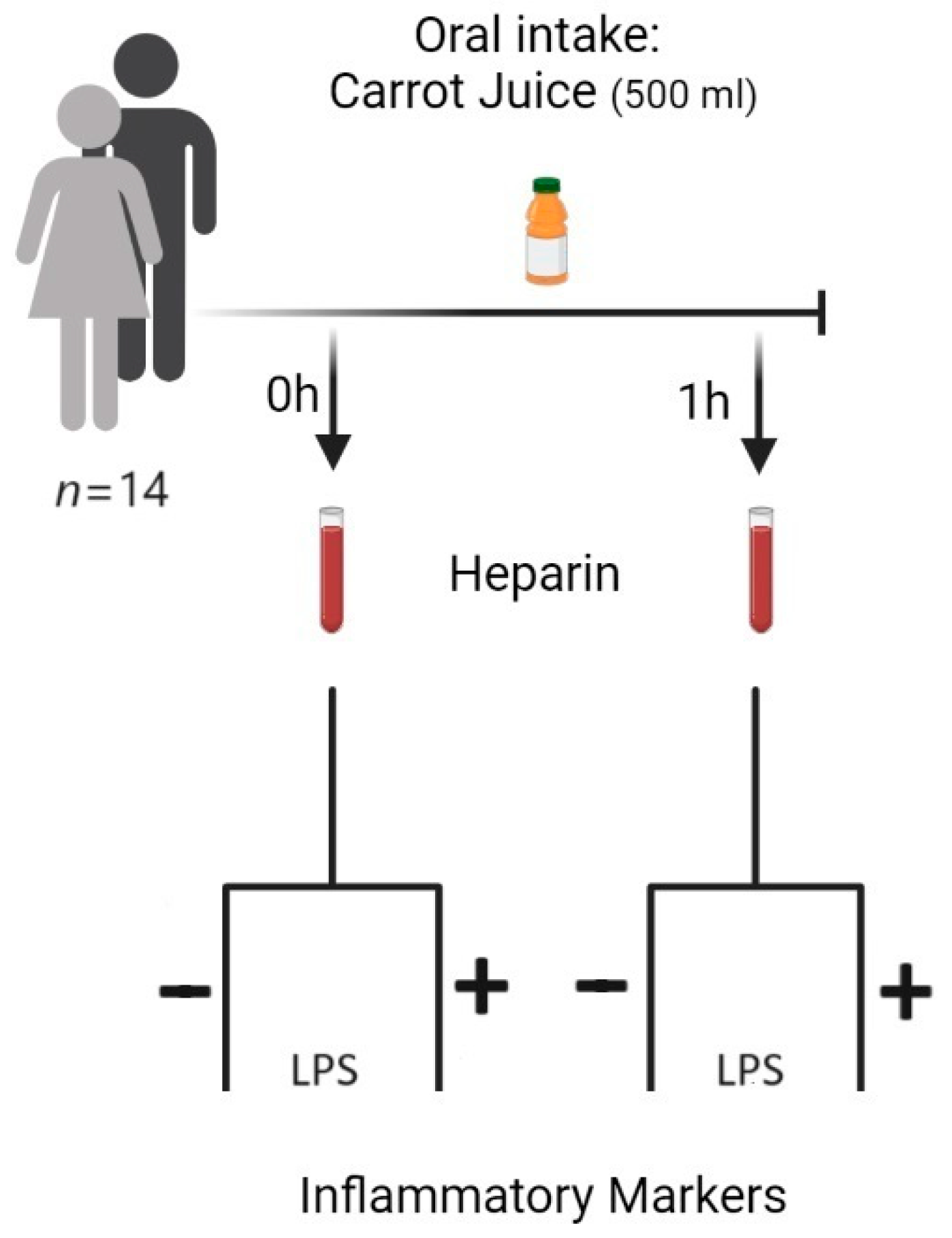
2.1. Study Subjects
2.2. Carrot Juice
2.3. Blood Sampling
2.4. LPS Mediated Stimulation of Leucocytes/Monocytes in Human Blood
2.5. Analysis of Cytokines and Chemokines in Plasma
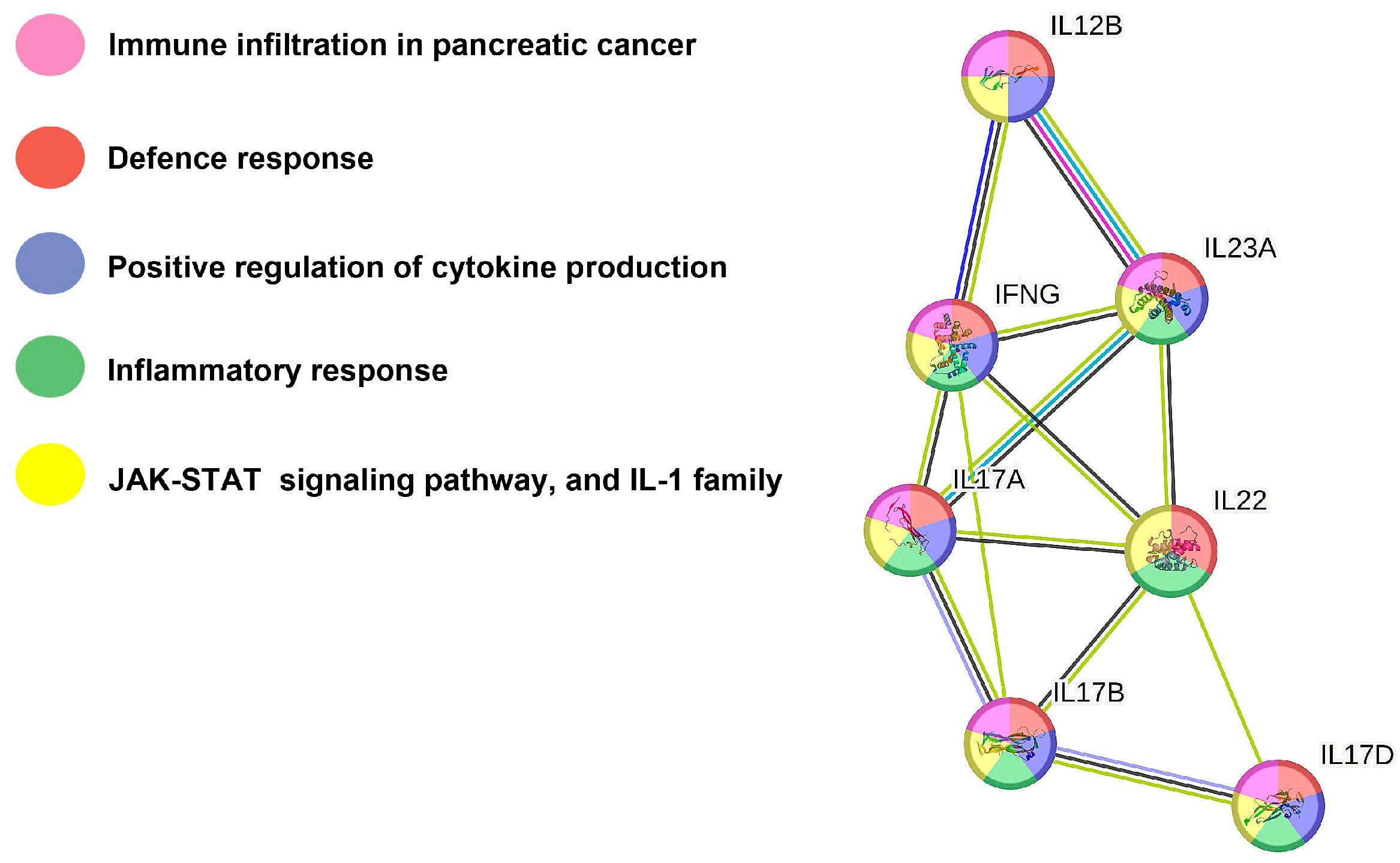
2.6. Protein–Protein Interaction Network Analysis
2.7. Statistical Analysis
3. Results
3.1. Carrot Juice Intake Affects Cytokine and Chemokine Concentrations in Human Plasma
3.2. Effect of Carrot Juice Intake in LPS-Stimulated Human Plasma
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deding, U.; Clausen, B.H.; Al-Najami, I.; Baatrup, G.; Jensen, B.L.; Kobaek-Larsen, M. Effect of oral intake of carrot juice on cyclooxygenases and cytokines in healthy human blood stimulated by lipopolysaccharide. Nutrients 2023, 15, 632. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P. Bioactive C17 and C18 acetylenic oxylipins from terrestrial plants as potential lead compounds for anticancer drug development. Molecules 2020, 25, 2568. [Google Scholar] [CrossRef] [PubMed]
- Deding, U.; Baatrup, G.; Christensen, L.P.; Kobaek-Larsen, M. Carrot intake and risk of colorectal cancer: A prospective cohort study of 57,053 Danes. Nutrients 2020, 12, 332. [Google Scholar] [CrossRef]
- Kobaek-Larsen, M.; Baatrup, G.; Khataei Notabi, M.; El-Houri, R.B.; Pipo-Olle, E.; Christensen Arnspang, E.; Christensen, L.P. Dietary polyacetylenic oxylipins falcarinol and falcarindiol prevent inflammation and colorectal neoplastic transformation: A mechanistic and dose-response study in a rat model. Nutrients 2019, 11, 2223. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Shao, F.; Zhang, F.; Miao, Q. Association between dietary carrot intake and breast cancer: A meta-analysis. Medicine 2018, 97, e12164. [Google Scholar] [CrossRef] [PubMed]
- Stefanson, A.L.; Bakovic, M. Falcarinol is a potent inducer of heme oxygenase-1 and was more effective than sulforaphane in attenuating intestinal inflammation at diet-achievable doses. Oxid. Med. Cell. Longev. 2018, 2018, 3153527. [Google Scholar] [CrossRef]
- El-Houri, R.B.; Kotowska, D.; Christensen, K.B.; Bhattacharya, S.; Oksbjerg, N.; Wolber, G.; Kristiansen, K.; Christensen, L.P. Polyacetylenes from carrots (Daucus carota) improve glucose uptake in vitro in adipocytes and myotubes. Food Funct. 2015, 6, 2135–2144. [Google Scholar] [CrossRef]
- Fallahzadeh, H.; Jalali, A.; Momayyezi, M.; Bazm, S. Effect of carrot intake in the prevention of gastric cancer: A meta-analysis. J. Gastric Cancer 2015, 15, 256–261. [Google Scholar] [CrossRef][Green Version]
- Xu, X.; Cheng, Y.; Li, S.; Zhu, Y.; Xu, X.; Zheng, X.; Mao, Q.; Xie, L. Dietary carrot consumption and the risk of prostate cancer. Eur. J. Nutr. 2014, 53, 1615–1623. [Google Scholar] [CrossRef]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef]
- Bae, K.E.; Choi, Y.W.; Kim, S.T.; Kim, Y.K. Components of rhizome extract of Cnidium officinale Makino and their in vitro biological effects. Molecules 2011, 16, 8833–8847. [Google Scholar] [CrossRef] [PubMed]
- Purup, S.; Larsen, E.; Christensen, L.P. Differential effects of falcarinol and related aliphatic C17-polyacetylenes on intestinal cell proliferation. J. Agric. Food Chem. 2009, 57, 8290–8296. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, K.H. Purification and identification of falcarinol, polyacetylene family, from Glehnia littoralis capable of cytotoxicity on jurkat T lymphoma. Cancer Prev. Res. 2008, 13, 216–221. [Google Scholar]
- Metzger, B.T.; Barnes, D.M.; Reed, J.D. Purple carrot (Daucus carota L.) polyacetylenes decrease lipopolysaccharide-induced expression of inflammatory proteins in macrophage and endothelial cells. J. Agric. Food Chem. 2008, 56, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Matsunaga, H.; Saita, T.; Mori, M.; Katano, M.; Okabe, H. Antiproliferative constituents in Umbelliferae plants II. Screening for polyacetylenes in some Umbelliferae plants, and isolation of panaxynol and falcarindiol from the root of Heracleum moellendorffii. Biol. Pharm. Bull. 1998, 21, 257–261. [Google Scholar] [CrossRef] [PubMed]
- Christensen, L.P.; Brandt, K. Bioactive polyacetylenes in food plants of the Apiaceae family: Occurrence, bioactivity, and analysis. J. Pharm. Biomed. Anal. 2006, 41, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Kong, C.S.; Umb, Y.R.; Lee, J.I.; Kim, Y.A.; Yea, S.S.; Seo, Y. Constituents isolated from Glehnia littoralis suppress proliferations of human cancer cells and MMP expression in HT1080 cells. Food Chem. 2010, 120, 385–394. [Google Scholar] [CrossRef]
- Zidorn, C.; Jöhrer, K.; Ganzera, M.; Schubert, B.; Sigmund, E.M.; Mader, J.; Greil, R.; Ellmerer, E.P.; Stuppner, H. Polyacetylenes from the Apiaceae vegetables carrot, celery, fennel, parsley, and parsnip and their cytotoxic activities. J. Agric. Food Chem. 2005, 53, 2518–2523. [Google Scholar] [CrossRef]
- Kuo, Y.C.; Lin, Y.L.; Huang, C.P.; Shu, J.W.; Tsai, W.J. A tumor cell growth inhibitor from Saposhnikovae divaricata. Cancer Investig. 2002, 20, 955–964. [Google Scholar] [CrossRef]
- Bernart, M.W.; Cardellina, J.H., 2nd; Balaschak, M.S.; Alexander, M.R.; Shoemaker, R.H.; Boyd, M.R. Cytotoxic falcarinol oxylipins from Dendropanax arboreus. J. Nat. Prod. 1996, 59, 748–753. [Google Scholar] [CrossRef]
- Alanko, J.; Kurahashi, Y.; Yoshimoto, T.; Yamamoto, S.; Baba, K. Panaxynol, a polyacetylene compound isolated from oriental medicines, inhibits mammalian lipoxygenases. Biochem. Pharmacol. 1994, 48, 1979–1981. [Google Scholar] [CrossRef] [PubMed]
- Appendino, G.; Tagliapietra, S.; Nano, G.M.; Picci, V. An antiplatelet acetylene from the leaves of Ferula communis. Fitoterapia 1993, 64, 179. [Google Scholar]
- Matsunaga, H.; Katano, M.; Yamamoto, H.; Fujito, H.; Mori, M.; Takata, K. Cytotoxic activity of polyacetylene compounds in Panax ginseng C. A. Meyer. Chem. Pharm. Bull. 1990, 38, 3480–3482. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; El-Rayes, B.F. Cyclooxygenase-2 in gastrointestinal malignancies. Cancer 2019, 125, 1221–1227. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.A.; Dubois, R.N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat. Rev. Cancer 2001, 1, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Dawid, C.; Dunemann, F.; Schwab, W.; Nothnagel, T.; Hofmann, T. Bioactive C17-polyacetylenes in carrots (Daucus carota L.): Current knowledge and future perspectives. J. Agric. Food Chem. 2015, 63, 9211–9222. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, U.; Kobaek-Larsen, M.; Kjoller, K.D.; Antonsen, S.; Baatrup, G.; Trelle, M.B. Quantification of the anti-neoplastic polyacetylene falcarinol from carrots in human serum by LC-MS/MS. J. Chromatogr. B 2022, 1210, 123440. [Google Scholar] [CrossRef] [PubMed]
- Shiao, Y.J.; Lin, Y.L.; Sun, Y.H.; Chi, C.W.; Chen, C.F.; Wang, C.N. Falcarindiol impairs the expression of inducible nitric oxide synthase by abrogating the activation of IKK and JAK in rat primary astrocytes. Br. J. Pharmacol. 2005, 144, 42–51. [Google Scholar] [CrossRef]
- Heydenreuter, W.; Kunold, E.; Sieber, S.A. Alkynol natural products target ALDH2 in cancer cells by irreversible binding to the active site. Chem. Commun. 2015, 51, 15784–15787. [Google Scholar] [CrossRef]
- Czyzewska, M.M.; Chrobok, L.; Kania, A.; Jatczak, M.; Pollastro, F.; Appendino, G.; Mozrzymas, J.W. Dietary acetylenic oxylipin falcarinol differentially modulates GABAA receptors. J. Nat. Prod. 2014, 77, 2671–2677. [Google Scholar] [CrossRef]
- Ohnuma, T.; Nakayama, S.; Anan, E.; Nishiyama, T.; Ogura, K.; Hiratsuka, A. Activation of the Nrf2/ARE pathway via S-alkylation of cysteine 151 in the chemopreventive agent-sensor Keap1 protein by falcarindiol, a conjugated diacetylene compound. Toxicol. Appl. Pharmacol. 2010, 244, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Ann. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, B.; Thiesson, H.C.; Henriksen, C.; Therland, K.; Falk, C.; Poulsen, T.; Fogh, B.; Madsen, K.; Walther, S.; Jensen, B.L. Differential effects of immunosuppressive drugs on COX-2 activity in vitro and in kidney transplant patients in vivo. Nephrol. Dial. Transplant. 2009, 24, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, P.; Panara, M.R.; Greco, A.; Fusco, O.; Natoli, C.; Iacobelli, S.; Cipollone, F.; Ganci, A.; Créminon, C.; Maclouf, J.; et al. Biochemical and pharmacological characterization of the cyclooxygenase activity of human blood prostaglandin endoperoxide synthases. J. Pharmacol. Exp. Ther. 1994, 271, 1705–1712. [Google Scholar] [PubMed]
- Clausen, B.H.; Wirenfeldt, M.; Hogedal, S.S.; Frich, L.H.; Nielsen, H.H.; Schroder, H.D.; Ostergaard, K.; Finsen, B.; Kristensen, B.W.; Lambertsen, K.L. Characterization of the TNF and IL-1 systems in human brain and blood after ischemic stroke. Acta Neuropathol. Commun. 2020, 8, 81. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Morris, J.H.; Cook, H.; Kuhn, M.; Wyder, S.; Simonovic, M.; Santos, A.; Doncheva, N.T.; Roth, A.; Bork, P.; et al. The STRING database in 2017: Quality-controlled protein-protein association networks, made broadly accessible. Nucl. Acids Res. 2017, 45, D362–D368. [Google Scholar] [CrossRef]
- van der Horst, D.; Carter-Timofte, M.E.; van Grevenynghe, J.; Laguette, N.; Dinkova-Kostova, A.T.; Olagnier, D. Regulation of innate immunity by Nrf2. Curr. Opin. Immunol. 2022, 78, 102247. [Google Scholar] [CrossRef]
- Battino, M.; Giampieri, F.; Pistollato, F.; Sureda, A.; de Oliveira, M.R.; Pittalà, V.; Fallarino, F.; Nabavi, S.F.; Atanasov, A.G.; Nabavi, S.M. Nrf2 as regulator of innate immunity: A molecular Swiss army knife! Biotechnol. Adv. 2018, 36, 358–370. [Google Scholar] [CrossRef]
- Ahmed, S.M.U.; Luo, L.; Namani, A.; Wang, X.J.; Tang, X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 585–597. [Google Scholar] [CrossRef]
- Saha, S.; Buttari, B.; Panieri, E.; Profumo, E.; Saso, L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules 2020, 25, 5474. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, E.; Suzuki, T.; Funayama, R.; Nagashima, T.; Hayashi, M.; Sekine, H.; Tanaka, N.; Moriguchi, T.; Motohashi, H.; Nakayama, K.; et al. Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat. Commun. 2016, 7, 11624. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, J.; Fu, M.; Zhao, X. The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Target. Ther. 2021, 6, 402. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Xu, M.; Mizoguchi, I.; Furusawa, J.; Kaneko, K.; Watanabe, K.; Mizuguchi, J.; Itoh, M.; Kawakami, Y.; Yoshimoto, T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013, 2013, 968549. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Chen, S.; Qian, H.; Huang, W. Interleukin-23: As a drug target for autoimmune inflammatory diseases. Immunology 2012, 135, 112–124. [Google Scholar] [CrossRef] [PubMed]
- Mills, K.H.G. IL-17 and IL-17-producing cells in protection versus pathology. Nat. Rev. Immunol. 2023, 23, 38–54. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 family of cytokines in health and disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Pappu, R.; Ramirez-Carrozzi, V.; Sambandam, A. The interleukin-17 cytokine family: Critical players in host defence and inflammatory diseases. Immunology 2011, 134, 8–16. [Google Scholar] [CrossRef]
- Beringer, A.; Noack, M.; Miossec, P. IL-17 in chronic inflammation: From discovery to targeting. Trends Mol. Med. 2016, 22, 230–241. [Google Scholar] [CrossRef]
- Chung, A.S.; Wu, X.; Zhuang, G.; Ngu, H.; Kasman, I.; Zhang, J.; Vernes, J.M.; Jiang, Z.; Meng, Y.G.; Peale, F.V.; et al. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat. Med. 2013, 19, 1114–1123. [Google Scholar] [CrossRef]
- Kolls, J.K.; Lindén, A. Interleukin-17 family members and inflammation. Immunity 2004, 21, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Dong, C. IL-17 cytokines in immunity and inflammation. Emerg. Microbes Infect. 2013, 2, e60. [Google Scholar] [CrossRef] [PubMed]
- Amatya, N.; Garg, A.V.; Gaffen, S.L. IL-17 signaling: The yin and the yang. Trends Immunol. 2017, 38, 310–322. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Miller, W.H., Jr. Reversal of autoimmune toxicity and loss of tumor response by Interleukin-17 blockade. N. Engl. J. Med. 2017, 376, 1989–1991. [Google Scholar] [CrossRef] [PubMed]
- Kryczek, I.; Wei, S.; Szeliga, W.; Vatan, L.; Zou, W. Endogenous IL-17 contributes to reduced tumor growth and metastasis. Blood 2009, 114, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, J.M.; Lee, Y.H.; Shi, Y.; Wang, X.; Angkasekwinai, P.; Nallaparaju, K.C.; Flaherty, S.; Chang, S.H.; Watarai, H.; Dong, C. Interleukin-17B antagonizes interleukin-25-mediated mucosal inflammation. Immunity 2015, 42, 692–703. [Google Scholar] [CrossRef]
- Starnes, T.; Broxmeyer, H.E.; Robertson, M.J.; Hromas, R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J. Immunol. 2002, 169, 642–646. [Google Scholar] [CrossRef]
- Ouyang, W.; O’Garra, A. IL-10 family cytokines IL-10 and IL-22: From basic science to clinical translation. Immunity 2019, 50, 871–891. [Google Scholar] [CrossRef]
- Dudakov, J.A.; Hanash, A.M.; van den Brink, M.R.M. Interleukin-22: Immunobiology and pathology. Ann. Rev. Immun. 2015, 33, 747–785. [Google Scholar] [CrossRef]
- Kaalby, L.; Al-Najami, I.; Deding, U.; Berg-Beckhoff, G.; Steele, R.J.C.; Kobaek-Larsen, M.; Shaukat, A.; Rasmussen, M.; Baatrup, G. Cause of death, mortality, and occult blood in colorectal cancer screening. Cancers 2022, 14, 246. [Google Scholar] [CrossRef]
- Deding, U.; Kaalby, L.; Steele, R.; Al-Najami, I.; Kobaek-Larsen, M.; Plantener, E.; Madsen, J.B.; Madsen, J.S.; Bjørsum-Meyer, T.; Baatrup, G. Faecal haemoglobin concentration predicts all-cause mortality. Eur. J. Cancer 2023, 184, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Chyuan, I.T.; Lai, J.H. New insights into the IL-12 and IL-23: From a molecular basis to clinical application in immune-mediated inflammation and cancers. Biochem. Pharmacol. 2020, 175, 113928. [Google Scholar] [CrossRef] [PubMed]
- Duvallet, E.; Semerano, L.; Assier, E.; Falgarone, G.; Boissier, M.C. Interleukin-23: A key cytokine in inflammatory diseases. Ann. Med. 2011, 43, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Brevi, A.; Cogrossi, L.L.; Grazia, G.; Masciovecchio, D.; Impellizzieri, D.; Lacanfora, L.; Grioni, M.; Bellone, M. Much more than IL-17A: Cytokines of the IL-17 family between microbiota and cancer. Front. Immunol. 2020, 11, 565470. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.; Tuzova, M.; Cruikshank, W.; Center, D. Regulation of cellular processes by interleukin-16 in homeostasis and cancer. J. Cell. Physiol. 2014, 29, 139–147. [Google Scholar] [CrossRef]
- Seegert, D.; Rosenstiel, P.; Pfahler, H.; Pfefferkorn, P.; Nikolaus, S.; Schreiber, S. Increased expression of IL-16 in inflammatory bowel disease. Gut 2001, 48, 326–332. [Google Scholar] [CrossRef]
- Mathy, N.L.; Scheuer, W.; Lanzendörfer, M.; Honold, K.; Ambrosius, D.; Norley, S.; Kurth, R. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 2000, 100, 63–69. [Google Scholar] [CrossRef]
- Di Paolo, N.C.; Shayakhmetov, D.M. Interleukin 1alpha and the inflammatory process. Nat. Immunol. 2016, 17, 906–913. [Google Scholar] [CrossRef]
- Perera, P.Y.; Lichy, J.H.; Waldmann, T.A.; Perera, L.P. The role of interleukin-15 in inflammation and immune responses to infection: Implications for its therapeutic use. Microbes Infect. 2012, 14, 247–261. [Google Scholar] [CrossRef]
- Perera, L.P. Interleukin 15: Its role in inflammation and immunity. Arch. Immunol. Ther. Exp. 2000, 48, 457–464. [Google Scholar]
- Hu, Y.; Liu, D.; Cui, P.; Zhang, W.; Chen, H.; Piao, C.; Lu, Y.; Liu, X.; Wang, Y.; Liu, J.; et al. IL-15-induced lymphocytes as adjuvant cellular immunotherapy for gastric cancer. Investig. New Drugs 2021, 39, 1538–1548. [Google Scholar] [CrossRef] [PubMed]
- Mager, L.F.; Wasmer, M.H.; Rau, T.T.; Krebs, P. Cytokine-induced modulation of colorectal cancer. Front. Oncol. 2016, 6, 96. [Google Scholar] [CrossRef] [PubMed]
- Schroder, K.; Hertzog, P.J.; Ravasi, T.; Hume, D.A. Interferon-gamma: An overview of signals, mechanisms and functions. J. Leukoc. Biol. 2004, 75, 163–189. [Google Scholar] [CrossRef] [PubMed]
- Alspach, E.; Lussier, D.M.; Schreiber, R.D. Interferon gamma and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb. Perspect. Biol. 2019, 11, a028480. [Google Scholar] [CrossRef]
| Biomarker | Plasma from Whole Blood before and after Intake of Carrot Juice | Plasma from LPS (10 μg/mL, 24 h)-Stimulated Whole Blood before and after Intake of Carrot Juice | ||||
|---|---|---|---|---|---|---|
| Time of Sampling | 0 h | 1 h | 0 h | 1 h | ||
| Mean (pg/mL) | Mean (pg/mL) | p-Value | Mean (pg/mL) | Mean (pg/mL) | p-Value | |
| Pro-Inflammatory Cytokines | ||||||
| IFN-γ | 26.5 ± 16.6 | 26.9 ± 16.5 | NSE | 252.7 ± 235.7 | 337.4 ± 278.2 | 0.0166 |
| IL-15 | 1.5 ± 0.35 | 1.8 ± 0.47 | 0.0105 | 2.0 ± 0.68 | 2.2 ± 0.83 | NSE |
| IL-17A | 7.3 ± 2.1 | 7.2 ± 2.7 | NSE | 96.6 ± 69.0 | 149.9 ± 83.9 | 0.0023 |
| IL-17B | 6.3 ± 7.6 | 7.2 ± 7.4 | NSE | 15.1 ± 9.7 | 19.2 ± 11.5 | 0.0134 |
| IL-17D | 39.4 ± 18.0 | 45.3 ± 18.6 | NSE | 48.9 ± 27.7 | 62.3 ± 24.8 | 0.0085 |
| IL-23 | 2.5 ± 1.9 | 4.5 ± 4.7 | NSE | 135.9 ± 184.4 | 207.9 ± 204.2 | 0.0002 |
| Anti-Inflammatory Cytokines | ||||||
| IL-12/IL-23p40 | 133.3 ± 41.9 | 141.3 ± 32.3 | NSE | 3011 ± 2395 | 4131 ± 2594 | 0.0245 |
| IL-22 | 4.4 ± 2.5 | 4.9 ± 2.6 | NSE | 8.0 ± 4.4 | 11.2 ± 8.2 | 0.0215 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobaek-Larsen, M.; Deding, U.; Al-Najami, I.; Clausen, B.H.; Christensen, L.P. Carrot Juice Intake Affects the Cytokine and Chemokine Response in Human Blood after Ex Vivo Lipopolysaccharide-Induced Inflammation. Nutrients 2023, 15, 5002. https://doi.org/10.3390/nu15235002
Kobaek-Larsen M, Deding U, Al-Najami I, Clausen BH, Christensen LP. Carrot Juice Intake Affects the Cytokine and Chemokine Response in Human Blood after Ex Vivo Lipopolysaccharide-Induced Inflammation. Nutrients. 2023; 15(23):5002. https://doi.org/10.3390/nu15235002
Chicago/Turabian StyleKobaek-Larsen, Morten, Ulrik Deding, Issam Al-Najami, Bettina Hjelm Clausen, and Lars Porskjær Christensen. 2023. "Carrot Juice Intake Affects the Cytokine and Chemokine Response in Human Blood after Ex Vivo Lipopolysaccharide-Induced Inflammation" Nutrients 15, no. 23: 5002. https://doi.org/10.3390/nu15235002
APA StyleKobaek-Larsen, M., Deding, U., Al-Najami, I., Clausen, B. H., & Christensen, L. P. (2023). Carrot Juice Intake Affects the Cytokine and Chemokine Response in Human Blood after Ex Vivo Lipopolysaccharide-Induced Inflammation. Nutrients, 15(23), 5002. https://doi.org/10.3390/nu15235002





