Alterations in Placental Inflammation-Related Gene Expression Partially Mediate the Effects of Prenatal Alcohol Consumption on Maternal Iron Homeostasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample
2.2. Ascertainment of Maternal Alcohol, Cigarette Smoking, and Drug Use
2.3. Demographics and Potential Covariates
2.4. Dietary, Hematological, and Biochemical Iron Indicators
2.5. Placental Gene Expression
2.6. Curated Gene List and Principal Component Analysis (PCA)
2.7. Statistical Analyses
3. Results
3.1. Sample Characteristics
3.2. Relation of PAE to PCs
3.3. Relation of PCs to Maternal and Infant Iron Outcomes
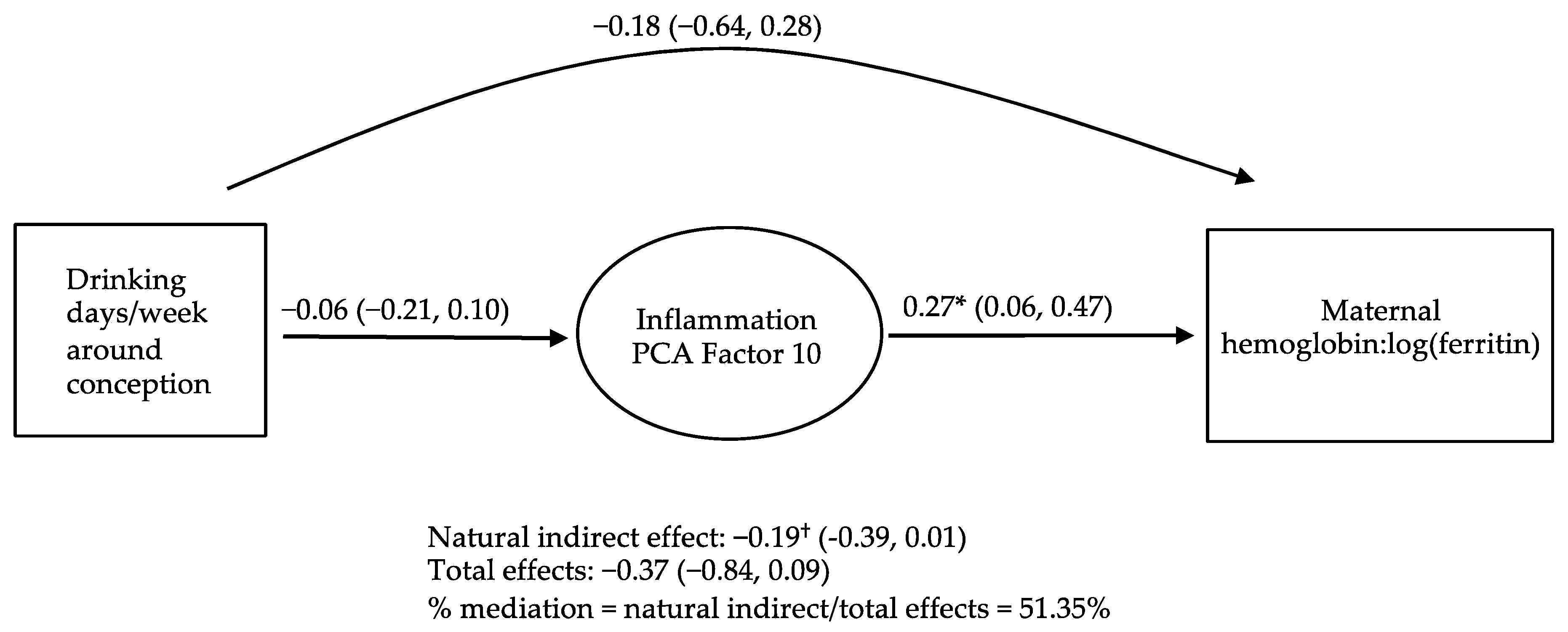
3.4. Causal Inference Analyses Examining PCs as Potential Mediators of the Relations of PAE to Maternal and Infant Iron Outcomes
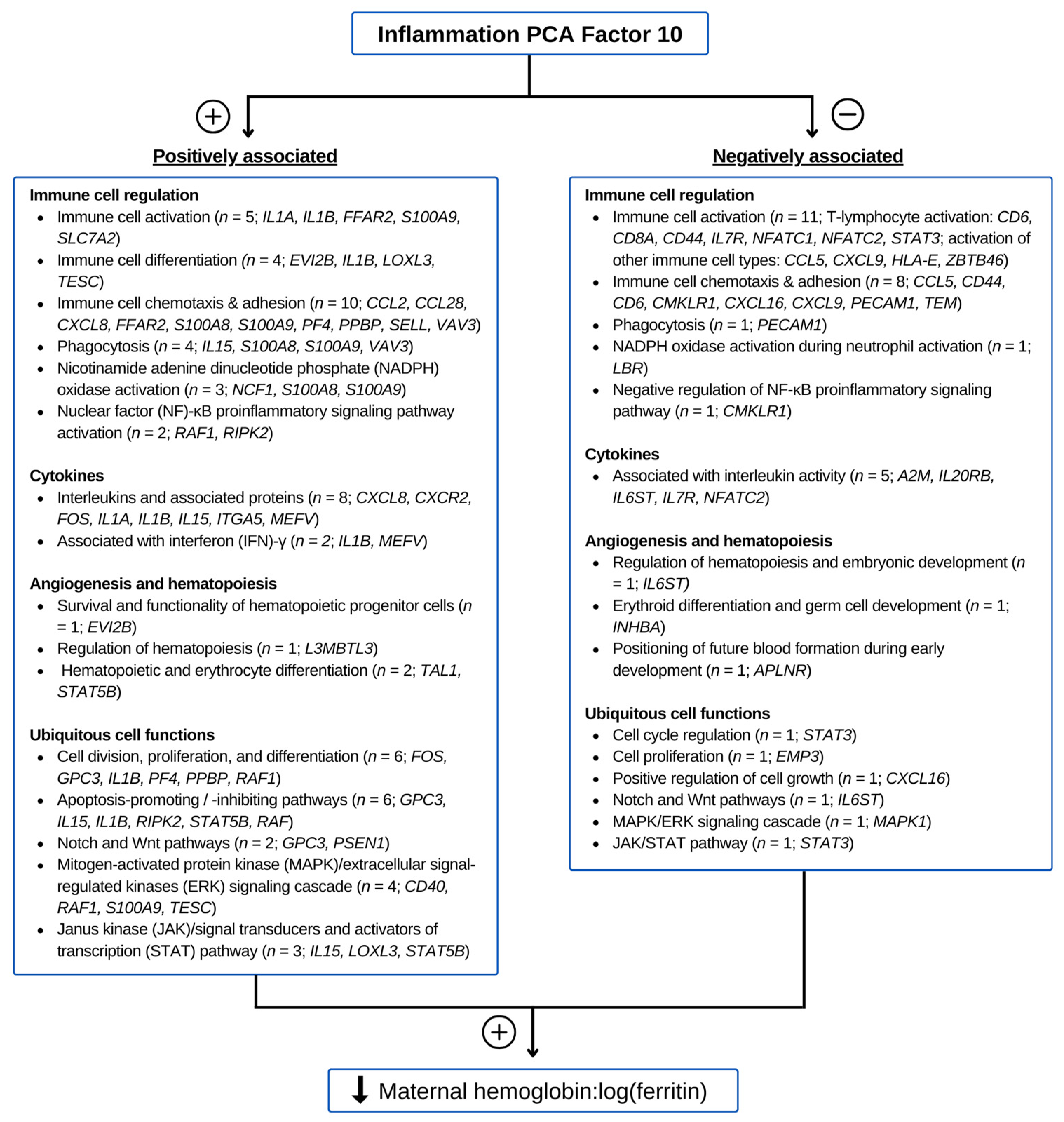
3.5. Functions of Genes Contributing to Inflammation PC 10
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- May, P.A.; Baete, A.; Russo, J.; Elliott, A.J.; Blankenship, J.; Kalberg, W.O.; Buckley, D.; Brooks, M.; Hasken, J.; Abdul-Rahman, O.; et al. Prevalence and characteristics of fetal alcohol spectrum disorders. Pediatrics 2014, 134, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Hoyme, H.E.; Kalberg, W.O.; Elliott, A.J.; Blankenship, J.; Buckley, D.; Marais, A.-S.; Manning, M.A.; Robinson, L.K.; Adam, M.P.; Abdul-Rahman, O.; et al. Updated clinical guidelines for diagnosing fetal alcohol spectrum disorders. Pediatrics 2016, 138, e20154256. [Google Scholar] [CrossRef] [PubMed]
- Hoyme, H.E.; May, P.A.; Kalberg, W.O.; Kodituwakku, P.; Gossage, J.P.; Trujillo, P.M.; Buckley, D.G.; Miller, J.H.; Aragon, A.S.; Khaole, N.; et al. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: Clarification of the 1996 Institute of Medicine criteria. Pediatrics 2005, 115, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef]
- May, P.A.; Chambers, C.D.; Kalberg, W.O.; Zellner, J.; Feldman, H.; Buckley, D.; Kopald, D.; Hasken, J.M.; Xu, R.; Honerkamp-Smith, G.; et al. Prevalence of Fetal Alcohol Spectrum Disorders in 4 US Communities. JAMA 2018, 319, 474–482. [Google Scholar] [CrossRef]
- May, P.A.; Blankenship, J.; Marais, A.S.; Gossage, J.P.; Kalberg, W.O.; Barnard, R.; De Vries, M.; Robinson, L.K.; Adnams, C.M.; Buckley, D.; et al. Approaching the prevalence of the full spectrum of fetal alcohol spectrum disorders in a South African population-based study. Alcohol. Clin. Exp. Res. 2013, 37, 818–830. [Google Scholar] [CrossRef]
- Carter, R.C.; Jacobson, J.L.; Molteno, C.D.; Jiang, H.; Meintjes, E.M.; Jacobson, S.W.; Duggan, C. Effects of heavy prenatal alcohol exposure and iron deficiency anemia on child growth and body composition through age 9 years. Alcohol. Clin. Exp. Res. 2012, 36, 1973–1982. [Google Scholar] [CrossRef]
- Carter, R.C.; Wainwright, H.; Molteno, C.D.; Georgieff, M.K.; Dodge, N.C.; Warton, F.; Meintjes, E.M.; Jacobson, J.L.; Jacobson, S.W. Alcohol, methamphetamine, and marijuana exposure have distinct effects on the human placenta. Alcohol. Clin. Exp. Res. 2016, 40, 753–764. [Google Scholar] [CrossRef]
- Oei, J.L. Alcohol use in pregnancy and its impact on the mother and child. Addiction 2020, 115, 2148–2163. [Google Scholar] [CrossRef]
- Denny, C.H.; Acero, C.S.; Naimi, T.S.; Kim, S.Y. Consumption of alcohol beverages and binge drinking among pregnant women aged 18–44 years-united states, 2015–2017. Morb. Mortal. Wkly. Rep. 2019, 68, 365–368. [Google Scholar] [CrossRef]
- Carter, R.C.; Georgieff, M.K.; Ennis, K.M.; Dodge, N.C.; Wainwright, H.; Meintjes, E.M.; Duggan, C.P.; Molteno, C.D.; Jacobson, J.L.; Jacobson, S.W. Prenatal alcohol-related alterations in maternal, placental, neonatal, and infant iron homeostasis. Am. J. Clin. Nutr. 2021, 114, 1107–1122. [Google Scholar] [CrossRef]
- Ganz, T. Hepcidin and iron regulation, 10 years later. Blood 2011, 117, 4425–4433. [Google Scholar] [CrossRef]
- Sangkhae, V.; Nemeth, E. Regulation of the iron homeostatic hormone hepcidin. Adv. Nutr. 2017, 8, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Sangkhae, V.; Nemeth, E. Placental iron transport: The mechanism and regulatory circuits. Free Radic. Biol. Med. 2019, 133, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.C.; Jacobson, S.W.; Molteno, C.D.; Jacobson, J.L. Fetal alcohol exposure, iron-deficiency anemia, and infant growth. Pediatrics 2007, 120, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Bodnar, T.S.; Raineki, C.; Wertelecki, W.; Yevtushok, L.; Plotka, L.; Zymak-Zakutnya, N.; Honerkamp-Smith, G.; Wells, A.; Rolland, M.; Woodward, T.S.; et al. Altered maternal immune networks are associated with adverse child neurodevelopment: Impact of alcohol consumption during pregnancy. Brain Behav. Immun. 2018, 73, 205–215. [Google Scholar] [CrossRef]
- Sowell, K.D.; Uriu-Adams, J.Y.; Van de Water, J.; Chambers, C.D.; Coles, C.D.; Kable, J.A.; Yevtushok, L.; Zymak-Zakutnya, N.; Wertelecki, W.; Keen, C.L.; et al. Implications of altered maternal cytokine concentrations on infant outcomes in children with prenatal alcohol exposure. Alcohol 2018, 68, 49–58. [Google Scholar] [CrossRef]
- Saini, N.; Helfrich, K.K.; Kwan, S.T.C.; Huebner, S.M.; Abazi, J.; Flentke, G.R.; Blohowiak, S.E.; Kling, P.J.; Smith, S.M. Alcohol’s Dysregulation of Maternal-Fetal IL-6 and p-STAT3 Is a Function of Maternal Iron Status. Alcohol. Clin. Exp. Res. 2019, 43, 2332–2343. [Google Scholar] [CrossRef]
- Ahluwalia, B.; Wesley, B.; Adeyiga, O.; Smith, D.M.; Da-Silva, A.; Rajguru, S. Alcohol modulates cytokine secretion and synthesis in human fetus: An in vivo and in vitro study. Alcohol 2000, 21, 207–213. [Google Scholar] [CrossRef]
- Ohtake, T.; Saito, H.; Hosoki, Y.; Inoue, M.; Miyoshi, S.; Suzuki, Y.; Fujimoto, Y.; Kohgo, Y. Hepcidin Is Down-Regulated in Alcohol Loading. Alcohol. Clin. Exp. Res. 2007, 31, S2–S8. [Google Scholar] [CrossRef]
- Carter, R.C.; Dodge, N.C.; Molteno, C.D.; Meintjes, E.M.; Jacobson, J.L.; Jacobson, S.W. Mediating and moderating effects of iron homeostasis alterations on fetal alcohol-related growth and neurobehavioral deficits. Nutrients 2022, 14, 4432. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.W.; Chiodo, L.M.; Sokol, R.J.; Jacobson, J.L. Validity of maternal report of prenatal alcohol, cocaine, and smoking in relation to neurobehavioral outcome. Pediatrics 2002, 109, 815–825. [Google Scholar] [CrossRef]
- Hollingshead, A.B. Four factor index of social status. Yale J. Sociol. 2011, 8, 21–51. [Google Scholar]
- Beard, J.L. Iron deficiency: Assessment during pregnancy and its importance in pregnant adolescents. Am. J. Clin. Nutr. 1994, 59, 502S–508S; discussion 508S–510S. [Google Scholar] [CrossRef] [PubMed]
- Carriaga, M.T.; Skikne, B.S.; Finley, B.; Cutler, B.; Cook, J.D. Serum transferrin receptor for the detection of iron deficiency in pregnancy. Am. J. Clin. Nutr. 1991, 54, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- WHO; UNICEF; UNU. Iron Deficiency Anemia: Prevention, Assessment and control—Report of a Joint WHO/UNICEF/UNU Consultation; World Health Organization: Geneva, Switzerland, 1998.
- Ganz, T.; Olbina, G.; Girelli, D.; Nemeth, E.; Westerman, M. Immunoassay for human serum hepcidin. Blood 2008, 112, 4292–4297. [Google Scholar] [CrossRef]
- Stodgell, C.J.; Miller, R.K.; Salamone, L.; Murray, J.; Chen, J.; Lambertini, L.; Schadt, E.; Littman, L.; Landrigan, P.; Aagaard, K.; et al. Lack of Correlation between placental gene expression and RNA integrity number (RIN) or time to collection. Placenta 2014, 35, A46–A47. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Hoffman, G.E.; Schadt, E.E. variancePartition: Interpreting drivers of variation in complex gene expression studies. BMC Bioinform. 2016, 17, 483. [Google Scholar] [CrossRef]
- Leek, J.T.; Johnson, W.E.; Parker, H.S.; Jaffe, A.E.; Storey, J.D. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics 2012, 28, 882–883. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [PubMed]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Stelzer, G.; Rosen, N.; Plaschkes, I.; Zimmerman, S.; Twik, M.; Fishilevich, S.; Stein, T.I.; Nudel, R.; Lieder, I.; Mazor, Y.; et al. The GeneCards suite: From gene data mining to disease genome sequence analyses. Curr. Protoc. Bioinform. 2016, 54, 1.30.1–1.30.33. [Google Scholar] [CrossRef] [PubMed]
- Pearl, J. Direct and Indirect Effects; Breese, J., Koller, D., Eds.; Morgan Kaufmann Publishers Inc.: San Francisco, CA, USA, 2001. [Google Scholar]
- Robins, J.M.; Greenland, S. Identifiability and exchangeability for direct and indirect effects. Epidemiology 1992, 3, 143–155. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Williams, J.B.W. Structured Clinical Interview for DSM-5 Disorders; American Psychiatric Association: Washington, DC, USA, 2021. [Google Scholar]
- Oken, E.; Kleinman, K.P.; Rich-Edwards, J. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003, 3, 6. [Google Scholar] [CrossRef]
- Verga Falzacappa, M.V.; Vujic Spasic, M.; Kessler, R.; Stolte, J.; Hentze, M.W.; Muckenthaler, M.U. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood 2007, 109, 353–358. [Google Scholar] [CrossRef]
- Wrighting, D.M.; Andrews, N.C. Interleukin-6 induces hepcidin expression through STAT3. Blood 2006, 108, 3204–3209. [Google Scholar] [CrossRef]
- Miller, A.M.; Horiguchi, N.; Jeong, W.I.; Radaeva, S.; Gao, B. Molecular mechanisms of alcoholic liver disease: Innate immunity and cytokines. Alcohol. Clin. Exp. Res. 2011, 35, 787–793. [Google Scholar] [CrossRef]
- Bodnar, T.S.; Raineki, C.; Wertelecki, W.; Yevtushok, L.; Plotka, L.; Granovska, I.; Zymak-Zakutnya, N.; Pashtepa, A.; Wells, A.; Honerkamp-Smith, G.; et al. Immune network dysregulation associated with child neurodevelopmental delay: Modulatory role of prenatal alcohol exposure. J. Neuroinflamm. 2020, 17, 39. [Google Scholar] [CrossRef] [PubMed]
- Ruffaner-Hanson, C.; Noor, S.; Sun, M.S.; Solomon, E.; Marquez, L.E.; Rodriguez, D.E.; Allan, A.M.; Caldwell, K.K.; Bakhireva, L.N.; Milligan, E.D. The maternal-placental-fetal interface: Adaptations of the HPA axis and immune mediators following maternal stress and prenatal alcohol exposure. Exp. Neurol. 2022, 355, 114121. [Google Scholar] [CrossRef] [PubMed]
- Young, S.L.; Saif, Z.; Meakin, A.S.; McMaster, E.S.; Hayes, N.; Gallo, L.A.; Reid, N.; Moritz, K.M.; Clifton, V.L. Alterations to Placental Glucocorticoid Receptor Expression with Alcohol Consumption. Reprod. Sci. 2021, 28, 1390–1402. [Google Scholar] [CrossRef] [PubMed]
- Teo, S.M.; Murrin, C.M.; Mehegan, J.; Douglass, A.; Hebert, J.R.; Segurado, R.; Kelleher, C.C.; Phillips, C.M. Associations between the maternal healthy lifestyle score and its individual components during early pregnancy with placental outcomes. Placenta 2023, 139, 75–84. [Google Scholar] [CrossRef]
- Wang, N.; Tikellis, G.; Sun, C.; Pezic, A.; Wang, L.; Wells, J.C.K.; Cochrane, J.; Ponsonby, A.L.; Dwyer, T. The effect of maternal prenatal smoking and alcohol consumption on the placenta-to-birth weight ratio. Placenta 2014, 35, 437–441. [Google Scholar] [CrossRef]
- Rufer, E.S.; Tran, T.D.; Attridge, M.M.; Andrzejewski, M.E.; Flentke, G.R.; Smith, S.M. Adequacy of maternal iron status protects against behavioral, neuroanatomical, and growth deficits in fetal alcohol spectrum disorders. PLoS ONE 2012, 7, e47499. [Google Scholar] [CrossRef]
- Rufer, E.S.; Tran, T.D.; Attridge, M.E.; Andrzejewski, M.E.; Smith, S.M. Subclinical maternal iron inadequacy exacerbates neurobehavioral deficits caused by developmental ethanol exposure. Alcohol. Clin. Exp. Res. 2010, 34, 292a. [Google Scholar]
- Mullarky, I.K.; Szaba, F.M.; Kummer, L.W.; Wilhelm, L.B.; Parent, M.A.; Johnson, L.L.; Smiley, S.T. Gamma interferon suppresses erythropoiesis via interleukin-15. Infect. Immun. 2007, 75, 2630–2633. [Google Scholar] [CrossRef]
- Lee, P.; Peng, H.; Gelbart, T.; Wang, L.; Beutler, E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. Proc. Natl. Acad. Sci. USA 2005, 102, 1906–1910. [Google Scholar] [CrossRef]
- Kawasumi, H.; Gono, T.; Kawaguchi, Y.; Kaneko, H.; Katsumata, Y.; Hanaoka, M.; Kataoka, S.; Yamanaka, H. IL-6, IL-8, and IL-10 are associated with hyperferritinemia in rapidly progressive interstitial lung disease with polymyositis/dermatomyositis. Biomed Res. Int. 2014, 2014, 815245. [Google Scholar] [CrossRef]
- Safran, M.; Rosen, N.; Twik, M.; BarShir, R.; Iny Stein, T.; Dahary, D. The GeneCards Suite. In Practical Guide to Life Science Databases; Springer: Singapore, 2022; pp. 27–56. [Google Scholar]
- Hayward, P.; Kalmar, T.; Arias, A.M. Wnt/Notch signalling and information processing during development. Development 2008, 135, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.A. The Jak/STAT pathway. Cold Spring Harb. Perspect. Biol. 2012, 4, a011205. [Google Scholar] [CrossRef] [PubMed]
- Seif, F.; Khoshmirsafa, M.; Aazami, H.; Mohsenzadegan, M.; Sedighi, G.; Bahar, M. The role of JAK-STAT signaling pathway and its regulators in the fate of T helper cells. Cell Commun. Signal. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
| Controls (n = 35) | Heavy-Drinking Mothers (n = 33) | ||
|---|---|---|---|
| M (SD) or n (%) | M (SD) or n (%) | p a | |
| Maternal characteristics | |||
| Age at conception (years) | 25.1 (4.9) | 29.3 (5.9) | 0.002 |
| Gravidity (no. previous pregnancies (%)) | 1.4 (1.2) | 2.2 (1.5) | 0.014 |
| Education (years of school completed) | 10.1 (1.4) | 9.5 (1.2) | 0.089 |
| Socioeconomic status [23] | 22.1 (6.9) | 19.7 (6.0) | 0.066 |
| No. (%) taking maternal prenatal iron supplementation | 23 (71.9) | 32 (91.4) | 0.037 |
| Alcohol and drug use | |||
| Around conception: | |||
| Oz AA/day | 0.0 (0.0) | 1.4 (1.0) | <0.001 |
| Oz AA/drinking day | 0.1 (0.3) | 4.1 (2.4) | <0.001 |
| Drinking days/week | 0.0 (0.1) | 2.2 (1.2) | <0.001 |
| Averaged across pregnancy: | |||
| Oz AA/day | 0.0 (0.1) | 0.8 (0.7) | <0.001 |
| Oz AA/drinking day | 0.1 (0.5) | 4.3 (1.7) | <0.001 |
| Drinking days/week | 0.0 (0.0) | 1.4 (1.0) | <0.001 |
| Current alcohol dependence b | 0 (0.0) | 6 (18.8) | 0.011 |
| No. (%) reporting cigarette smoking | 22 (66.7) | 24 (68.6) | 0.065 |
| Cigarettes/day among smokers | 5.8 (4.3) | 7.0 (4.3) | 0.352 |
| No. (%) reporting marijuana use | 4 (12.1) | 4 (11.4) | 0.929 |
| Marijuana days/month among users | 4.0 (4.7) | 9.7 (9.0) | 0.047 |
| No. (%) reporting methamphetamine use | 11 (15.5) | 12 (13.8) | 0.763 |
| Methamphetamine days/month among users | 4.2 (6.6) | 7.5 (7.1) | 0.525 |
| Maternal iron measures | |||
| Ferritin (logged ug/L values) | 2.4 (0.5) | 3.0 (0.7) | <0.001 |
| Hemoglobin (g/dL):log(ferritin ug/L) | 4.6 (0.8) | 4.0 (0.8) | 0.002 |
| Urinary hepcidin (ng):creatinine (mg) | 26.5 (5.4) | 24.9 (6.3) | 0.276 |
| Infant characteristics | |||
| Weeks of gestation at delivery | 39.3 (1.7) | 39.2 (1.8) | 0.440 |
| Sex, no. female (%) | 14 (42.4) | 13 (37.1) | 0.656 |
| Birthweight (g) | 3100.9 (496.1) | 2964.0 (532.2) | 0.139 |
| No. (%) small for gestational age c | 6 (18.2) | 11 (31.4) | 0.207 |
| Neonatal iron measures | |||
| Hemoglobin (g/dL) d | 15.3 (2.6) | 14.9 (1.8) | 0.219 |
| Ferritin (logged ug/L values) e | 5.2 (0.7) | 5.3 (0.6) | 0.156 |
| Hemoglobin (g/dL):log(ferritin) f | 3.0 (0.5) | 2.8 (0.5) | 0.088 |
| 6.5-month infant iron measures | |||
| Hemoglobin (g/dL) | 10.8 (1.0) | 10.7 (1.2) | 0.314 |
| Iron deficiency (yes = 1, no = 0) | 9 (27.3) | 12 (34.3) | 0.532 |
| Iron deficiency anemia (yes = 1, no = 0) | 4 (12.1) | 7 (20.0) | 0.378 |
| Iron Metabolism PCA Factor: | Factor 8 | Factor 11 a | Factor 13 b,c | Factor 18 d | Factor 20 e | Factor 42 | ||||||
| r | ß | r | ß | r | ß | r | ß | r | ß | r | ß | |
| Alcohol use around conception: | ||||||||||||
| Oz AA/day (logged values) | - | - | - | - | - | - | - | - | 0.22 † | 0.19 | 0.22 † | 0.22 † |
| Oz AA/drinking day | −0.21 † | −0.22 † | - | - | - | - | - | - | - | - | 0.22 † | 0.22 † |
| Drinking days/week | - | - | −0.23 † | −0.23 † | - | - | - | - | - | - | - | - |
| Alcohol use across pregnancy: | ||||||||||||
| Oz AA/day (logged values) | - | - | - | - | - | - | −0.20 † | −0.27 * | 0.22 † | 0.18 | - | - |
| Oz AA/drinking day | −0.28 * | −0.27 * | - | - | 0.23 † | 0.14 | - | - | - | - | - | - |
| Drinking days/week | - | - | - | - | - | - | −0.20 † | −0.26 * | 0.20 † | 0.15 | - | - |
| Iron Metabolism PCA Factor: | Factor 44 c,f | Factor 49 | Factor 59 f | |||||||||
| r | ß | r | ß | r | ß | |||||||
| Alcohol use around conception: | ||||||||||||
| Oz AA/day (logged values) | - | - | - | - | - | - | ||||||
| Oz AA/drinking day | - | - | - | - | - | - | ||||||
| Drinking days/week | −0.25 * | −0.21 † | - | - | - | - | ||||||
| Alcohol use across pregnancy: | ||||||||||||
| Oz AA/day (logged values) | - | - | - | - | −0.20 † | −0.13 | ||||||
| Oz AA/drinking day | - | - | - | - | - | - | ||||||
| Drinking days/week | - | - | 0.20 † | 0.19 | −0.25 * | −0.19 | ||||||
| Inflammation PCA Factor: | Factor 5 | Factor 10 e | Factor 12 | Factor 22 d | Factor 34 d | Factor 35 | ||||||
| r | ß | r | ß | r | ß | r | ß | r | r | ß | ||
| Alcohol use around conception: | ||||||||||||
| Oz AA/day (logged values) | - | - | −0.29 * | −0.28 * | - | - | 0.26 * | 0.24 † | - | - | −0.29 * | −0.25 * |
| Oz AA/drinking day | −0.26 * | −0.27 * | −0.30 ** | −0.29 * | - | - | 0.32 ** | 0.29 * | - | - | −0.26 * | −0.21 † |
| Drinking days/week | - | - | −0.32 ** | −0.30 ** | 0.25 * | 0.23 † | - | - | - | - | −0.31 ** | −0.27 * |
| Alcohol use across pregnancy: | - | - | ||||||||||
| Oz AA/day (logged values) | - | - | −0.24 * | −0.22 † | 0.21 † | 0.19 | - | - | - | - | −0.35 ** | −0.31 ** |
| Oz AA/drinking day | −0.25 * | −0.27 * | −0.27 * | −0.24 * | - | - | - | - | 0.22 † | 0.20 † | −0.28 * | −0.25 * |
| Drinking days/week | - | - | −0.21 † | −0.19 | 0.24 * | 0.22 † | - | - | - | - | −0.38 *** | −0.34 ** |
| Inflammation PCA Factor: | Factor 40 | Factor 45 | Factor 46 | Factor 57 | ||||||||
| r | ß | r | ß | r | ß | r | ß | |||||
| Alcohol use around conception: | ||||||||||||
| Oz AA/day (logged values) | 0.26 * | 0.26 * | 0.28 * | 0.23 † | - | - | - | - | ||||
| Oz AA/drinking day | 0.22 † | 0.22 † | 0.28 * | 0.23 † | −0.22 † | −0.22 † | - | - | ||||
| Drinking days/week | 0.22 † | 0.21 † | 0.24 * | 0.19 | - | - | - | - | ||||
| Alcohol use across pregnancy: | ||||||||||||
| Oz AA/day (logged values) | 0.21 † | 0.20 | 0.24 * | 0.18 | - | - | - | - | ||||
| Oz AA/drinking day | - | - | 0.23 † | 0.17 | - | - | - | - | ||||
| Drinking days/week | 0.21 † | 0.20 | - | - | - | - | 0.23 † | 0.16 | ||||
| Iron Metabolism PCA Factor: | Factor 8 | Factor 11 a | Factor 13 b,c | Factor 18 d | Factor 20 e | |||||
| r | ß | r | ß | r | ß | r | ß | r | ß | |
| Maternal iron measures: | ||||||||||
| Ferritin (logged ug/L values) b | - | - | - | - | - | - | - | - | - | - |
| Hemoglobin (g/dL):log(ferritin ug/L) b | - | - | - | - | - | - | - | - | - | - |
| Urinary hepcidin (ng):creatinine (mg) g | - | - | −0.31 † | −0.29 | - | - | - | - | −0.38 * | −0.44 * |
| Neonatal iron measures: | ||||||||||
| Hemoglobin (g/dL) h | - | - | - | - | - | - | −0.23† | −0.19 | - | - |
| Ferritin (logged ug/L values) h | - | - | - | - | - | - | - | - | - | - |
| Hemoglobin (g/dL):log(ferritin) h | - | - | - | - | - | - | - | - | - | - |
| 6.5-month infant iron measures: | ||||||||||
| Hemoglobin (g/dL) h | - | - | - | - | - | - | 0.24 * | 0.28 * | - | - |
| Iron deficiency (Yes = 1, No = 0) g,i | - | - | - | - | 0.31 ** | 0.27 † | - | - | - | - |
| Iron deficiency anemia (Yes = 1, No = 0) f,g,i | - | - | - | - | 0.21 † | 0.18 | −0.22 † | −0.32 * | - | - |
| Iron Metabolism PCA Factor: | Factor 42 | Factor 44 c,f | Factor 49 | Factor 59 f | ||||||
| r | ß | r | ß | r | ß | r | ß | |||
| Maternal iron measures: | ||||||||||
| Ferritin (logged ug/L values) b | - | - | - | - | - | - | - | - | ||
| Hemoglobin (g/dL):log(ferritin ug/L) b | - | - | - | - | - | - | - | - | ||
| Urinary hepcidin (ng):creatinine (mg) g | - | - | −0.39 * | −0.36 † | - | - | - | - | ||
| Neonatal iron measures: | ||||||||||
| Hemoglobin (g/dL) h | - | - | 0.22 † | 0.22 † | - | - | −0.24 † | −0.09 | ||
| Ferritin (logged ug/L values) h | - | - | 0.26 † | 0.22 | - | - | - | - | ||
| Hemoglobin (g/dL):log(ferritin) h | - | - | 0.26 † | 0.21 | - | - | - | - | ||
| 6.5-month infant iron measures: | ||||||||||
| Hemoglobin (g/dL) h | - | - | - | - | 0.21 † | 0.24 † | - | - | ||
| Iron deficiency (Yes = 1, No = 0) g,i | - | - | - | - | - | - | - | - | ||
| Iron deficiency anemia (Yes = 1, No = 0) f,g,i | - | - | - | - | - | - | 0.23 † | 0.24 † | ||
| Inflammation PCA Factor: | Factor 5 | Factor 10 e | Factor 12 | Factor 22 d | Factor 34 d | |||||
| r | ß | r | ß | r | ß | r | ß | r | ß | |
| Maternal iron measures: | ||||||||||
| Ferritin (logged ug/L values) b | - | - | −0.26 * | −0.23 * | - | - | - | - | - | - |
| Hemoglobin (g/dL):log(ferritin ug/L) b | −0.22† | −0.19 | 0.39 *** | 0.34 ** | - | - | - | - | - | - |
| Urinary hepcidin (ng):creatinine (mg) g | - | - | - | - | - | - | −0.31 † | −0.31 | - | - |
| Neonatal iron measures: | ||||||||||
| Hemoglobin (g/dL) h | - | - | - | - | - | - | - | - | - | - |
| Ferritin (logged ug/L values) h | - | - | - | - | - | - | - | - | - | - |
| Hemoglobin (g/dL):log(ferritin) h | - | - | - | - | - | - | - | - | - | - |
| 6.5-month infant iron measures: | ||||||||||
| Hemoglobin (g/dL) h | - | - | - | - | - | - | - | - | - | - |
| Iron deficiency (Yes = 1, No = 0) g,i | - | - | - | - | 0.22 † | 0.23 † | 0.30 ** | 0.27 * | - | - |
| Iron deficiency anemia (Yes = 1, No = 0) f,g,i | - | - | - | - | 0.27 * | 0.26 * | - | - | - | - |
| Inflammation PCA Factor: | Factor 35 | Factor 40 | Factor 45 6 | Factor 46 | Factor 57 d,e | |||||
| r | ß | r | ß | r | ß | r | ß | r | ß | |
| Maternal iron measures: | ||||||||||
| Ferritin (logged ug/L values) b | - | - | - | - | - | - | - | - | - | - |
| Hemoglobin (g/dL):log(ferritin ug/L) b | - | - | - | - | - | - | - | - | - | - |
| Urinary hepcidin (ng):creatinine (mg) g | - | - | 0.30 † | 0.30 † | - | - | - | - | - | - |
| Neonatal iron measures: | ||||||||||
| Hemoglobin (g/dL) h | - | - | - | - | - | - | - | - | 0.23 † | 0.08 |
| Ferritin (logged ug/L values) h | - | - | - | - | - | - | - | - | 0.24 † | 0.12 |
| Hemoglobin (g/dL):log(ferritin) h | - | - | - | - | - | - | - | - | - | - |
| 6.5-month infant iron measures: | ||||||||||
| Hemoglobin (g/dL) h | - | - | - | - | −0.23 † | −0.25 * | −0.20 † | −0.20 | - | - |
| Iron deficiency (Yes = 1, No = 0) g,i | - | - | - | - | - | - | - | - | - | - |
| Iron deficiency anemia (Yes = 1, No = 0) f,g,i | - | - | - | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masehi-Lano, J.J.; Deyssenroth, M.; Jacobson, S.W.; Jacobson, J.L.; Molteno, C.D.; Dodge, N.C.; Wainwright, H.C.; Meintjes, E.M.; Lesseur, C.; Cheng, H.; et al. Alterations in Placental Inflammation-Related Gene Expression Partially Mediate the Effects of Prenatal Alcohol Consumption on Maternal Iron Homeostasis. Nutrients 2023, 15, 4105. https://doi.org/10.3390/nu15194105
Masehi-Lano JJ, Deyssenroth M, Jacobson SW, Jacobson JL, Molteno CD, Dodge NC, Wainwright HC, Meintjes EM, Lesseur C, Cheng H, et al. Alterations in Placental Inflammation-Related Gene Expression Partially Mediate the Effects of Prenatal Alcohol Consumption on Maternal Iron Homeostasis. Nutrients. 2023; 15(19):4105. https://doi.org/10.3390/nu15194105
Chicago/Turabian StyleMasehi-Lano, Jacqueline J., Maya Deyssenroth, Sandra W. Jacobson, Joseph L. Jacobson, Christopher D. Molteno, Neil C. Dodge, Helen C. Wainwright, Ernesta M. Meintjes, Corina Lesseur, Haoxiang Cheng, and et al. 2023. "Alterations in Placental Inflammation-Related Gene Expression Partially Mediate the Effects of Prenatal Alcohol Consumption on Maternal Iron Homeostasis" Nutrients 15, no. 19: 4105. https://doi.org/10.3390/nu15194105
APA StyleMasehi-Lano, J. J., Deyssenroth, M., Jacobson, S. W., Jacobson, J. L., Molteno, C. D., Dodge, N. C., Wainwright, H. C., Meintjes, E. M., Lesseur, C., Cheng, H., Li, Q., Hao, K., Chen, J., & Carter, R. C. (2023). Alterations in Placental Inflammation-Related Gene Expression Partially Mediate the Effects of Prenatal Alcohol Consumption on Maternal Iron Homeostasis. Nutrients, 15(19), 4105. https://doi.org/10.3390/nu15194105






