Co-Administration of Roxadustat and Zinc Stabilizes Both Serum Copper and Zinc Concentrations in Patients Undergoing Hemodialysis
Highlights
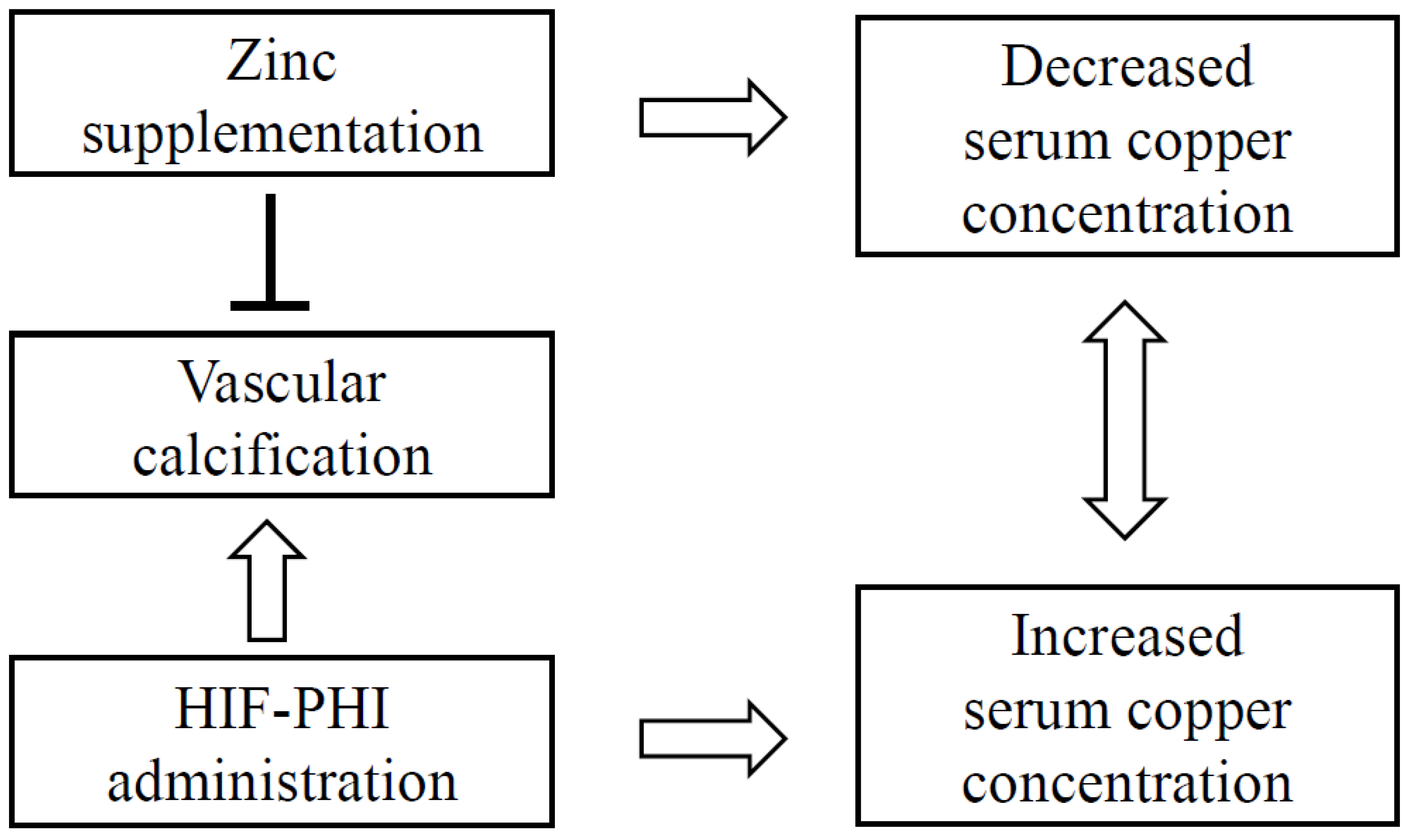
- Most hemodialysis patients require zinc supplementation due to hypozincemia, which may reduce their serum copper levels, which are instead increased by HIF-PH inhibitors.
- Combining HIF-PH inhibitors with zinc supplementation helps normalize both copper and zinc levels. Therefore, this combination therapy allows hemodialysis patients to safely receive zinc supplementation without reducing their serum copper levels, preventing the excessive increases caused by HIF-PH inhibitors alone.
Abstract
:1. Introduction
2. Methods
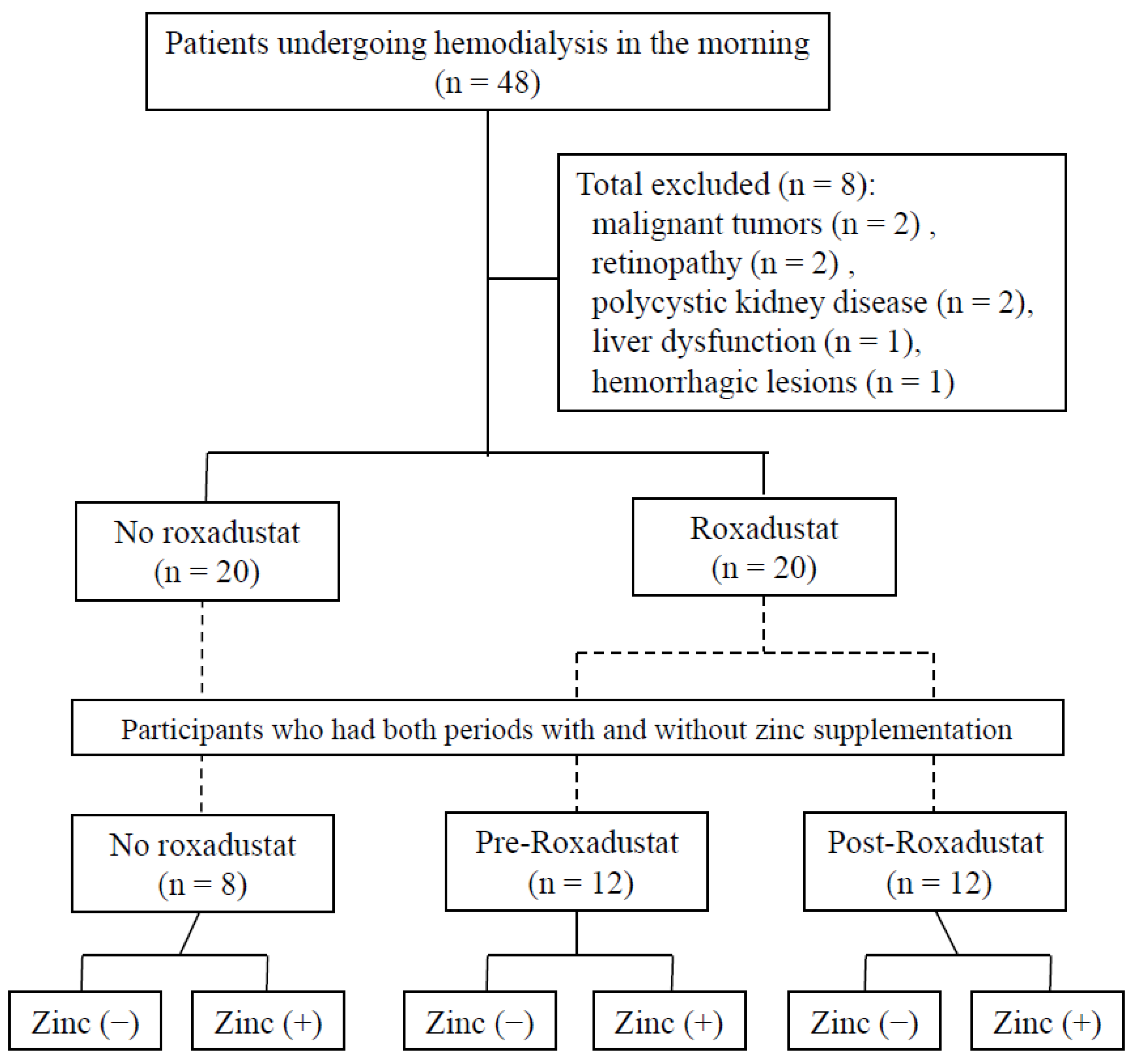
2.1. Participants
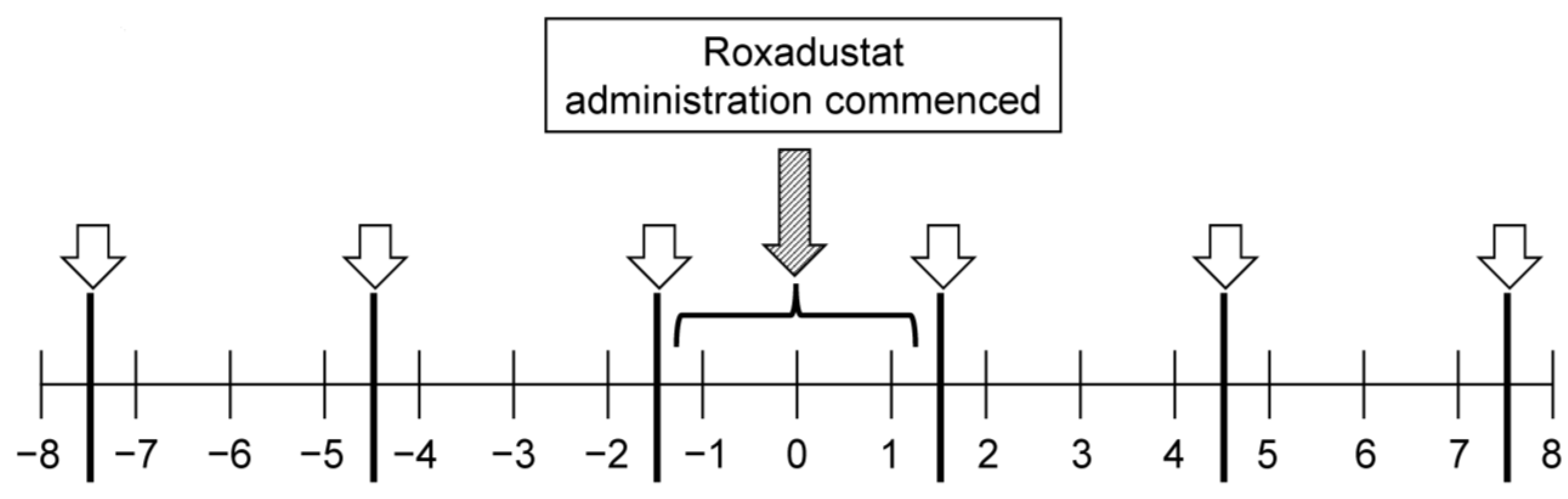
2.2. Observation Period
2.3. Measurements
2.4. Data Collection
2.5. Statistical Analyses
2.6. Treatments
3. Results
3.1. Characteristics of the Participants
3.2. Serum Zinc and Copper Concentrations of Participants Who Were or Were Not Administering Roxadustat
3.3. Relationship between Roxadustat Dose and Serum Copper Concentration
3.4. Assessment of the Level of Zinc Supplementation
3.5. Normalization of High Serum Copper and Low Serum Zinc Concentrations Following the Initiation of, or an Increase in, the Level of Zinc Supplementation
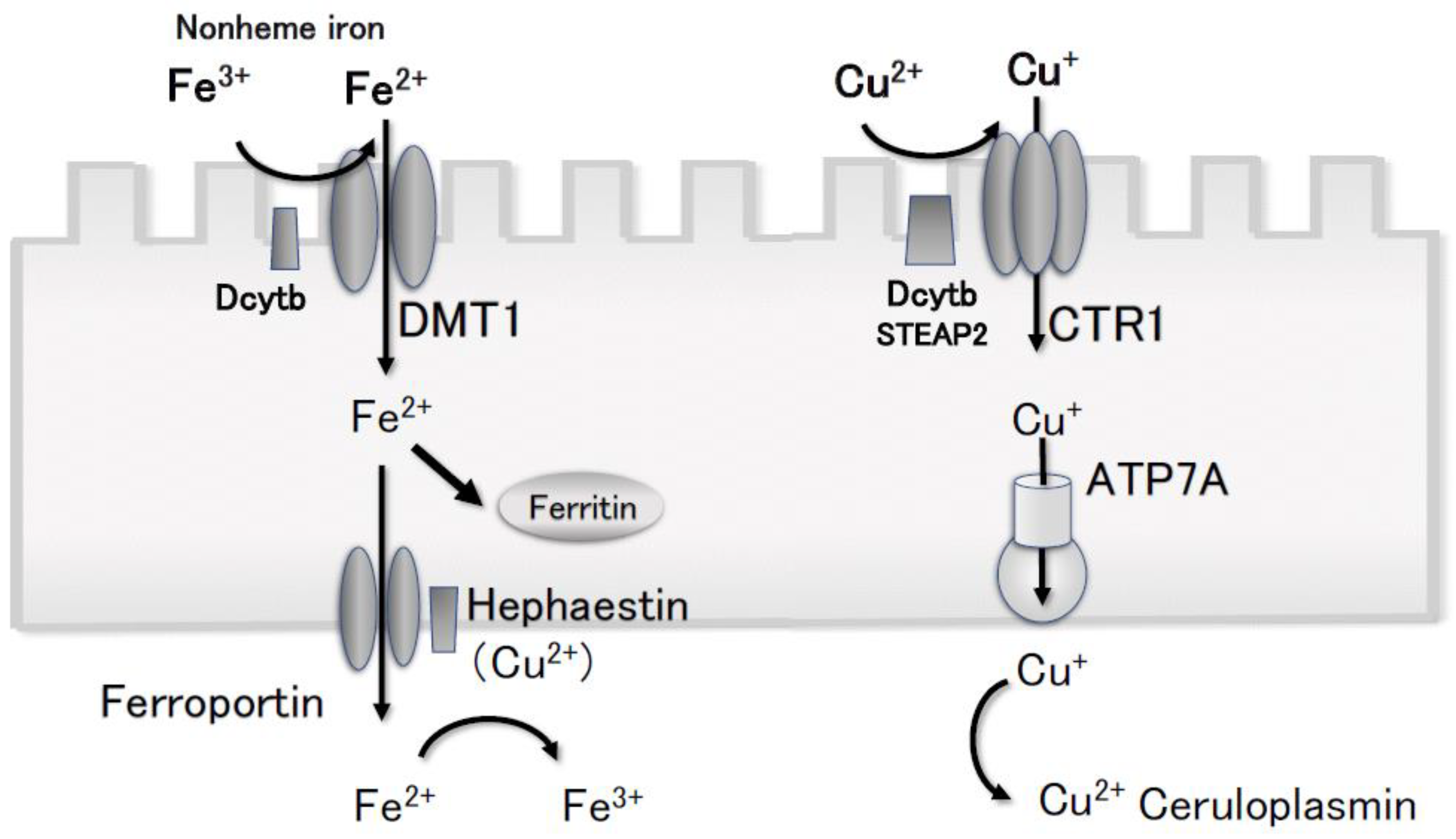
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Yap, D.Y.H.; McMahon, L.P.; Hao, C.; Hu, N.; Okada, H.; Suzuki, Y.; Kim, S.G.; Lim, S.K.; Vareesangthip, K.; Hung, C.; et al. APSN HIF-PHI Recommendation Committee. Recommendations by the Asian Pacific Society of Nephrology (APSN) on the appropriate use of HIF-PH inhibitors. Nephrology 2021, 26, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, H.; Kurihara, S.; Anayama, M.; Makino, Y.; Nagasaw, M. Four cases of serum copper excess in patients with renal anemia receiving a hypoxia-inducible factor-prolyl hydroxylase inhibitor: A possible safety concern. Case Rep. Nephrol. Dial. 2022, 12, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Post-Marketing Surveillance of EVRENZO® Tablets (Roxadustat) in Patients with Renal Anemia. ClinicalTrials.gov Identifier: NCT04408820. [Internet]. Available online: https://amn.astellas.jp/content/dam/jp/amn/jp/ja/di/doc/Pdfs/DocNo202110238_y.pdf?redirect=false (accessed on 12 January 2023).
- Knobeloch, L.; Ziarnik, M.; Howard, J.; Theis, B.; Farmer, D.; Anderson, H.; Proctor, M. Gastrointestinal upsets associated with ingestion of copper-contaminated water. Environ. Health Perspect. 1994, 102, 958–961. [Google Scholar] [CrossRef] [PubMed]
- Bozalioğlu, S.; Ozkan, Y.; Turan, M.; Simşek, B. Prevalence of zinc deficiency and immune response in short-term hemodialysis. J. Trace Elem. Med. Biol. 2005, 18, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Wang, M.-Q.; Hu, R.; Yang, Y.; Huang, Y.-S.; Xian, S.-X.; Lu, L. Effect of zinc supplementation on maintenance hemodialysis patients: A systematic review and meta-analysis of 15 randomized controlled trials. Biomed. Res. Int. 2017, 2017, 1024769. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.W.; Giroux, A.; L’Abbé, M.R. The effect of dietary zinc on intestinal copper absorption. Am. J. Clin. Nutr. 1981, 34, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Pethő, D.; Gáll, T.; Zavaczki, E.; Nyitrai, M.; Posta, J.; Zarjou, A.; Agarwal, A.; Balla, G.; Balla, J. Zinc inhibits HIF-prolyl hydroxylase inhibitor-aggravated VSMC calcification induced by high phosphate. Front. Physiol. 2020, 10, 1584. [Google Scholar] [CrossRef]
- Klevay, L.M. Alzheimer’s disease as copper deficiency. Med. Hypotheses 2008, 70, 802–807. [Google Scholar] [CrossRef]
- Siotto, M.; Simonelli, I.; Pasqualetti, P.; Mariani, S.; Caprara, D.; Bucossi, S.; Ventriglia, M.; Molinario, R.; Antenucci, M.; Rongioletti, M.; et al. Association Between Serum Ceruloplasmin Specific Activity and Risk of Alzheimer’s Disease. J. Alzheimer’s Dis. 2016, 50, 1181–1189. [Google Scholar] [CrossRef]
- Royer, A.; Sharman, T. Copper Toxicity [Updated 27 March 2023]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557456/ (accessed on 12 January 2023).
- Squitti, R.; Simonelli, I.; Ventriglia, M.; Siotto, M.; Pasqualetti, P.; Rembach, A.; Doecke, J.; Bush, A.I. Meta-analysis of serum non-ceruloplasmin copper in Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 38, 809–822. [Google Scholar] [CrossRef]
- Takahashi, A. Role of zinc and copper in erythropoiesis in patients on hemodialysis. J. Ren. Nutr. 2022, 32, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Nishime, K.; Kondo, M.; Saito, K.; Miyawaki, H.; Nakagawa, T. Zinc Burden Evokes Copper Deficiency in the Hypoalbuminemic Hemodialysis Patients. Nutrients 2020, 12, 577. [Google Scholar] [CrossRef] [PubMed]
- Garai, K.; Sahoo, B.; Kaushalya, S.K.; Desai, R.; Maiti, S. Zinc lowers amyloid-beta toxicity by selectively precipitating aggregation intermediates. Biochemistry 2007, 46, 10655–10663. [Google Scholar] [CrossRef] [PubMed]
- Miller, Y.; Ma, B.; Nussinov, R. Zinc ions promote Alzheimer Abeta aggregation via population shift of polymorphic states. Proc. Natl. Acad. Sci. USA 2010, 107, 9490–9495. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.C.; Yu, W.C.; Shih, Y.H.; Chen, C.Y.; Guo, Z.H.; Huang, S.J.; Chan, J.C.C.; Chen, Y.R. Zinc ion rapidly induces toxic, off-pathway amyloid-β oligomers distinct from amyloid-β derived diffusible ligands in Alzheimer’s disease. Sci. Rep. 2018, 8, 4772. [Google Scholar] [CrossRef] [PubMed]
- Kanabrocki, E.L.; Sothern, R.B.; Ryan, M.D.; Kahn, S.; Augustine, G.; Johnson, C.; Foley, S.; Gathing, A.; Eastman, G.; Friedman, N.; et al. Circadian characteristics of serum calcium, magnesium and eight trace elements and of their metallo-moieties in urine of healthy middle-aged men. Clin. Ter. 2008, 159, 329–346. [Google Scholar] [PubMed]
- Shibata, S.; Kitamura, M. Modern diagnostic testing system. In Routine Clinical Biochemical Quantitative Methods; Nakayama Shoten Co, Ltd.: Tokyo, Japan, 1964; pp. 159–162. (In Japanese) [Google Scholar]
- Kodama, H.; Tanaka, M.; Naito, Y.; Katayama, K.; Moriyama, M. Japan’s practical guidelines for zinc deficiency with a particular focus on taste disorders, inflammatory bowel disease, and liver cirrhosis. Int. J. Mol. Sci. 2020, 21, 2941. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nishi, S.; Tomo, T.; Masakane, I.; Saito, K.; Nangaku, M.; Hattori, M.; Suzuki, T.; Morita, S.; Ashida, A.; et al. 2015 Japanese Society for Dialysis Therapy: Guidelines for renal anemia in chronic kidney disease. Ren. Replace. Ther. 2017, 3, 36. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Z.; Fang, M.; Han, Y.; Wang, G.; Wang, S.; Xue, M.; Li, Y.; Zhang, L.; Wu, J.; et al. Transferrin plays a central role in coagulation balance by interacting with clotting factors. Cell Res. 2020, 30, 119–132. [Google Scholar] [CrossRef]
- Tang, X.; Fang, M.; Cheng, R.; Zhang, Z.; Wang, Y.; Shen, C.; Han, Y.; Lu, Q.; Du, Y.; Liu, Y.; et al. Iron-Deficiency and Estrogen Are Associated With Ischemic Stroke by Up-Regulating Transferrin to Induce Hypercoagulability. Circ. Res. 2020, 127, 651–663. [Google Scholar] [CrossRef]
- Ikee, R.; Tsunoda, M.; Sasaki, N.; Sato, N.; Hashimoto, N. Clinical factors associated with serum copper levels and potential effect of sevelamer in hemodialysis patients. Int. Urol. Nephrol. 2013, 45, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Takagi, K.; Masuda, K.; Yamazaki, M.; Kiyohara, C.; Itoh, S.; Wasaki, M.; Inoue, H. Metal ion and vitamin adsorption profiles of phosphate binder ion-exchange resins. Clin. Nephrol. 2010, 73, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.T.; Maitani, T. Metal-dependent properties of metallothionein. Replacement in vitro of zinc in zinc-thionein with copper. Biochem. J. 1981, 199, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Mori, K.; Shoji, T.; Emoto, M. Association of Zinc Deficiency with Development of CVD Events in Patients with CKD. Nutrients 2021, 13, 1680. [Google Scholar] [CrossRef] [PubMed]
- Mastrogiannaki, M.; Matak, P.; Keith, B.; Simon, M.C.; Vaulont, S.; Peyssonnaux, C. HIF-2alpha, but not HIF-1alpha, promotes iron absorption in mice. J. Clin. Investig. 2009, 119, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.; Qu, A.; Anderson, E.R.; Matsubara, T.; Martin, A.; Gonzalez, F.J.; Shah, Y.M. Hypoxia-inducible factor-2α mediates the adaptive increase of intestinal ferroportin during iron deficiency in mice. Gastroenterology 2011, 140, 2044–2055. [Google Scholar] [CrossRef] [PubMed]
- White, C.; Kambe, T.; Fulcher, Y.G.; Sachdev, S.W.; Bush, A.I.; Fritsche, K.; Lee, J.; Quinn, T.P.; Petris, M.J. Copper transport into the secretory pathway is regulated by oxygen in macrophages. J. Cell Sci. 2009, 122, 1315–1321. [Google Scholar] [CrossRef] [PubMed]
- Pourvali, K.; Matak, P.; Latunde-Dada, G.O.; Solomou, S.; Mastrogiannaki, M.; Peyssonnaux, C.; Sharp, P.A. Basal expression of copper transporter 1 in intestinal epithelial cells is regulated by hypoxia-inducible factor 2α. FEBS Lett. 2012, 586, 2423–2427. [Google Scholar] [CrossRef]
- Xie, L.; Collins, J.F. Transcriptional regulation of the Menkes copper ATPase (Atp7a) gene by hypoxia-inducible factor (HIF2{alpha}) in intestinal epithelial cells. Am. J. Physiol. Cell Physiol. 2011, 300, C1298–C1305. [Google Scholar] [CrossRef]
- Ari, E.; Kaya, Y.; Demir, H.; Asicioglu, E.; Keskin, S. The correlation of serum trace elements and heavy metals with carotid artery atherosclerosis in maintenance hemodialysis patients. Biol. Trace Elem. Res. 2011, 144, 351–359. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Bello, A.; Field, C.J.; Gill, J.S.; Hemmelgarn, B.R.; Holmes, D.T.; Jindal, K.; Klarenbach, S.W.; Manns, B.J.; et al. Concentrations of trace elements and clinical outcomes in hemodialysis patients: A prospective cohort study. CJASN 2018, 13, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Tahir, N.; Ashraf, A.; Waqar, S.H.; Rafae, A.; Kantamneni, L.; Sheikh, T.; Khan, R. Copper deficiency, a rare but correctable cause of pancytopenia: A review of literature. Expert Rev. Hematol. 2022, 15, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N. Copper Deficiency Myelopathy (Human Swayback). Mayo Clin. Proc. 2006, 81, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Djafarian, K.; Mojtahed, A.; Varkaneh, H.K.; Shab-Bidar, S. The effect of zinc supplementation on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Pharmacol. 2018, 834, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Cui, W.; Tan, Y.; Luo, P.; Chen, Q.; Zhang, C.; Qu, W.; Miao, L.; Cai, L. Zinc is essential for the transcription function of Nrf2 in human renal tubule cells in vitro and mouse kidney in vivo under the diabetic condition. J. Cell. Mol. Med. 2014, 18, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.M.; Yoon, J.S. Glycemic control of type 2 diabetic patients after short-term zinc supplementation. Nutr. Res. Pract. 2008, 2, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Ninomiya, T.; Ohara, T.; Doi, Y.; Uchida, K.; Shirota, T.; Yonemoto, K.; Kitazono, T.; Kiyohara, Y. Dietary patterns and risk of dementia in an elderly Japanese population: The Hisayama Study. Am. J. Clin. Nutr. 2013, 97, 1076–1082. [Google Scholar] [CrossRef]
- Canevelli, M.; Lucchini, F.; Quarata, F.; Bruno, G.; Cesari, M. Nutrition and Dementia: Evidence for Preventive Approaches? Nutrients 2016, 8, 144. [Google Scholar] [CrossRef]
- Rembach, A.; Doecke, J.D.; Roberts, B.R.; Watt, A.D.; Faux, N.G.; Volitakis, I.; Pertile, K.K.; Rumble, R.L.; Trounson, B.O.; Fowler, C.J.; et al. Longitudinal analysis of serum copper and ceruloplasmin in Alzheimer’s disease. J. Alzheimer’s Dis. 2013, 34, 171–182. [Google Scholar] [CrossRef]
- Bagheri, S.; Squitti, R.; Haertlé, T.; Siotto, M.; Saboury, A.A. Role of Copper in the Onset of Alzheimer’s Disease Compared to Other Metals. Front. Aging Neurosci. 2018, 9, 446. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Gianattasio, K.Z.; Bennett, E.E.; Stewart, J.D.; Xu, X.; Park, E.S.; Smith, R.L.; Ying, Q.; Whitsel, E.A.; Power, M.C. The Associations of Dietary Copper with Cognitive Outcomes. Am. J. Epidemiol. 2022, 191, 1202–1211. [Google Scholar] [CrossRef] [PubMed]
- Barnard, N.D.; Bush, A.I.; Ceccarelli, A.; Cooper, J.; de Jager, C.A.; Erickson, K.I.; Fraser, G.; Kesler, S.; Levin, S.M.; Lucey, B.; et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol. Aging 2014, 35 (Suppl. S2), S74–S78. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Abe, M.; Okada, K.; Tei, R.; Maruyama, N.; Kikuchi, F.; Higuchi, T.; Soma, M. Oral zinc supplementation reduces the erythropoietin responsiveness index in patients on hemodialysis. Nutrients 2015, 7, 3783–3795. [Google Scholar] [CrossRef] [PubMed]
- Shiota, J. Effect of zinc supplementation on bone formation in hemodialysis patients with normal or low turnover bone. Ren. Fail. 2015, 37, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Mokas, S.; Larivière, R.; Lamalice, L.; Gobeil, S.; Cornfield, D.N.; Agharazii, M.; Richard, D.E. Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int. 2016, 90, 598–609. [Google Scholar] [CrossRef]
- Voelkl, J.; Tuffaha, R.; Luong, T.T.; Zickler, D.; Masyout, J.; Feger, M.; Verheyen, N.; Blaschke, F.; Kuro-o, M.; Tomaschitz, A.; et al. Zinc inhibits phosphate-induced vascular calcification through TNFAIP3-mediated suppression of NF-κB. J. Am. Soc. Nephrol. 2018, 29, 1636–1648. [Google Scholar] [CrossRef]

| No Roxadustat | Roxadustat | p Value | |
|---|---|---|---|
| Number of participants | 20 | 20 | |
| Male sex, n (%) | 8 (40.0) | 12 (60.0) | 0.206 |
| Age, mean ± SD (years) | 77.1 ± 12.3 | 73.0 ± 11.4 | 0.274 |
| Duration of dialysis, mean ± SD (years) | 7.0± 5.2 | 6.9 ± 4.7 | 0.953 |
| Etiology, n (%) | 0.725 | ||
| Diabetic nephropathy | 9 (45.0) | 7 (35.0) | |
| Nephrosclerosis | 7 (35.0) | 8 (40.0) | |
| Chronic glomerulonephritis | 2 (10.0) | 4 (20.0) | |
| Other | 2 (10.0) | 1 (5.0) |
| No Roxadustat | Roxadustat | p Value | |
|---|---|---|---|
| Number of participants | 8 | 12 | |
| Male sex, n (%) | 5 (62.5) | 6 (50.0) | 0.582 |
| Age, mean ± SD (years) | 81.8 ± 6.6 | 76.0 ± 6.1 | 0.061 |
| Duration of dialysis, mean ± SD (years) | 7.2 ± 5.0 | 7.3± 5.6 | 0.970 |
| Etiology, n (%) | 0.222 | ||
| Diabetic nephropathy | 5 (62.5) | 4 (33.3) | |
| Nephrosclerosis | 1 (12.5) | 6 (50.0) | |
| Chronic glomerulonephritis | 1 (12.5) | 2 (16.7) | |
| Uncertain | 1 (12.5) | 0 (0.00) |
| No Roxadustat | Pre-Roxadustat | Post-Roxadustat | ANOVA p-Value | Roxadustat Dose (mg) | ||
|---|---|---|---|---|---|---|
| [Zn] (µmol/L) | Zinc (−) | 9.6 ± 1.2 | 8.6 ± 1.6 | 7.0 ± 1.4 | <0.001 | 59.0 ± 26.0 |
| Zinc (+) | 13.1 ± 4.1 | 11.1 ± 2.1 | 10.6 ± 3.17 | 0.068 | 59.4 ± 25.5 | |
| p-value | <0.001 | <0.001 | <0.001 | 0.483 | ||
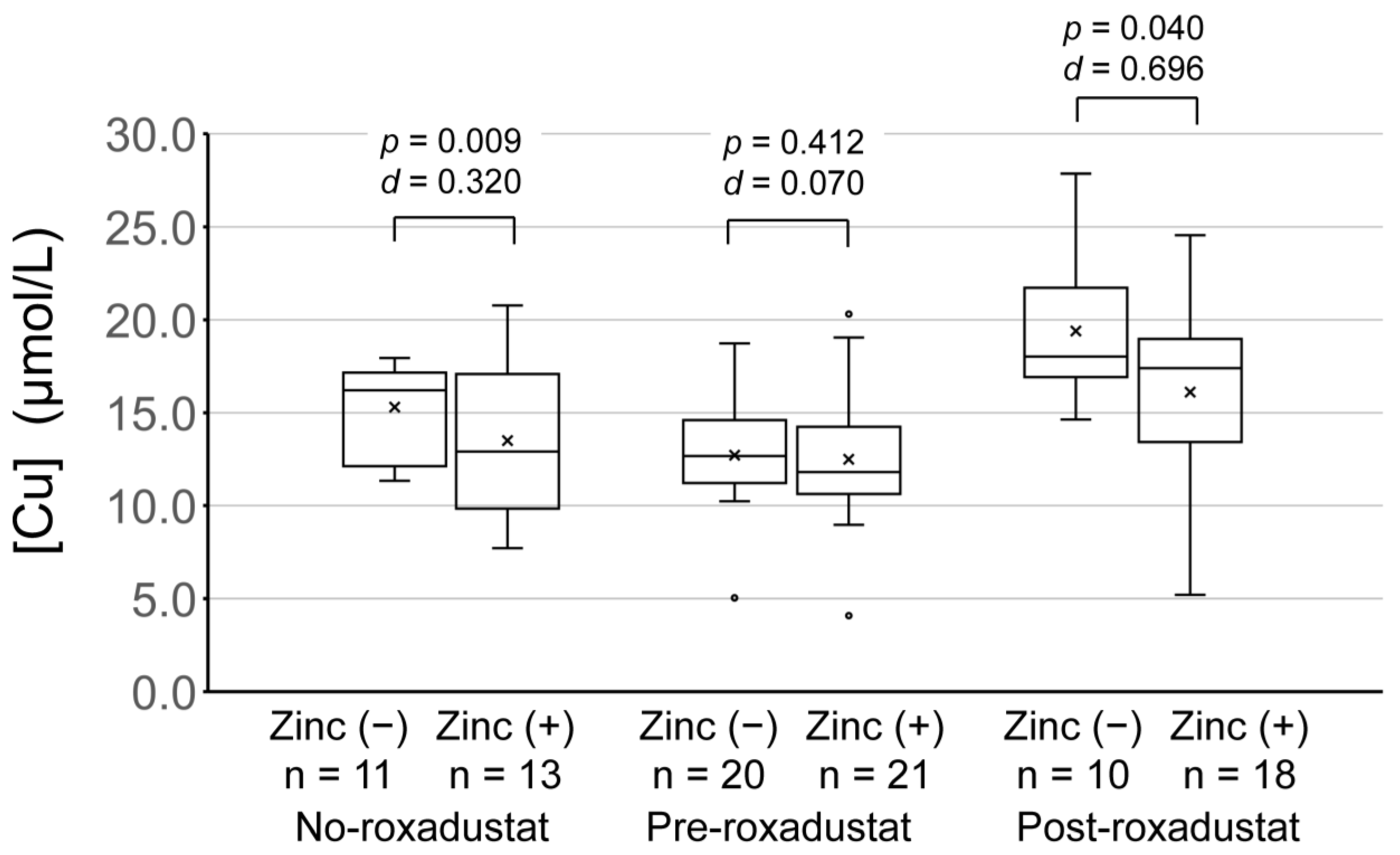
| [Cu] (µmol/L) | Zinc (−) | 15.3 ± 2.47 | 12.7 ± 2.8 | 19.4 ± 4.1 | <0.005 | |
| Zinc (+) | 12.4 ± 3.1 | 12.5 ± 3.5 | 16.1 ± 5.3 | <0.005 | ||
| p-value | 0.009 | 0.412 | 0.040 | |||
| Cohen’s d | 0.320 | 0.070 | 0.696 | |||
| Zinc dose (mg) | 28.5 ± 4.6 | 35.1 ± 10.4 | 36.2 ± 6.7 * | 0.026 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, A. Co-Administration of Roxadustat and Zinc Stabilizes Both Serum Copper and Zinc Concentrations in Patients Undergoing Hemodialysis. Nutrients 2023, 15, 4887. https://doi.org/10.3390/nu15234887
Takahashi A. Co-Administration of Roxadustat and Zinc Stabilizes Both Serum Copper and Zinc Concentrations in Patients Undergoing Hemodialysis. Nutrients. 2023; 15(23):4887. https://doi.org/10.3390/nu15234887
Chicago/Turabian StyleTakahashi, Akira. 2023. "Co-Administration of Roxadustat and Zinc Stabilizes Both Serum Copper and Zinc Concentrations in Patients Undergoing Hemodialysis" Nutrients 15, no. 23: 4887. https://doi.org/10.3390/nu15234887
APA StyleTakahashi, A. (2023). Co-Administration of Roxadustat and Zinc Stabilizes Both Serum Copper and Zinc Concentrations in Patients Undergoing Hemodialysis. Nutrients, 15(23), 4887. https://doi.org/10.3390/nu15234887





