Effect of a Diet-Induced Obesity on the Progeny Response in a Murine Model
Abstract
:1. Introduction
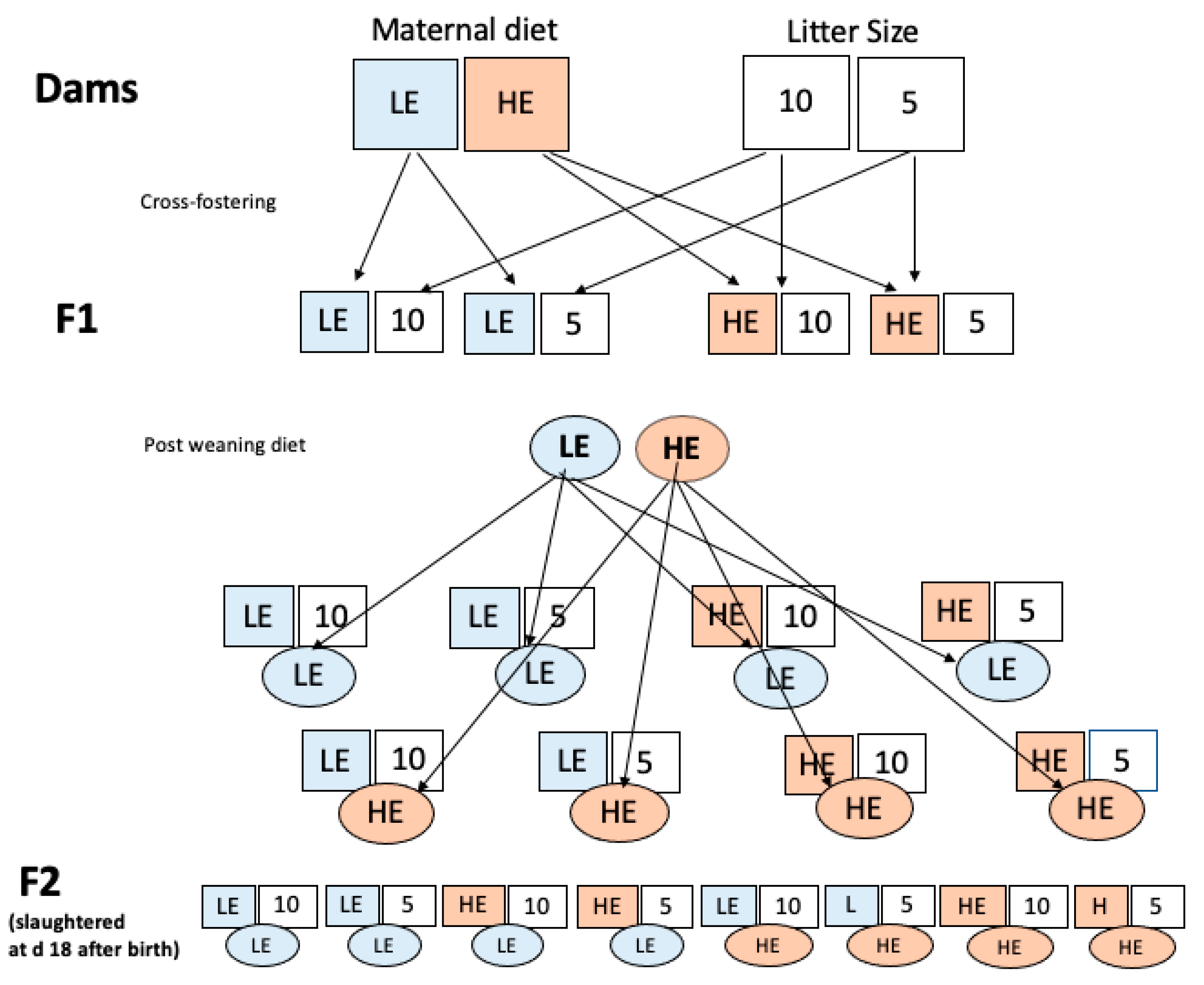
2. Materials and Methods
2.1. Location
2.2. Animals and Sampling
2.3. Statistical Design
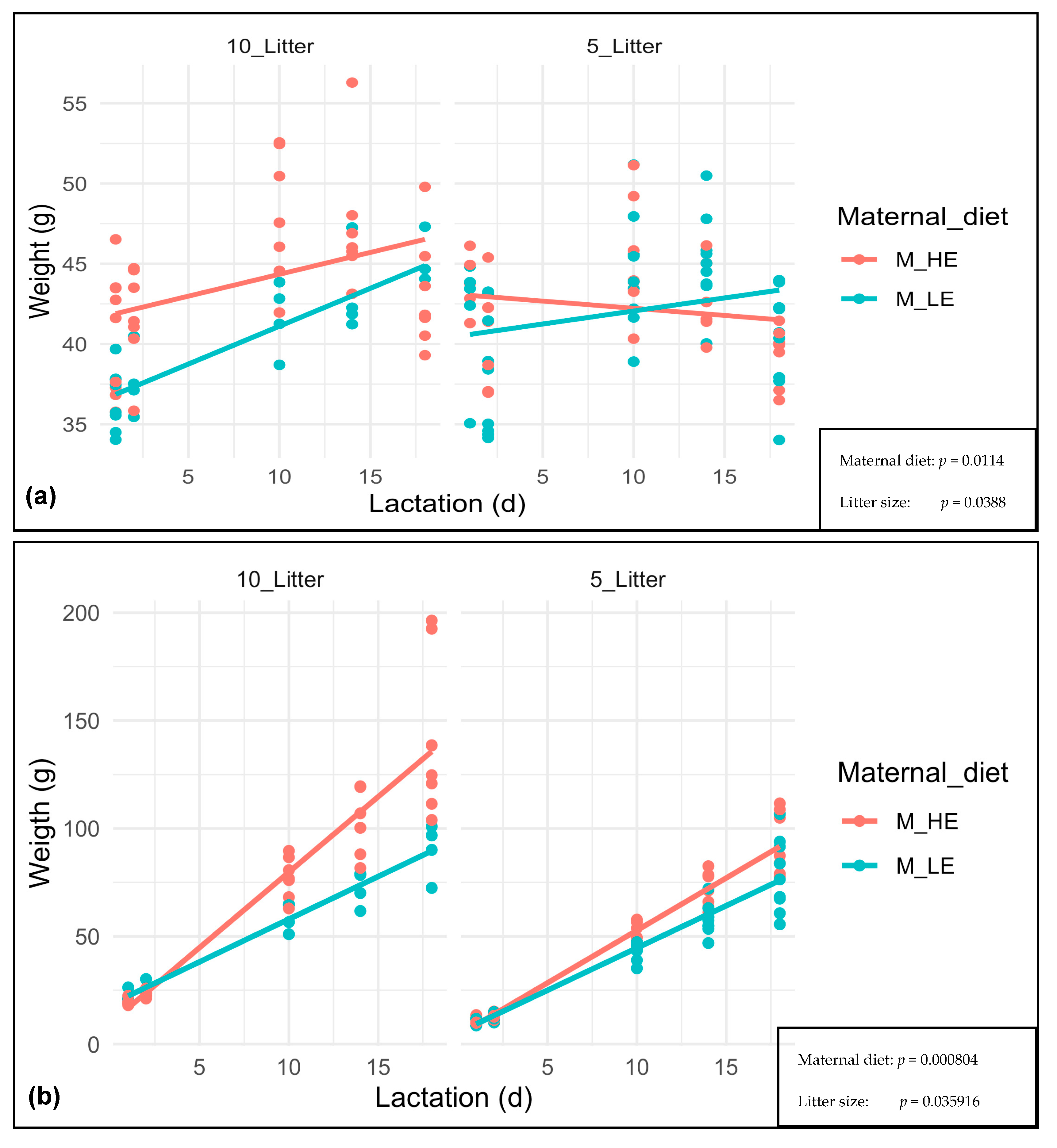
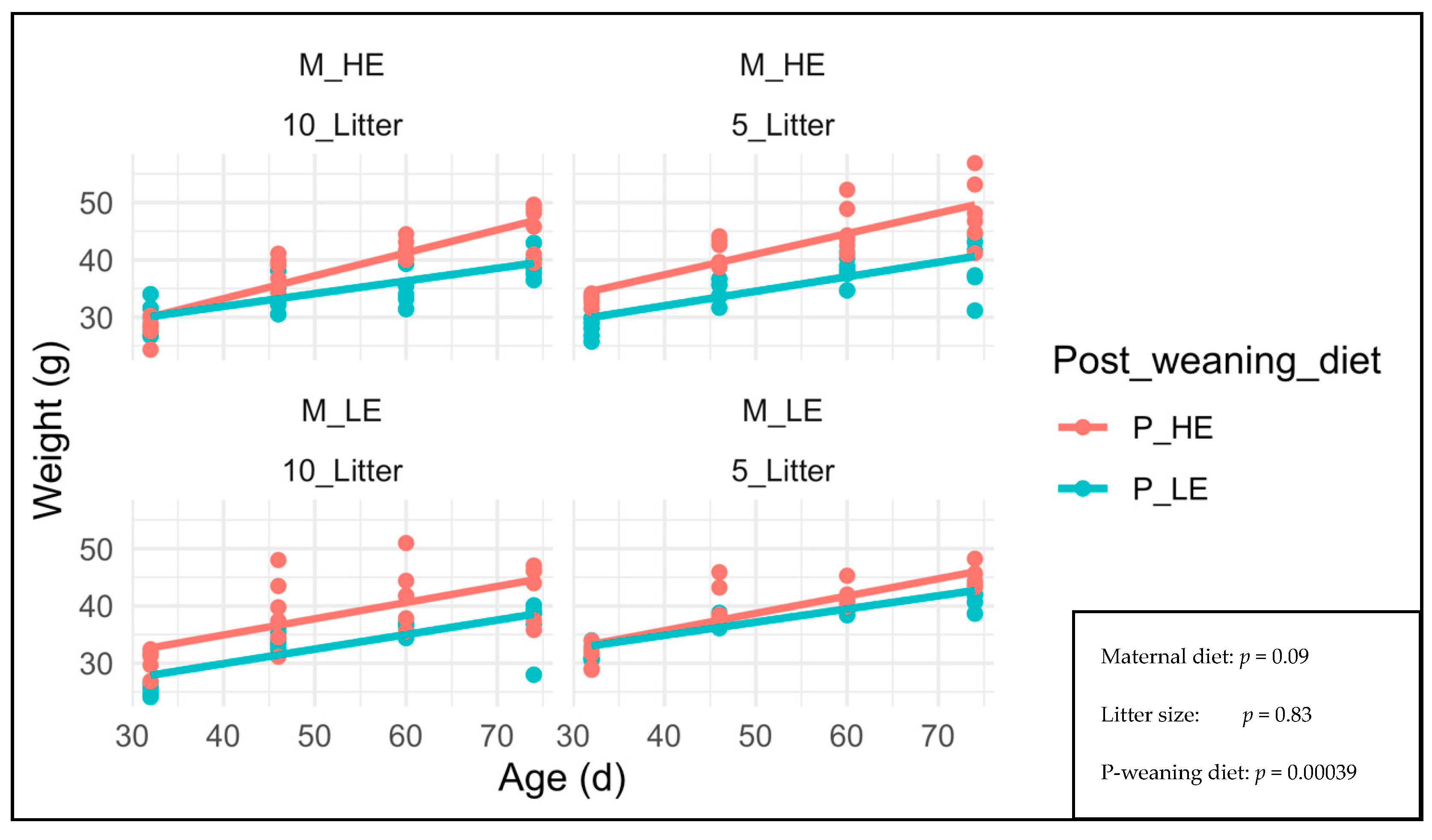
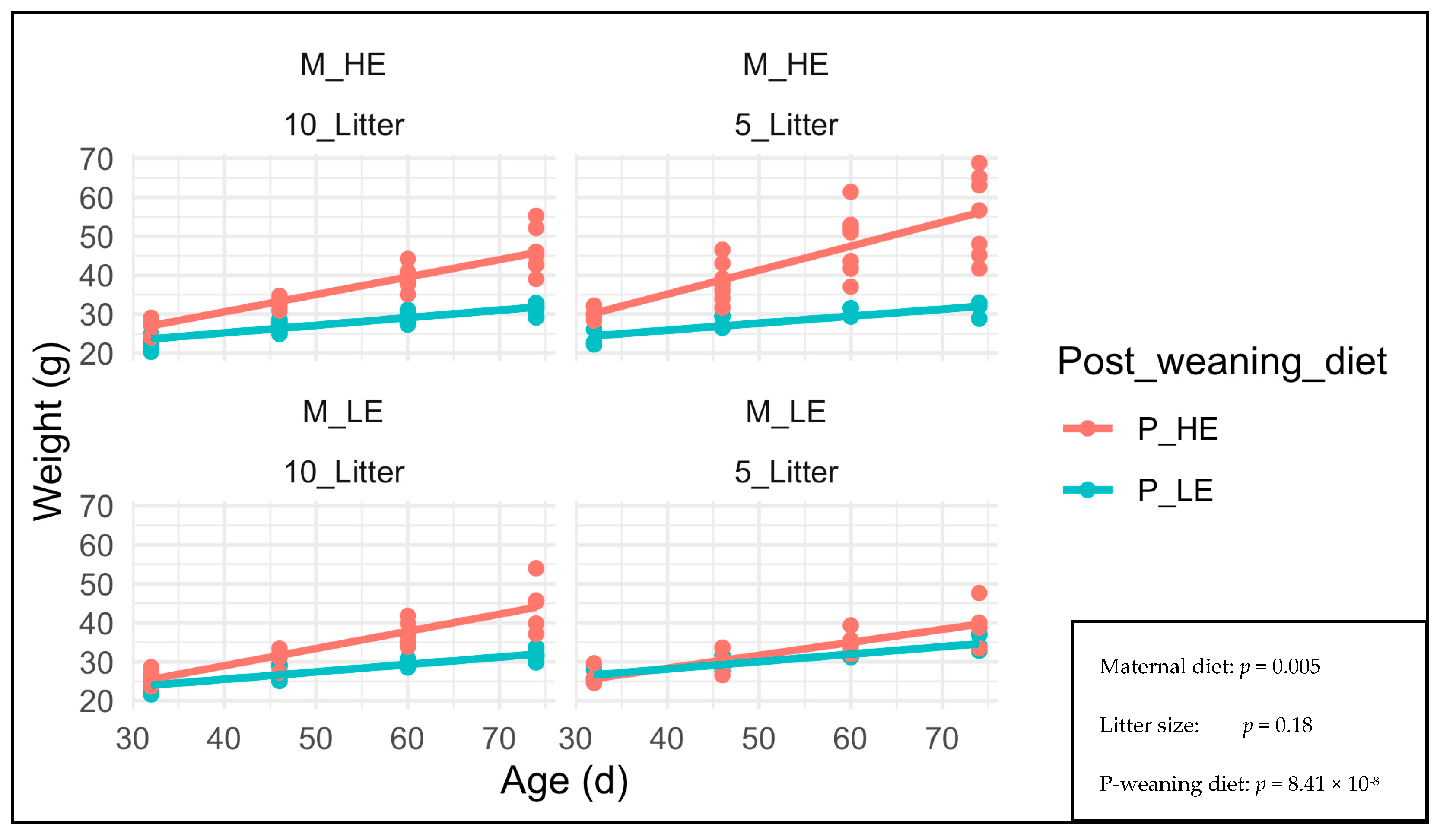
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chooi, C.; Ding, C.; Magkos, F. The epidemiology of obesity. Methabolism 2019, 92, 6–10. [Google Scholar] [CrossRef]
- Heslehurst, N.; Ells, L.J.; Simpson, H.; Batterham, A.; Wilkinson, J.; Summerbell, C.D. Trends in maternal obesity incidence rates, demographic predictors, and health inequalities in 36,821 women over a 15-year period. BJOG 2007, 114, 187–194. [Google Scholar] [CrossRef]
- Catalano, P.M.; Ehrenberg, H.M. The short- and longterm implications of maternal obesity on the mother and her offspring. BJOG 2006, 113, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Alfaradhi, M.Z.; Ozanne, S.E. Developmental programming in response to maternal overnutrition. Front. Genet. 2011, 2, 27. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta 2014, 1842, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Zheng, J.; Li, M.; Yu, M.; Ping, F.; Wang, T.; Wang, X. A maternal high-fat diet induces DNA methylation changes that contribute to glucose intolerance in offspring. Front. Endocrinol. 2019, 10, 871. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 444, 860–867. [Google Scholar] [CrossRef]
- Álvarez-Chávez, A.; Canto, P. Influence of maternal obesity on the skeletal muscle of offspring. Boletín Médico Del. Hosp. Infant. México 2022, 79, 284–292. [Google Scholar] [CrossRef]
- Parra-Vargas, M.; Ramon-Krauel, M.; Lerin, C.; Jimenez-Chillaron, J.C. Size Does Matter: Litter Size Strongly Determines Adult Metabolism in Rodents. Cell Metab. 2020, 32, 334–340. [Google Scholar] [CrossRef]
- Martins, T.; Castro-Ribeiro, C.; Lemos, S.; Ferreira, T.; Nascimento-Gonçalves, E.; Rosa, E.; Oliveira, P.; Antunes, A. Murine Models of Obesity. Obesities 2022, 2, 127–147. [Google Scholar] [CrossRef]
- Schoonejans, J.; Ozanne, S. Developmental programming by maternal obesity: Lessons from animal models. Diabet. Med. 2021, 38, e1469. [Google Scholar] [CrossRef] [PubMed]
- Sejrsen, K.; Purup, S. Influence of prepubertal feeding level on milk yield potential of dairy heifers: A review. J. Anim. Sci. 1997, 75, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Segovia, S.A.; Vickers, M.H.; Gray, C.; Reynolds, C.M. Maternal obesity, inflammation, and developmental programming. BioMed Res. Int. 2014, 2014, 418975. [Google Scholar] [CrossRef] [PubMed]
- Ailhaud, G.; Grimaldi, P.; N’egrel, R. Cellular and molecular aspects of adipose tissue development. Annu. Rev. Nutr. 1992, 12, 207–233. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of fat cell turnover in humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef]
- Kleinert, M.; Clemmensen, C.; Hofmann, S.; Moore, M.; Renner, S.; Woods, S.; Huypens, P.; Beckers, J.; de Angelis, M.; Schurmann, A.; et al. Animal models of obesity and diabetes mellitus. Nat. Rev. Endocrinol. 2018, 14, 140–162. [Google Scholar] [CrossRef]
- Kulhanek, D.; Weigel, R.; Paulsen, M.E. Maternal high-fat–high-carbohydrate diet-induced obesity is associated with increased appetite in peripubertal male but not female C57Bl/6J Mice. Nutrients 2020, 12, 2919. [Google Scholar] [CrossRef]
- Ito, J.; Nakagawa, K.; Kato, S.; Miyazawa, T.; Kimura, F.; Miyazawa, T. The combination of maternal and offspring high-fat diets causes marked oxidative stress and development of metabolic syndrome in mouse offspring. Life Sci. 2016, 151, 70–75. [Google Scholar] [CrossRef]
- Frommelt, L.; Bielohuby, M.; Stoehr, B.J.M.; Menhofer, D.; Bidlingmaier, M.; Kienzle, E. Effects of low-carbohydrate, high-fat diets on apparent digestibility of minerals and trace elements in rats. Nutrition 2014, 30, 869–875. [Google Scholar] [CrossRef]
- Iguchi, T.; Wantanabe, H.; Ohta, Y.; Blumberg, B. Developmental effect: Oestrogen induced vaginal changes and adipogenesis. Int. J. Androl. 2008, 31, 263–268. [Google Scholar] [CrossRef]
- Dyck, D.J.; Heigenhauser, G.J.; Bruce, C.R. Role of adipokines as regulators of skeletal muscle fatty acid metabolism and insulin sensitivity. Acta Physiol. 2006, 186, 5–16. [Google Scholar] [CrossRef]
- Daghestani, M.H.; Ozand, P.T.; Al-Himadi, A.R.; Al-Odaib, A.N. Hormonal levels of leptin, insulin, ghrelin, and neuropeptide Y in lean, overweight and obese Saudi females. Saudi Med. J. 2007, 28, 1191–1197. [Google Scholar] [PubMed]
- Shin, J.H.; Hur, J.Y.; Seo, H.S.; Jeong, Y.A.; Lee, J.K.; Oh, M.J.; Kim, T.; Saw, H.S.; Kim, S.H. The ratio of estrogen receptor to estrogen receptor in adipose tissue is associated with leptin production and obesity. Steroids 2007, 72, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Muhlhausler, B.S.; Duffield, J.A.; McMillen, I.C. Increased maternal nutrition stimulates peroxisome proliferator activated receptor-γ, adiponectin, and leptin messenger ribonucleic acid expression in adipose tissue before birth. Endocrinology 2007, 148, 878–885. [Google Scholar] [CrossRef]
- Schubert, M. Detection of Meat and Fat Quality in Pork and Beef Using X-ray. Master’s Thesis, Faculty of Life Sciences, University of Copenhagen, København, Denmark, 2009. [Google Scholar]
- Kruse, M.; Fiallo, A.; Tao, J.; Susztak, K.; Amann, E.K.; Katz, I.B.; Charron, M.J. A high fat diet during pregnancy and lactation induces cardiac and renal abnormalities in GLUT4 +/− male mice. Kidney Blood Press. Res. 2017, 42, 468–482. [Google Scholar] [CrossRef]
- Subbaramaiah, K.; Howe, L.R.; Bhardwaj, P.; Du, B.; Gravaghi, C.; Yantiss, R.K.; Zhou, X.K.; Blaho, V.A.; Hla, T.; Yang, P.; et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev. Res. 2004, 4, 329–346. [Google Scholar] [CrossRef]
- Haku, M. Breast-feeding: Factor associated with the continuation of breast-feeding, the current situation in Japan, and recommendations for further research. J. Med. Investig. 2007, 54, 224–234. [Google Scholar] [CrossRef]
- Donath, S.M.; Amir, L.H. Does maternal obesity adversely affect breast-feeding initiation and duration? J. Paediatr. Child. Health 2000, 36, 482–486. [Google Scholar] [CrossRef]
- Rasmussen, K.M.; Hilson, J.A.; Kjolhede, C.L. Obesity may impair lactogenesis II. J. Nutr. 2011, 131, 3009–3011. [Google Scholar] [CrossRef]
- Li, R.; Jewell, S.; Grummer-Strawn, L. Maternal obesity and breast-feeding practices. Am. J. Clin. Nutr. 2003, 77, 931–936. [Google Scholar] [CrossRef]
- Radulescu, L.; Munteanu, O.; Popa, F.; Cirstoiu, M. The implications and consequences of maternal obesity on fetal intrauterine growth restriction. J. Med. Life 2013, 6, 292–298. [Google Scholar] [PubMed]
- Luzzo, K.M.; Wang, Q.; Purcell, S.H.; Chi, M.; Jimenez, P.T.; Grindler, N.; Schedl, T.; Moley, K.H. High fat diet induced developmental defects in themouse: Oocytemeiotic aneuploidy and fetal growth retardation/brain defects. PLoS ONE 2012, 7, e49217. [Google Scholar] [CrossRef]
- Flint, D.J.; Travers, M.T.; Barber, M.C.; Binart, N.; Kelly, P.A. Diet-induced obesity impairs mammary development and lactogenesis in murine mammary gland. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 1179–1187. [Google Scholar] [CrossRef]
- Buonfiglio, D.; Buonfiglio, C.; Ramos-Lobo, A.M.; Freitas, V.M.; Zampieri, T.T.; Nagaishi, V.S.; Magalhães, M.; Cipolla-Neto, J.; Cella, N.; Donato, J., Jr. Obesity impairs lactation performance in mice by inducing prolactin resistance. Sci. Rep. 2016, 6, 22421. [Google Scholar] [CrossRef]
- Chechi, K.; Cheema, S.K. Maternal diet rich in saturated fats has deleterious effects on plasma lipids of mice. Exp. Clin. Cardiol. 2006, 11, 129–135. [Google Scholar]
- Howie, G.J.; Sloboda, D.M.; Kamal, T.; Vickers, M.H. Maternal nutritional history predicts obesity in adult offspring independent of postnatal diet. J. Physiol. 2009, 587, 905–915. [Google Scholar] [CrossRef]
- Boney, C.M.; Verma, A.; Tucker, R.; Vohr, V.R. Metabolic syndrome in childhood: Association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005, 115, 290–296. [Google Scholar] [CrossRef]
- Harder, T.; Rodekamp, E.; Schellong, K.; Dudenhausen, J.W.; Plagemann, A. Birth weight and subsequent risk of type 2 diabetes: A meta-analysis. Am. J. Epidemiol. 2007, 165, 849–857. [Google Scholar] [CrossRef]
- Glastras, S.J.; Tsang, M.; The, R.; Chen, H.; McGrath, R.T.; Zaky, A.A.; Pollock, C.A.; Saad, S. Maternal obesity promotes diabetic nephropathy in rodent Offspring. Sci. Rep. 2016, 9, 27769. [Google Scholar] [CrossRef]
- Moussa, H.N.; Alrais, M.A.; Leon, M.G.; Abbas, E.L.; Sibai, B.M. Obesity epidemic: Impact from preconception to postpartum. Future Sci. OA 2016, 2, FSO137. [Google Scholar] [CrossRef]
- Bilbo, S.D.; Tsang, V. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 2010, 24, 2104–2115. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sloboda, D.M.; Vickers, M.H. Maternal obesity and developmental programming of metabolic disorders in offspring: Evidence from animal models. Exp. Diabetes Res. 2011, 2011, 592408. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, K.M.; Kjolhede, C.L. Prepregnant overweight and obesity diminish the prolactin response to suckling in the first week postpartum. Pediatrics 2004, 113, 465–471. [Google Scholar] [CrossRef]
- Bellisario, V.; Berry, A.; Capoccia, S.; Raggi, C.; Panetta, P.; Branchi, I.; Piccaro, G.; Giorgio, M.; Pelicci, P.G.; Cirulli, F. Gender-dependent resiliency to stressful and metabolic challenges following prenatal exposure to high-fat diet in the p66(Shc−/−) mouse. Front. Behav. Neurosci. 2014, 8, 285. [Google Scholar] [CrossRef] [PubMed]
- Bellisario, V.; Panetta, P.; Balsevich, G.; Baumann, V.; Noble, J.; Raggi, C.; Nathan, O.; Berry, A.; Seckl, J.; Schmidt, M.; et al. Maternal high-fat diet acts as a stressor increasing maternal glucocorticoids’ signaling to the fetus and disrupting maternal behavior and brain activation in C57BL/6J mice. Psychoneuroendocrinology 2015, 60, 138–150. [Google Scholar] [CrossRef]
- Yan, X.; Tong, J.F.; Zhu, M.J.; Ford, S.P.; Nathanielsz, P.W.; Du, M. Maternal obesity induces inflammation and adipogenesis in late gestation fetal sheep muscle. Diabetes 2009, 58, 85. [Google Scholar]
- Samuelsson, A.M.; Matthews, P.A.; Argenton, M.; Christie, M.R.; McConnell, J.M.; Jansen, E.H.; Piersma, A.H.; Ozanne, S.E.; Twinn, D.F.; Remacle, C.; et al. Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance: A novel murine model of developmental programming. Hypertension 2008, 51, 383–392. [Google Scholar] [CrossRef]
- Williams, G. Aromatase up-regulation, insulin and raised intracellular oestrogens in men, induce adiposity, metabolic syndrome and prostate disease, via aberrant ER-a and GPER signaling. Mol. Cell Endocrinol. 2012, 351, 269–278. [Google Scholar] [CrossRef]
- Oben, J.A.; Mouralidarane, A.; Samuelsson, A.M.; Matthews, P.J.; Morgan, M.L.; McKee, C.; Soeda, J.; Fernandez-Twinn, D.S.; Martin-Gronert, M.S.; Ozanne, S.E.; et al. Maternal obesity during pregnancy and lactation programs the development of offspring non-alcoholic fatty liver disease in mice. J. Hepatol. 2010, 52, 913–920. [Google Scholar] [CrossRef]
- Park, E.J.; Lee, J.H.; Yu, G.Y.; He, G.; Ali, S.R.; Holzer, R.G.; Osterreicher, C.H.; Takahashi, H.; Karin, M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell 2010, 140, 197–208. [Google Scholar] [CrossRef]
- Ashino, N.G.; Saito, K.N.; Souza, F.D.; Nakutz, F.S.; Roman, E.A.; Velloso, L.A.; Torsoni, A.S.; Torsoni, M.A. Maternal high fat feeding through pregnancy and lactation predisposes mouse offspring to molecular insulin resistance and fatty liver. J. Nutr. Biochem. 2012, 23, 341–348. [Google Scholar] [CrossRef]
- Gregorio, B.M.; Souza-Mello, V.; Carvalho, J.J.; Mandarim-De-Lacerda, A.; Aguila, M.B. Maternal high-fat intake predisposes nonalcoholic fatty liver disease in C57BL/6 offspring. Am. J. Obs. Gynecol. 2010, 203, e495. [Google Scholar] [CrossRef]
- Vickers, M.H.; Breier, B.H.; Cutfield, W.S.; Hofman, P.L.; Gluckman, P.D. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am. J. Physiol. Endocrinol. Metab. 2000, 279, 83–87. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Committee on an Evidence Framework for Obesity Prevention Decision Making; Kumanyika, S.K.; Parker, L.; Sim, L.J. Obesity Prevention Strategies in Concept and Practice; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallardo Paffetti, M.; Cárcamo, J.G.; Azócar-Aedo, L.; Parra, A. Effect of a Diet-Induced Obesity on the Progeny Response in a Murine Model. Nutrients 2023, 15, 4970. https://doi.org/10.3390/nu15234970
Gallardo Paffetti M, Cárcamo JG, Azócar-Aedo L, Parra A. Effect of a Diet-Induced Obesity on the Progeny Response in a Murine Model. Nutrients. 2023; 15(23):4970. https://doi.org/10.3390/nu15234970
Chicago/Turabian StyleGallardo Paffetti, Maria, Juan G. Cárcamo, Lucía Azócar-Aedo, and Angel Parra. 2023. "Effect of a Diet-Induced Obesity on the Progeny Response in a Murine Model" Nutrients 15, no. 23: 4970. https://doi.org/10.3390/nu15234970
APA StyleGallardo Paffetti, M., Cárcamo, J. G., Azócar-Aedo, L., & Parra, A. (2023). Effect of a Diet-Induced Obesity on the Progeny Response in a Murine Model. Nutrients, 15(23), 4970. https://doi.org/10.3390/nu15234970





