Combating Obesity: Harnessing the Synergy of Postbiotics and Prebiotics for Enhanced Lipid Excretion and Microbiota Regulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AYS Non-Targeted Metabolic Assay
2.2.1. Sample Pretreatment
2.2.2. Chromatography-Mass Spectrometry Analysis
2.3. Animal Experimental Design
2.4. Changes in Body Weight and Fecal Quality of Mice
2.5. Determination of Biochemical Indicators in Serum
2.6. Determination of Fecal Lipid Content in Mice
2.7. Intestinal Microbiota Analysis
2.8. Statistical Analysis
3. Results
3.1. AYS Metabolite Analysis
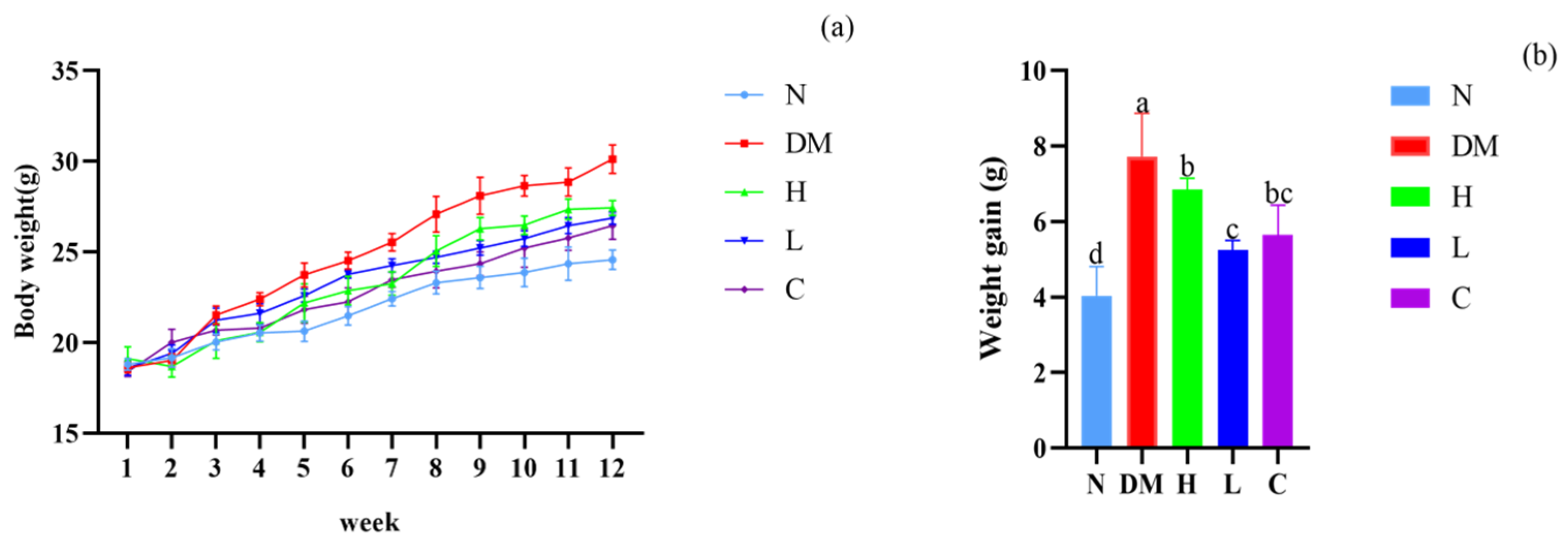
3.2. The Combination of Prebiotics and Postbiotics Reduces the Body Weight Gain in Mice
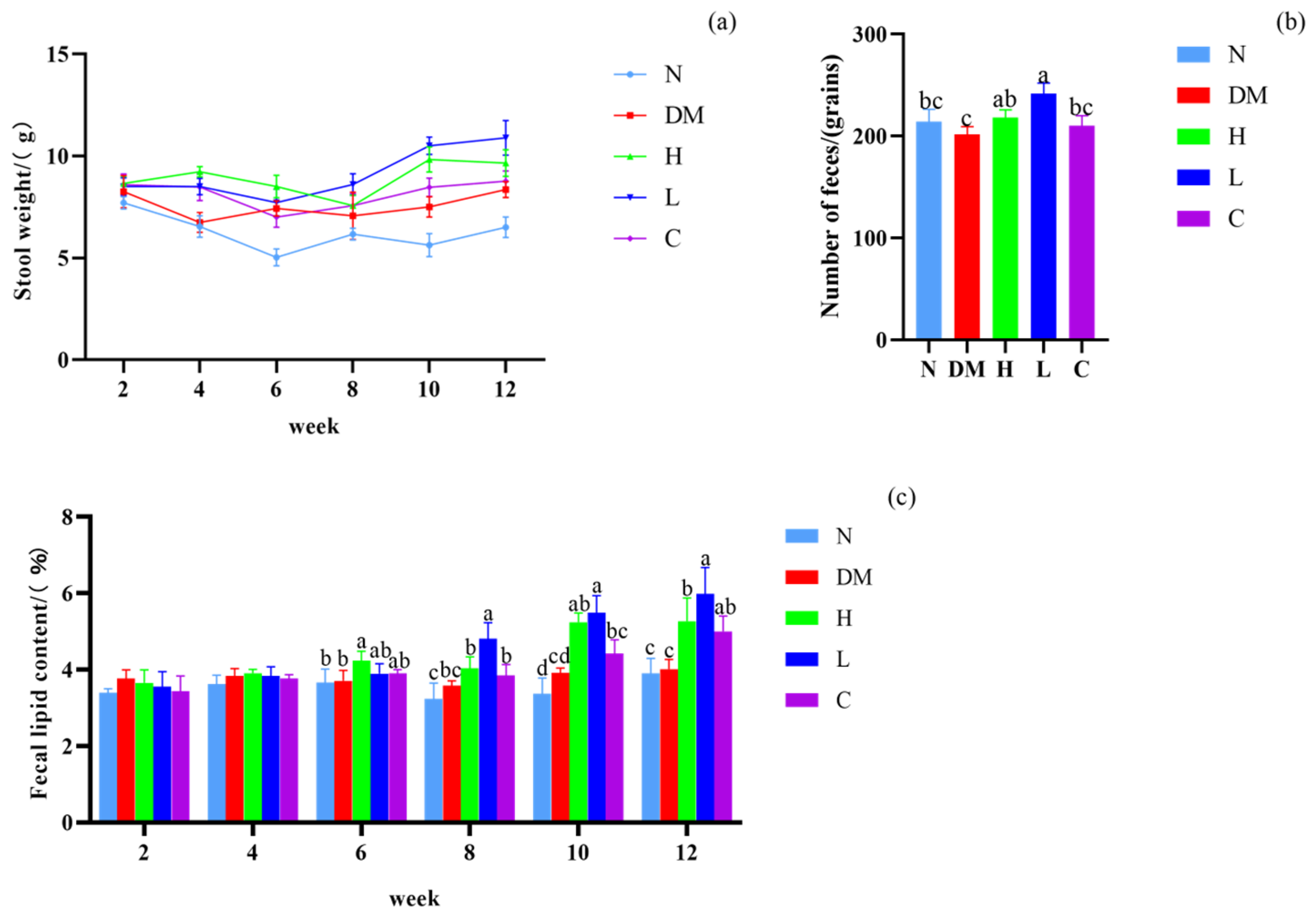
3.3. Effect on Fecal Lipid Content and Defecation of Mice
3.4. Effects on Serum Levels of TC, TG, LDL-C, and HDL-C in Mice
3.5. Effect on Intestinal Microbiota Composition
3.5.1. Alpha Diversity Analysis
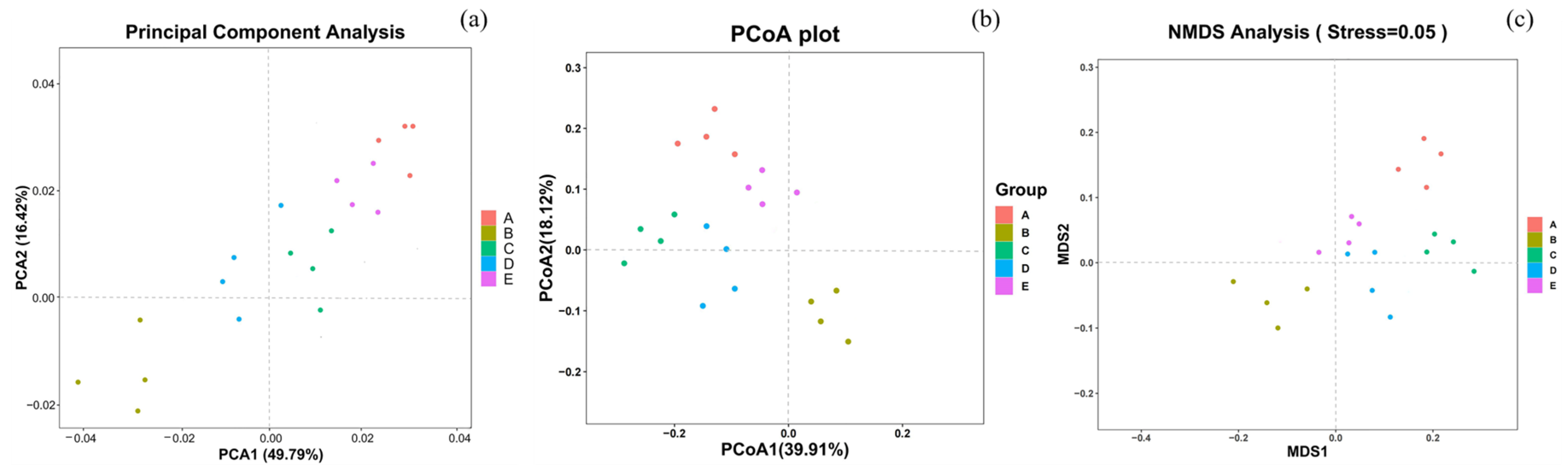
3.5.2. Beta Diversity Analysis
3.5.3. Analysis of Intestinal Microbiota Composition
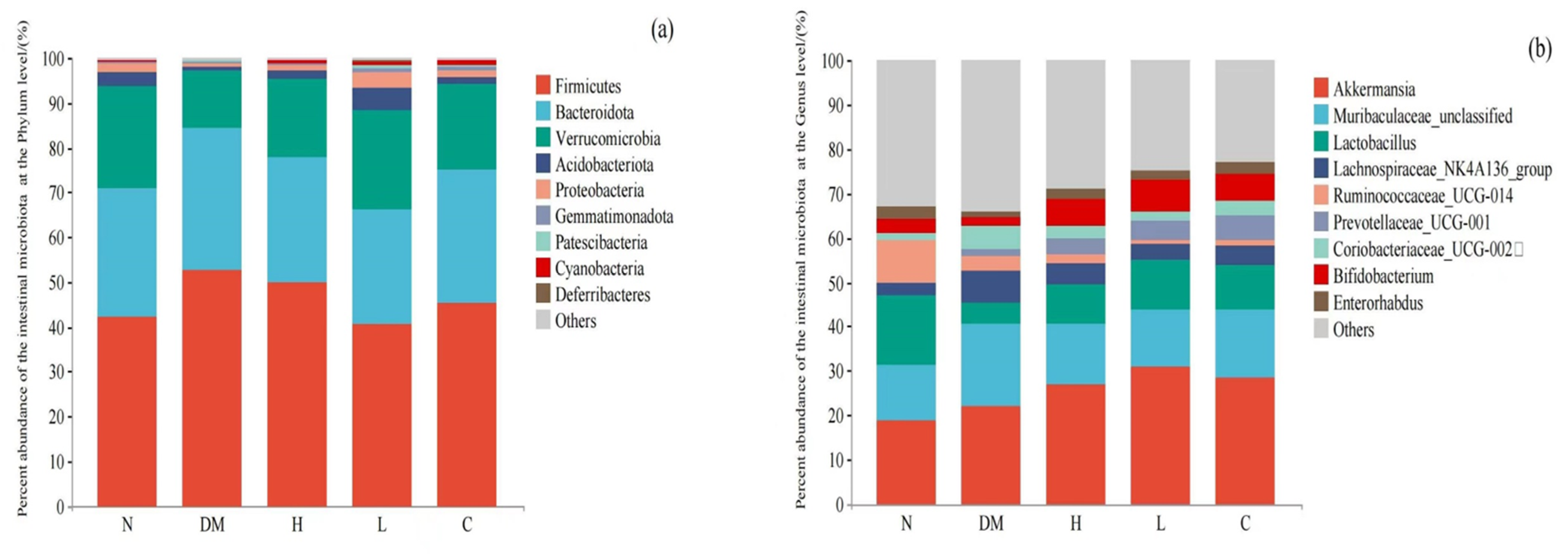
Composition of Intestinal Microbiota at Phylum Level
Composition of Intestinal Microbiota Based on Genus Level
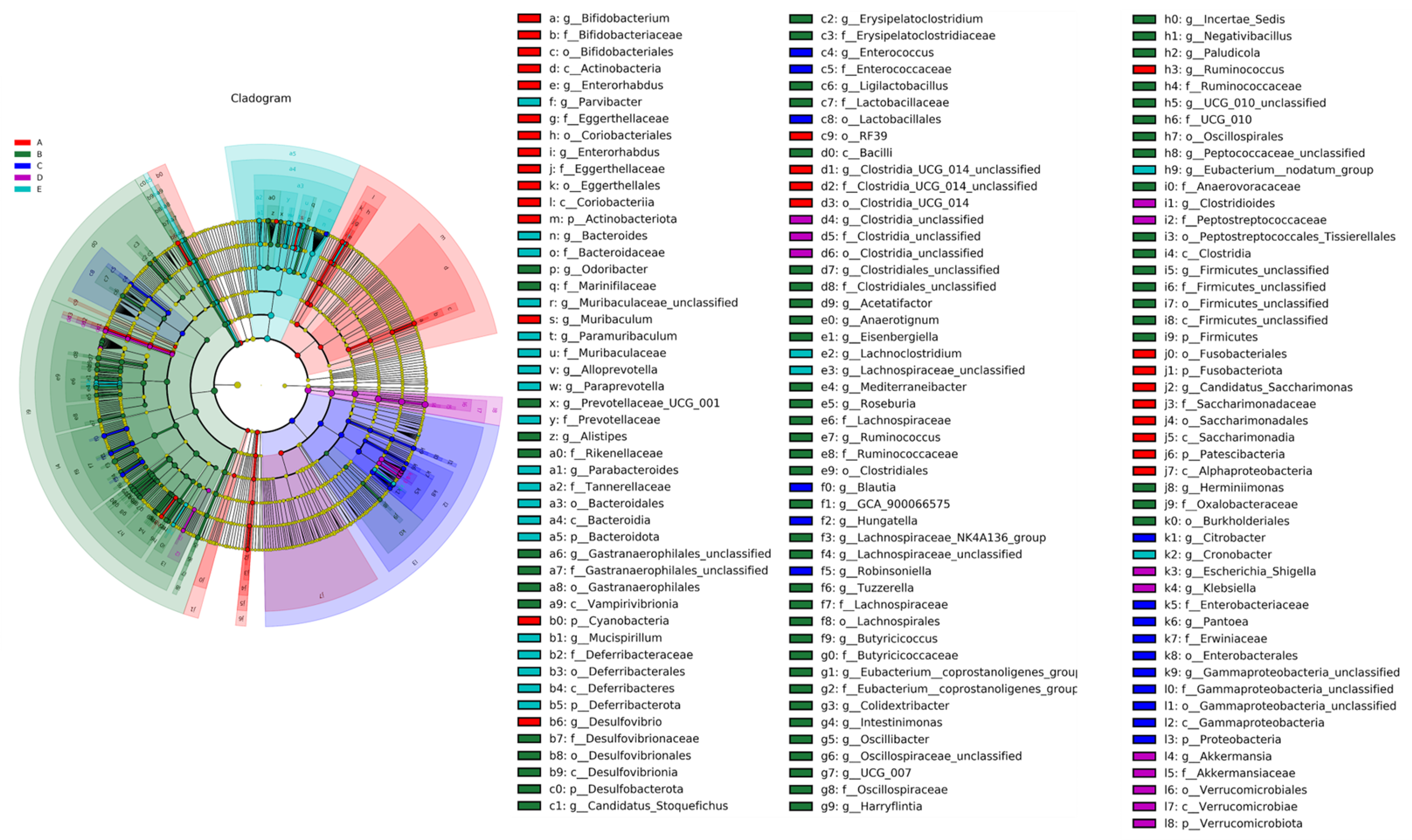
LEfSe Analysis of Mice Gut Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Amer Diabet, A. 8. Obesity management for the treatment of type 2 diabetes: Standards of medical care in diabetes-2021. Diabetes Care 2021, 44, S100–S110. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Zerdan, M.B.; Allam, S.; Zerdan, M.B.; Bouferraa, Y.; Assi, H.I. Metabolic Syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Rao, G. Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: A scientific statement from the american heart association. Circulation 2015, 132, E130. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA clinical practice update on lifestyle modification using diet and exercise to achieve weight loss in the management of nonalcoholic fatty liver disease: Expert review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef] [PubMed]
- Tak, Y.J.; Lee, S.Y. Long-term efficacy and safety of anti-obesity treatment: Where do we stand? Curr. Obes. Rep. 2021, 10, 14–30. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Makino, S.; Sato, A.; Goto, A.; Nakamura, M.; Ogawa, M.; Chiba, Y.; Hemmi, J.; Kano, H.; Takeda, K.; Okumura, K.; et al. Enhanced natural killer cell activation by exopolysaccharides derived from yogurt fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. J. Dairy Sci. 2016, 99, 915–923. [Google Scholar] [CrossRef]
- Rigo-Adrover, M.D.; Knipping, K.; Garssen, J.; van Limpt, K.; Knol, J.; Franch, A.; Castell, M.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. Prevention of rotavirus diarrhea in suckling rats by a specific fermented milk concentrate with prebiotic mixture. Nutrients 2019, 11, 189. [Google Scholar] [CrossRef] [PubMed]
- Amaretti, A.; di Nunzio, M.; Pompei, A.; Raimondi, S.; Rossi, M.; Bordoni, A. Antioxidant properties of potentially probiotic bacteria: In vitro and in vivo activities. Appl. Microbiol. Biotechnol. 2013, 97, 809–817. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of grohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- Sawada, H.; Furushiro, M.; Hirai, K.; Motoike, M.; Watanabe, T.; Yokokura, T. Purification and characterization of an antihypertensive compound from Lact. Casei. Agric. Biol. Chem. 1990, 54, 3211–3219. [Google Scholar] [CrossRef][Green Version]
- Nakamura, F.; Ishida, Y.; Sawada, D.; Ashida, N.; Sugawara, T.; Sakai, M.; Goto, T.; Kawada, T.; Fujiwara, S. Fragmented lactic acid bacterial cells activate peroxisome proliferator-activated receptors and ameliorate dyslipidemia in obese mice. J. Agric. Food Chem. 2016, 64, 2549–2559. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.H.; Zhang, C.H.; Dong, M.; Jiang, J.; Xu, H.D.; Yan, C.L.; Liu, X.; Zhou, H.; Zhang, H.; Chen, L.; et al. Myristoleic acid produced by enterococci reduces obesity through brown adipose tissue activation. Gut 2020, 69, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, J.; Igarashi, M.; Watanabe, K.; Karaki, S.; Mukouyama, H.; Kishino, S.; Li, X.; Ichimura, A.; Irie, J.; Sugimoto, Y.; et al. Gut microbiota confers host resistance to obesity by metabolizing dietary polyunsaturated fatty acids. Nat. Commun. 2019, 10, 4007. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.A.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Young, G.P.; Taranto, T.; Gibson, P.R.; Ward, P.B. Different fibers have different regional effects on luminal contents of rat colon. Gastroenterology 1991, 101, 1274–1281. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Natsume, M.; Yasuda, A.; Ishizaka, M.; Kawahata, K.; Koga, J. Fructooligosaccharides suppress high-fat diet-induced fat accumulation in C57BL/6J mice. Biofactors 2017, 43, 145–151. [Google Scholar] [CrossRef]
- Li, Y.; Wang, G.; Hao, Z.; Huang, J.; Gao, C.; Huang, J.; Li, H. Dietary fructooligosaccharides prevent obesity and regulate the gut microbiota in C57BL/6 mice. Food Sci. 2022, 43, 150–157. [Google Scholar]
- Kong, S.; Huang, X.J.; Cao, H.; Bai, Y.; Che, Q.S.; Nie, H.; Su, Z.Q. Anti-obesity effects of galacto-oligosaccharides in obese rats. Eur. J. Pharmacol. 2022, 917, 174728. [Google Scholar] [CrossRef]
- Hattori, H.; Moriyama, A.; Ohno, T.; Shibata, T.; Iwahashi, H.; Mitsunaga, T. Molecular networking-based lipid profiling and multi-omics approaches reveal new contributions of functional vanilloids to gut microbiota and lipometabolism changes. Food Chem. Mol. Sci. 2022, 5, 100123. [Google Scholar] [CrossRef] [PubMed]
- Branco, J.R.; Esteves, A.M.; Imbroisi, R.I.; Demaria, T.M.; Lisboa, P.C.; Lopes, B.P.; Moura, E.G.; Zancan, P.; Sola-Penna, M. Citrate enrichment in a western diet reduces weight gain via browning of adipose tissues without resolving diet-induced insulin resistance in mice. Food Funct. 2022, 13, 10947–10955. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Shen, Y.B.; Wu, G.C.; Qi, X.G.; Zhang, H.; Wang, L.; Qian, H.F. Determination of key active components in different edible oils affecting lipid accumulation and reactive oxygen species production in HepG2 cells. J. Agric. Food Chem. 2018, 66, 11943–11956. [Google Scholar] [CrossRef] [PubMed]
- Dyrbus, K.; Osadnik, T.; Desperak, P.; Desperak, A.; Gasior, M.; Banach, M. Evaluation of dyslipidaemia and the impact of hypolipidemic therapy on prognosis in high and very high risk patients through the Hyperlipidaemia Therapy in tERtiary Cardiological cEnTer (TERCET) Registry. Pharmacol. Res. 2018, 132, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.F.; Wang, Y.T.; Tan, S.M.; Wang, J. Effects of banana resistant starch on the biochemical indexes and intestinal flora of obese rats induced by a high-fat diet and their correlation analysis. Front. Bioeng. Biotechnol. 2021, 9, 575724. [Google Scholar] [CrossRef] [PubMed]
- Campbell, C.L.; Yu, R.Q.; Li, F.Z.; Zhou, Q.; Chen, D.Z.; Qi, C.; Yin, Y.; Sun, J. Modulation of fat metabolism and gut microbiota by resveratrol on high-fat diet-induced obese mice. Diabetes Metab. Syndr. Obes. -Targets Ther. 2019, 12, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.L.; Zhu, Y.Y.; Ma, Y.L.; Xiang, Q.S.; Shen, R.L.; Liu, Y.Q. Oat products modulate the gut microbiota and produce anti-obesity effects in obese rats. J. Funct. Foods 2016, 25, 408–420. [Google Scholar] [CrossRef]
- Jiang, P.R.; Zheng, W.Y.; Sun, X.N.; Jiang, G.P.; Wu, S.; Xu, Y.X. Sulfated polysaccharides from undaria pinnatifida improved high fat diet-induced metabolic syndrome, gut microbiota dysbiosis and inflammation in BALB/c mice. Int. J. Biol. Macromol. 2021, 167, 1587–1597. [Google Scholar] [CrossRef]
- Cao, W.X.; Chin, Y.X.; Chen, X.; Mi, Y.; Xue, C.H.; Wang, Y.M.; Tang, Q.J. The role of gut microbiota in the resistance to obesity in mice fed a high fat diet. Int. J. Food Sci. Nutr. 2020, 71, 453–463. [Google Scholar] [CrossRef]
- Ottman, N.; Geerlings, S.Y.; Aalvink, S.; De Vos, W.M.; Belzer, C. Action and function of Akkermansia muciniphila in microbiome ecology, health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 637–642. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, J.; Vigliotti, C.; Witjes, J.; Le, P.; Holleboom, A.G.; Verheij, J.; Nieuwdorp, M.; Clément, K. Gut microbiota and human NAFLD: Disentangling microbial signatures from metabolic disorders. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 279–297. [Google Scholar] [CrossRef]
- Maruvada, P.; Leone, V.; Kaplan, L.M.; Chang, E.B. The human microbiome and obesity: Moving beyond associations. Cell Host Microbe 2017, 22, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Bi, R.; Sun, Y. Recent advances on the interaction between fruits or vegetables and intestinal flora and its effect on health. J. Chin. Inst. Food Sci. Technol. 2022, 22, 387–407. [Google Scholar]
- Canuto, G.A.B.; Da Costa, J.L.; Da Cruz, P.L.R.; De Souza, A.R.L.; Faccio, A.T.; Klassen, A.; Rodrigues, K.; Tavares, M. Metabolomics:definitions, state-of-the-art and representative applications. Quim. Nova 2018, 41, 75–91. [Google Scholar] [CrossRef]
- Huber-Ruano, I.; Calvo, E.; Mayneris-Perxachs, J.; Rodriguez-Pena, M.M.; Ceperuelo-Mallafre, V.; Cedo, L.; Fernandez-Veledo, S. Orally administered Odoribacter laneus improves glucose control and inflammatory profile in obese mice by depleting circulating succinate. Microbiome 2022, 10, 135. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.M.; Lu, Y.L.; Ren, D.Y.; Chen, X.F.; Yang, X.B. Non-digestive stachyose enhances bioavailability of isoflavones for improving hyperlipidemia and hyperglycemia in mice fed with high fat diet. J. Food Drug Anal. 2021, 29, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Laybutt, D.R.; Youngson, N.A.; Morris, M.J. Concurrent betaine administration enhances exercise-induced improvements to glucose handling in obese mice. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2439–2449. [Google Scholar] [CrossRef] [PubMed]
- Ribo, S.; Sanchez-Infantes, D.; Martinez-Guino, L.; Garcia-Mantrana, I.; Ramon-Krauel, M.; Tondo, M.; Arning, E.; Nofrarías, M.; Osorio-Conles, Ó.; Fernández-Pérez, A.; et al. Increasing breast milk betaine modulates Akkermansia abundance in mammalian neonates and improves long-term metabolic health. Sci. Transl. Med. 2021, 13, 587. [Google Scholar] [CrossRef]
- Nazli, A.; Yang, P.C.; Jury, J.; Howe, K.; Watson, J.L.; Soderholm, J.D.; Sherman, P.M.; Perdue, M.H.; McKay, D.M. Epithelia under metabolic stress perceive commensal bacteria as a threat. Am. J. Pathol. 2004, 164, 947–957. [Google Scholar] [CrossRef]
- Hashmi, A.; Naeem, N.; Farooq, Z.; Masood, S.; Iqbal, S.; Naseer, R. Effect of prebiotic galacto-oligosaccharides on serum lipid profile of hypercholesterolemics. Probiotics Antimicrob. Proteins 2016, 8, 19–30. [Google Scholar] [CrossRef]
- Brochard, C.; Venara, A.; Bodere, A.; Ropert, A.; Bouguen, G.; Siproudhis, L. Pathophysiology of fecal incontinence in obese patients: A prospective case-matched study of 201 patients. Neurogastroenterol. Motil. 2017, 29, e13051. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.R.; Stadler, N.; Abbaci, A.; Liu, J.; Boullier, A.; Marie, N.; Biondi, O.; Moldes, M.; Morichon, R.; Feve, B.; et al. Effect of monoclonal antibody blockade of long fragment neurotensin on weight loss, behavior, and metabolic traits after high-fat diet induced obesity. Front. Endocrinol. 2021, 12, 739287. [Google Scholar] [CrossRef] [PubMed]
- Ijiri, D.; Nojima, T.; Kawaguchi, M.; Yamauchi, Y.; Fujita, Y.; Ijiri, S.; Ohtsuka, A. Effects of feeding outer bran fraction of rice on lipid accumulation and fecal excretion in rats. Biosci. Biotechnol. Biochem. 2015, 79, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Seo, K.H.; Lee, H.G.; Eor, J.Y.; Jeon, H.J.; Yokoyama, W.; Kim, H. Effects of kefir lactic acid bacteria-derived postbiotic components on high fat diet-induced gut microbiota and obesity. Food Res. Int. 2022, 157, 111445. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.Y.; Liu, Z.Y.; Zhu, Y.Q.; Wang, H.; Dai, Z.J.; Yang, X.H.; Ren, X.; Xue, Y.; Shen, Q. Cooked adzuki bean reduces high-fat diet-induced body weight gain, ameliorates inflammation, and modulates intestinal homeostasis in mice. Front. Nutr. 2022, 9, 918696. [Google Scholar] [CrossRef] [PubMed]
- Belzer, C.; De Vos, W.M. Microbes inside-from diversity to function: The case of Akkermansia. Isme J. 2012, 6, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Plovier, H.; Everard, A.; Druart, C.; Depommier, C.; Van Hul, M.; Geurts, L.; Chilloux, J.; Ottman, N.; Duparc, T.; Lichtenstein, L.; et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 2017, 23, 107–113. [Google Scholar] [CrossRef]


| Group | Diet | Treatment |
|---|---|---|
| N | Normal diet | 0.2 mL PBS per day |
| DM | High-fat diet | 0.2 mL PBS per day |
| H | High-fat diet | 200 μL of AYS per day |
| L | High-fat diet | 100 μL of AYS per day |
| C | High-fat diet | 200 μL postbiotics of Yicuizhi dilute solution per day |
| No. | Compounds | M/z | Rt (s) | B2 | Class |
|---|---|---|---|---|---|
| 1 | Val-Val | 217.1537 | 224.884 | 134,779.7 | Carboxylic acids and derivatives |
| 2 | Val-Asp | 233.1115 | 382.094 | 89,946.17 | Carboxylic acids and derivatives |
| 3 | Val-Glu | 247.1313 | 402.493 | 2501.2 | Carboxylic acids and derivatives |
| 4 | Val-Tyr | 281.1486 | 231.593 | 173,349.6 | Carboxylic acids and derivatives |
| 5 | Val-Pro | 215.1373 | 261.452 | 176,255.3 | Carboxylic acids and derivatives |
| 6 | Val-Phe | 265.1537 | 190.596 | 424,802.5 | Carboxylic acids and derivatives |
| 7 | Gly-Glu | 205.0854 | 440.611 | 28,407.88 | Carboxylic acids and derivatives |
| 8 | Gly-Val | 175.1071 | 390.344 | 11,429.14 | Carboxylic acids and derivatives |
| 9 | Gly-Arg | 232.1391 | 448.69 | 25,774.23 | Carboxylic acids and derivatives |
| 10 | Thr-Glu | 231.0964 | 306.569 | 20,522.67 | Carboxylic acids and derivatives |
| 11 | Thr-Leu | 233.1492 | 250.772 | 801,098.3 | Carboxylic acids and derivatives |
| 12 | Ile-Glu | 261.1436 | 359.036 | 30,986.21 | Carboxylic acids and derivatives |
| 13 | Ile-Met | 263.1417 | 193.436 | 280,259.7 | Carboxylic acids and derivatives |
| 14 | Leu-Phe | 279.1695 | 177.737 | 412,315.3 | Carboxylic acids and derivatives |
| 15 | Leu-Tyr | 295.1643 | 212.834 | 629,953.3 | Carboxylic acids and derivatives |
| 16 | Pro-Thr | 199.1063 | 119.86 | 64,398.15 | Carboxylic acids and derivatives |
| 17 | His-Phe | 285.1339 | 148.378 | 478,955.4 | Carboxylic acids and derivatives |
| 18 | Arg-Ala | 246.154 | 449.38 | 33,634.96 | Carboxylic acids and derivatives |
| 19 | Ala-Lys | 218.1479 | 437.541 | 166,954.3 | Carboxylic acids and derivatives |
| 20 | Tyr-Phe | 311.1371 | 39.745 | 26,125.42 | Carboxylic acids and derivatives |
| 21 | Succinate | 117.0193 | 432.639 | 11,464.94 | Carboxylic acids and derivatives |
| 22 | Citrate | 191.02 | 553.862 | 334,888.1 | Carboxylic acids and derivatives |
| 23 | Betaine | 118.086 | 274.181 | 7,530,456 | Carboxylic acids and derivatives |
| 24 | Agmatine | 131.1272 | 450.11 | 96,303.9 | Organonitrogen compounds |
| 25 | Stachyose | 667.227 | 488.158 | 210,245.2 | Organooxygen compounds |
| 26 | Ile-Lys | 260.1943 | 354.886 | 37,147.84 | Organooxygen compounds |
| 27 | Pantothenate | 220.1162 | 276.731 | 24,141.14 | Organooxygen compounds |
| 28 | Maltotriose | 503.1593 | 504.935 | 7323.386 | Organooxygen compounds |
| 29 | Pantothenate | 218.1041 | 277.019 | 150,933 | Organooxygen compounds |
| 30 | Sucrose | 341.1078 | 488.116 | 41,952.1 | Organooxygen compounds |
| 31 | Ribitol | 133.051 | 199.833 | 1,769,454 | Organooxygen compounds |
| 32 | Diacetyl | 87.04301 | 177.737 | 136,216.7 | Organooxygen compounds |
| 33 | Maltotriose | 522.198 | 494.367 | 65,036.57 | Organooxygen compounds |
| 34 | D-Mannose | 239.0775 | 296.488 | 78,534.97 | Organooxygen compounds |
| 35 | Hypoxanthine | 137.0436 | 258.532 | 721.8929 | Imidazopyrimidines |
| 36 | Adenine | 134.0478 | 158.426 | 2,083,855 | Imidazopyrimidines |
| 37 | Hypoxanthine | 135.0314 | 167.125 | 862,657.7 | Imidazopyrimidines |
| 38 | Uric acid | 167.0213 | 329.316 | 11,690.18 | Imidazopyrimidines |
| 39 | Xanthine | 151.0262 | 214.702 | 242,308.8 | Imidazopyrimidines |
| 40 | Cytosine | 112.0496 | 196.825 | 315,753.4 | Diazines |
| 41 | Uracil | 113.0333 | 159.348 | 35,649.18 | Diazines |
| 42 | Thymine | 125.0354 | 72.761 | 549,390.5 | Diazines |
| 43 | Uracil | 111.0196 | 284.668 | 19,261.86 | Diazines |
| 44 | Orotate | 155.0098 | 248.47 | 270,828.3 | Diazines |
| 45 | Nicotinate | 122.0245 | 227.3765 | 33,340.51 | Pyridines and derivatives |
| 46 | Pyridoxine | 170.0804 | 132.01 | 165,434.2 | Pyridines and derivatives |
| 47 | Nicotinate | 124.0377 | 232.263 | 90,057.04 | Pyridines and derivatives |
| 48 | Benzoic acid | 121.0293 | 15.862 | 22,075.6 | Benzene and substituted derivatives |
| 49 | Tyramine | 138.0904 | 214.194 | 25,018.14 | Benzene and substituted derivatives |
| 50 | Hippuric acid | 178.0505 | 193.264 | 609,644 | Benzene and substituted derivatives |
| 51 | Palmitic acid | 255.2328 | 49.642 | 6,242,620 | Fatty Acyls |
| 52 | Linoleic acid | 279.2339 | 36.733 | 1,609,868 | Fatty Acyls |
| 53 | Heptadecanoic acid | 269.2489 | 48.362 | 181,756.1 | Fatty Acyls |
| 54 | Riboflavin | 377.1541 | 250.772 | 3816.888 | Pteridines and derivatives |
| 55 | Riboflavin | 435.1591 | 262.95 | 3317.16 | Pteridines and derivatives |
| 56 | 1,2-dioleoyl-sn-glycerol-3-phosphatidylcholine | 786.5968 | 95.831 | 85,056.19 | Glycerophospholipids |
| 57 | sn-Glycerol 3-phosphoethanolamine | 216.0617 | 393.803 | 59,884.73 | Glycerophospholipids |
| 58 | Norharmane | 169.0754 | 72.333 | 164,364.3 | Indoles and derivatives |
| 59 | Indolelactic acid | 204.0666 | 159.156 | 112,7278 | Indoles and derivatives |
| 60 | Ile-Pro | 229.1539 | 272.911 | 246,708.1 | Pyrimidine nucleosides |
| 61 | Deoxythymidine 5′-phosphate (dTMP) | 321.0482 | 411.011 | 10,607.32 | Pyrimidine nucleotides |
| 62 | Cholesteryl sulfate | 465.3023 | 26.694 | 165,882.3 | Steroids and steroid derivatives |
| 63 | Cholic acid | 426.3198 | 226.884 | 5626.847 | Steroids and steroid derivatives |
| 64 | (S)-2-Hydroxyglutarate | 147.0304 | 392.132 | 111,556.1 | Hydroxy acids and derivatives |
| 65 | Dopamine | 136.0745 | 255.082 | 9297.395 | Phenols |
| 66 | Bestatin | 350.204 | 200.345 | 35,719.13 | Peptidomimetics |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zheng, Y.; Xie, K.; Hou, Y.; Liu, Q.; Jiang, Y.; Zhang, Y.; Man, C. Combating Obesity: Harnessing the Synergy of Postbiotics and Prebiotics for Enhanced Lipid Excretion and Microbiota Regulation. Nutrients 2023, 15, 4971. https://doi.org/10.3390/nu15234971
Zhao Y, Zheng Y, Xie K, Hou Y, Liu Q, Jiang Y, Zhang Y, Man C. Combating Obesity: Harnessing the Synergy of Postbiotics and Prebiotics for Enhanced Lipid Excretion and Microbiota Regulation. Nutrients. 2023; 15(23):4971. https://doi.org/10.3390/nu15234971
Chicago/Turabian StyleZhao, Yueming, Yaping Zheng, Kui Xie, Yanmei Hou, Qingjing Liu, Yujun Jiang, Yu Zhang, and Chaoxin Man. 2023. "Combating Obesity: Harnessing the Synergy of Postbiotics and Prebiotics for Enhanced Lipid Excretion and Microbiota Regulation" Nutrients 15, no. 23: 4971. https://doi.org/10.3390/nu15234971
APA StyleZhao, Y., Zheng, Y., Xie, K., Hou, Y., Liu, Q., Jiang, Y., Zhang, Y., & Man, C. (2023). Combating Obesity: Harnessing the Synergy of Postbiotics and Prebiotics for Enhanced Lipid Excretion and Microbiota Regulation. Nutrients, 15(23), 4971. https://doi.org/10.3390/nu15234971





