Abstract
Metabolic syndrome (MetS) is a growing global health problem. Evidence suggests that diets rich in phytochemical-containing herbs and spices can contribute to reducing the risk of chronic diseases. This review assesses the scope of evidence supporting the use of herbs and spices in the diet for the prevention or treatment of MetS and its associated health conditions. A search of the PubMed, Scopus and Google Scholar databases was carried out to assess the available clinical evidence for culinary doses of commonly used herbs and spices. Trials that were measuring health factors related to metabolic disorders in healthy individuals, or the health of individuals with MetS or associated diseases, were included. Out of a total of 1738 papers identified, there were 142 relevant studies on black pepper, chilli, cardamom, cinnamon, coriander, cumin, fennel, fenugreek, garlic, ginger, nigella seed, rosemary, sage and turmeric. No relevant research was found for cloves, mint, oregano, parsley or thyme. Cinnamon, fenugreek and ginger were the herbs/spices with the most published trials on them and that showed promise for glycaemic control. Cardamom appears to have potential to reduce inflammatory markers, and cinnamon, ginger and turmeric to reduce blood lipids. Patients with type 2 diabetes were the population most likely to be included in studies, but the preventative benefits of herbs/spices in healthy populations were also investigated, particularly for chilli, ginger and cinnamon. There is evidence for the beneficial effect of culinary doses of many common herbs/spices in the prevention and treatment of MetS and associated disorders.
1. Introduction
Metabolic syndrome (MetS) and its associated conditions, such as obesity, diabetes and cardiovascular disease, are a growing global health problem. Between 2000 and 2019, global diabetes rates grew by more than 1.5% annually and prevalence rates for all other metabolic diseases also increased [1]. Poor diet and physical inactivity are risk factors for the development of MetS and lifestyle changes are key for treatment [2]. MetS involves the dysregulation of blood glucose, insulin resistance, raised blood lipids, increased inflammation and high blood pressure [2]. Therefore, measuring these biomarkers in healthy individuals can indicate the risk of MetS developing or can be used to monitor progress in those with MetS.
Research indicates that the inclusion of herbs and spices in the diet, as is often the case in Mediterranean and Asian diets, may contribute to positive long-term health outcomes [3,4]. Herbs and spices are a particularly rich source of phytochemicals and the consumption of diets rich in phytochemicals has been linked with a reduced risk of cardiometabolic disease and obesity [5,6].
Many studies looking at the health benefits of herbs and spices use high-dose extracts; however, these do not reflect the main way that the general public might be able to take advantage of these relatively cheap additions to their diet. Are these more expensive high-dose formulations necessary for everyone to benefit from herbs and spices, or do culinary doses provide benefits too?
Some researchers have begun to investigate this question. Clinical studies using herb and spice mixes to improve physiological responses to food have indicated that the inclusion of herbs/spices in the diet may have preventative or therapeutic benefits [7,8,9,10,11,12,13,14,15]. The spice mixes used in these studies ranged in dose from 6 g to 23.5 g and positively impacted vascular function, blood glucose and insulin and blood lipids following meals. These effects may contribute to a reduced risk of MetS and its associated conditions when herbs and spices are consumed regularly. The reasons for the specific herb/spice mixes chosen and doses used were often not explained. Doses of 6 g of Italian herb mixes [8], 23.5 g of Asian spices [11] or a combination of Mediterranean herbs and Asian spices at doses of 14.5 g [10] and between 0.5 and 6.6 g [13,14] have been found to have benefits. The lack of consistency in herb/spice mix formulations makes it challenging to attribute benefits to a particular herb/spice, a combination of herb/spices or a dose.
Zanzer et al. assessed the effect of the concentrated liquid extracts of individual spices, but standardized them to equal polyphenol contents, enabling the different effects from each spice to be elucidated [15]. The dose of polyphenols provided from each extract corresponded to the amount found in 6 g of cinnamon. Cinnamon and turmeric positively impacted on blood sugar levels, and turmeric reduced appetite; however, ginger and star anise did not have any effect. Therefore, the individual effects from different herbs goes beyond a general benefit from polyphenol intake and needs to be clarified.
The purpose of a scoping review is to identify a body of evidence, explore how it has been reported, identify evidence gaps [16], and perhaps to inform the development of future systematic reviews [17]. The many different types of evidence available for the health benefits of herbs/spices mean that systematic review methods are not yet appropriate. This methodology uses a systematic approach to searching, but produces qualitative results to highlight available research and provide a base for determining what further research is needed.
Therefore, the aim of this review was to assess the clinical evidence available for the metabolic health benefits of culinary doses of a range of common herbs and spices and to investigate whether there was consistency in the doses used and outcomes found. The most promising herbs and spices in the diet for specific health outcomes or populations can be identified, as well as the future clinical research needed to confirm this effect.
2. Materials and Methods
A number of previous reviews have identified herbs/spices with beneficial evidence for general health and for MetS and its associated disorders more specifically [2,3,18,19,20,21,22,23,24]. These were assessed to determine a list of herbs/spices that were most likely to have adequate evidence for this scoping review: black pepper (Piper nigrum L.), cardamom (Elletaria cardamomum (L.) Maton), chilli (Capsicum frutescens L.), cinnamon (Cinnamomum sp.), cloves (Syzygium aromaticum (L.) Merr. and L.M.Perry), cumin (Cuminum cyminum L.), fennel (Foeniculum vulgare Mill.), fenugreek (Trigonella foenum-graecum L.), garlic (Allium sativum L.), ginger (Zingiber officinale Roscoe), mint (Mentha sp.), nigella seed (Nigella sativa L.), oregano (Origanum vulgare L.), parsley (Petroselinum crispum (Mill.) Fuss), rosemary (Salvia rosmarinus Spenn.), sage (Salvia officinalis L.), thyme (Thymus vulgaris L.) and turmeric (Curcuma longa L.). A scoping review methodology was then used, referring to the preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews [25].
2.1. Search Strategy
The PICO (population, intervention, comparison, outcome) strategy was used to formulate search terms. The research question was as follows: do herbs and spices affect symptoms of MetS in healthy or relevant diseased populations? The population was healthy individuals or those with MetS and related disorders. The intervention was single herbs or spices at culinary doses. The comparison was a control or no treatment. The outcome was a change in symptoms associated with MetS or a change in relevant biomarkers: blood glucose, lipids, insulin or inflammatory markers. PubMed and Scopus were searched in January 2023 with no date restrictions applied. A search of PubMed database was carried out using the search terms: (“black pepper” or “Piper nigrum” or “black seed” or “black cumin” or “Nigella sativa” or cardamom or “Elettaria cardamomum” or chilli or “Capsicum frutescens” or cinnamon or “Cinnamomum zeylanicum” or cloves or “Syzygium aromaticum” or coriander or “Coriandrum sativum” or cumin or “Cuminum cyminum” or fennel or “Foeniculum vulgare” or fenugreek or “Trigonella foenum-graecum” or garlic or “Allium sativum” or ginger or “Zingiber officinale” or mint or “Mentha” or oregano or “Origanum vulgare” or parsley or “Petroselinum crispum” or rosemary or “Rosmarinus officinalis” or sage or “Salvia officinalis” or thyme or “Thymus vulgaris” or turmeric or “Curcuma longa”) AND (metabol* or diabetes or obesity or cardiovascular or “blood glucose” or “blood sugar” or “blood lipids” or “blood fats” or “blood insulin”). The results were filtered by Clinical Trial as the article type. Each herb/spice was searched for separately in Scopus, with the following search term: TITLE-ABS-KEY ({herb/spice name}) AND TITLE-ABS-KEY (metabolic OR metabolism OR diabetes OR obesity OR cardiovascular) AND TITLE (clinical OR human). Additional studies were found using Google Scholar and hand searching reference lists from relevant reviews.
2.2. Inclusion and Exclusion Criteria
The inclusion criteria were the use of a whole herb/spice or powdered/ground herb/spice in food, drinks or encapsulated and at doses that could reasonably be achieved in the diet without negatively impacting palatability. Concentrated extracts or oils and combinations of herbs or spices were excluded. Studies of water infusions or herbal teas were included when the formulation and quantity was what might be reasonably consumed at home. Any studies that administered herbal formulations or more than one herb/spice at a time were excluded; however, those with multiple individual herbs/spices being investigated in separate arms of the study were included. To ensure a broad range of different studies, clinical trials that were measuring biomarkers related to metabolic disorder in healthy individuals or the health of individuals with metabolic disease or related conditions were included. Studies were included regardless of the age or health status of participants, as the intention was to determine the potential for herbs and spices to both prevent and treat metabolic disease. However, if the participants had a co-morbidity not related to MetS the study was excluded. Studies were included regardless of language. Any retrieved studies not in English were translated using Google Translate. Animal and in vitro studies were excluded. Reviews were excluded.
2.3. Study Selection and Data Collection
The articles identified from title and abstract screening were added into a Microsoft Excel spreadsheet, the papers were retrieved, and final inclusion decisions were made by reading the full text. Two reviewers (MM and VR) screened this list to decide on the final included studies. For each article, the following data were entered into the spreadsheet: study type, herb/spice investigated, population, dose of herb/spice, length of study and outcome measures. The evidence for each herb/spice was clustered into type of metabolic health measurement investigated, and whether there were positive findings or not. The Jadad Scale was used to score the methodological quality of the clinical trials. The Jadad scale was originally developed for assessing clinical trials in pain research, but has been widely adapted and is considered to offer the best validity and reliability evidence [26]. It has limitations in trials investigating food, due to the difficulty in blinding with fresh food, but was still considered the most appropriate. One point is scored for each of the following: a mention of randomisation; a description of an appropriate randomisation method; a mention of blinding; a description of an appropriate blinding method; and, all participants in the trial being accounted for in the results. Trials that scored 0–2 were considered low quality and those scoring 3–5 were considered high quality.
3. Results
The PubMed search identified 792 results, while the Scopus search produced 925 results. An additional 21 papers were found from Google Scholar and reference list scanning. Title and abstract screening led to 254 papers for full-text screening. This led to a total of 142 relevant papers for data extraction (Figure 1).
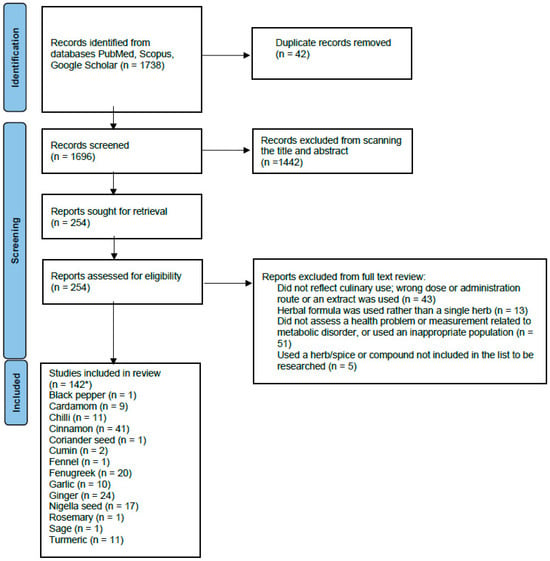
Figure 1.
PRISMA flow diagram of the screening process. * The number of studies for all the individual herbs has a total sum of more than 145, as some studies included more than one herb in each arm of the study.
Evidence was found for black pepper, chilli, cardamom, cinnamon, cloves, coriander, cumin, fennel, fenugreek, garlic, ginger, nigella seed, rosemary, sage and turmeric. No relevant research was found for mint, oregano, parsley or thyme. Table 1 summarises the included studies. The rationale behind the studies excluded at full-text screening is shown in Supplementary Table S1.

Table 1.
Summary of results from included studies.
3.1. Black Pepper, Cardamom and Chilli
There was one single-blind cross-over trial on black pepper in healthy adults. A dose of 1.3 g added to a meal had no impact on appetite or thermogenesis [138].
Ten studies (eight double-blind RCTs, one single-blind RCT and one clinical study) looked at the impact of cardamom on inflammation and a range of metabolic markers in individuals with hypertension [27], diabetes [29,32,59], prediabetes [28], poly-cystic ovarian syndrome (PCOS) [34,35] and non-alcoholic fatty liver disease (NAFLD) [30,33]. All the studies on cardamom used 3 g/day for between 8 and 20 weeks. Five out of six studies that investigated inflammatory markers found positive effects [28,30,31,32,34,35], two studies found benefits on blood glucose and insulin and two studies found no benefit from cardamom on blood lipids, while effects on blood pressure were variable.
Eleven clinical studies [9,36,37,38,39,40,41,42,43,44,45] investigated the effects of chilli on appetite, vascular function and blood glucose, insulin and lipids. Apart from one study looking at the effect of chilli on glucose and insulin in pregnant women with gestational diabetes [42], all the included studies were in healthy individuals.
Four of the intervention studies used doses of 30 g of fresh chilli [36,37,38,39], the other seven intervention studies used doses of 0.6 g [40], 1.25 g [42], 3.09 g [44,45] or 5 g [43], a meal with chilli containing 5.82 mg of capsaicinoids [9] or chilli capsules containing 10 mg of capsaicinoids [41].
Appetite and/or thermogenesis or metabolic rate were measured in eight out of thirteen of the studies and five of these found a beneficial effect. There were as many studies finding positive results as those showing no effect for the key metabolic biomarkers of blood glucose, insulin and lipids (see Figure 2).
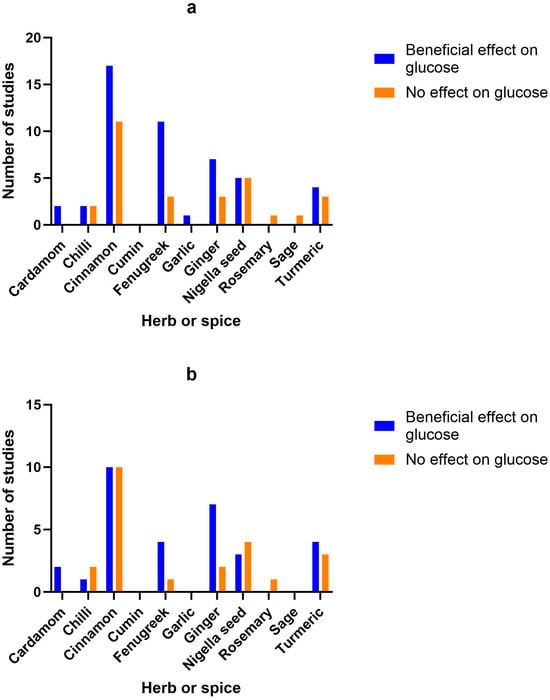
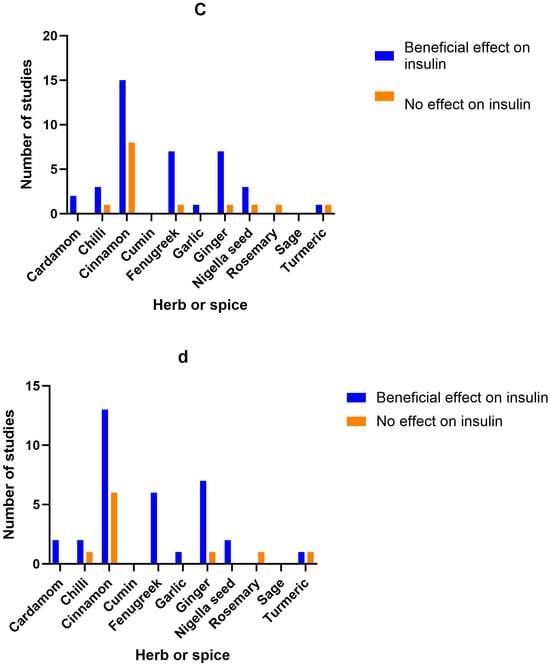
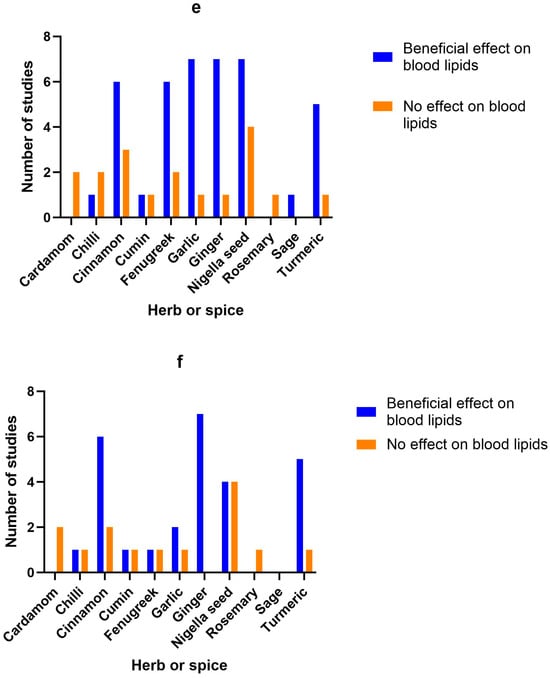
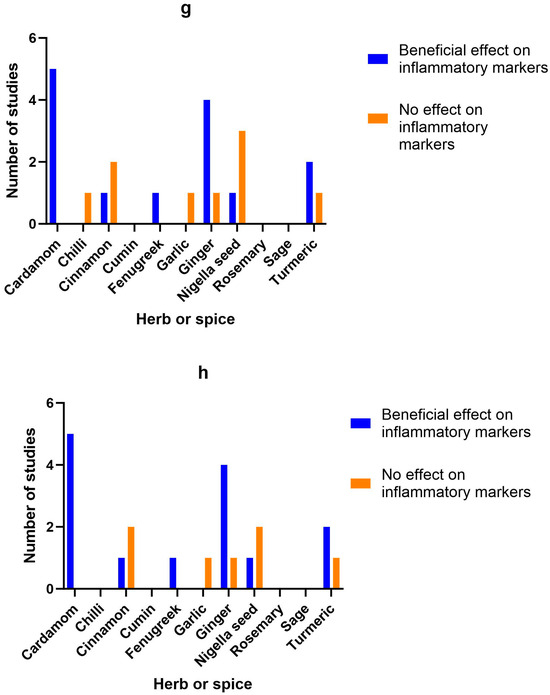
Figure 2.
Number of studies and high-quality studies demonstrating the effect of herbs/spices in specific cardiometabolic biomarkers. Figure 2 identifies the main health markers measured and whether effects were seen for each of the herbs and spices in all studies and only in high-quality studies: (a) Number of studies showing an effect or lack of effect for each of the herbs and spices on blood glucose; (b) Number of high-quality studies showing an effect or lack of effect for each of the herbs and spices on blood glucose; (c) Number of studies showing an effect or lack of effect for each of the herbs and spices on insulin; (d) Number of high-quality studies showing an effect or lack of effect for each of the herbs and spices on insulin; (e) Number of studies showing an effect or lack of effect for each of the herbs and spices on blood lipids; (f) Number of high-quality studies showing an effect or lack of effect for each of the herbs and spices on blood lipids; (g) Number of studies showing an effect or lack of effect for each of the herbs and spices on inflammatory markers; (h) Number of high-quality studies showing an effect or lack of effect for each of the herbs and spices on inflammatory markers.
One cross-over study found an improvement in diet-induced thermogenesis [36] one single-blind cross-over study found increased metabolic rate [44] and one single-blind cross-over study found decreased appetite from chilli [45]; however, four studies found no effect on metabolic rate or energy intake [9,39,40,41]. A reduction in insulin was found in three studies [9,38,42], while an increase was found in one [43]. There was no change in blood glucose levels from two randomized cross-over studies [37,39], while one double-blind RCT [42] and one clinical study [43] found significant reduction in glucose from chilli consumption. The quality of the clinical studies was generally low (8 low and 3 high according to Jadad scores), mainly due to the difficulty in blinding the consumption of chilli in food.
3.2. Cinnamon
There were 41 studies looking at the benefits of culinary doses of cinnamon. Ten in healthy individuals [46,48,49,50,54,55,56,57,81,82], nineteen in those with diabetes [47,53,59,60,61,63,64,66,67,68,69,70,71,74,75,76,78,79,84], six in women with PCOS [58,65,72,73,80,83], one in Asian Indians with MetS [77], one in patients with NAFLD [62], one in individuals with impaired glucose tolerance [51], one in sedentary women with obesity [52], one in women with dyslipidaemia [86] and one in prediabetic individuals [166]. Doses ranged from 1 to 6 g, either as a single dose or daily for between 2 weeks and 6 months. Whether a positive effect was seen or not did not appear to correlate with dosage.
Beneficial effects on glucose were found in 6 randomized cross-over studies, 1 randomized trial and 10 double-blind RCTs from doses of 0.5–6 g/day, 100 mL of cinnamon tea or single doses of 5–6 g of cinnamon [46,48,52,53,54,55,56,57,62,73,74,75,76,77,78,79,82], while 7 double-blind RCTs, 3 single-blind RCTs and 1 randomized cross-over study found no effect from a single dose of 1–6 g or 1–1.5 g/day [47,49,51,60,61,63,65,70,71,80,166].
Beneficial effects on insulin were found in 4 randomized cross-over studies, 10 double-blind RCTs and 1 single-blind RCT from doses of 1–3 g/day or single doses of 3–6 g of cinnamon [46,48,49,57,59,62,68,70,73,75,77,78,79,80,83], while 6 double-blind RCTs, 1 single-blind RCT and 1 randomized cross-over study found no effect from doses of 1–1.5 g/day or a single dose of 6 g [47,49,51,53,60,67,71,166].
3.3. Coriander Seed, Cumin and Fennel
One single-blind RCT found that 2 g/day of coriander seeds for 40 days improved average body mass index (BMI) from 27.3 to 26.7 and blood lipids (total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL)), as well as systolic blood pressure, in patients with hyperlipidaemia [116].
Two clinical studies looked at the benefit of cumin on anthropometric measures, blood insulin and blood lipid levels in overweight adults [85] and women with dyslipidaemia [86]. The randomised clinical trial by Zare et al. used a dose of 6 g/day for 3 months and this reduced all blood lipid measurements, as well as anthropometric measurements of weight, body mass index (BMI), waist circumference and fat [85], while Pishdad et al. used 3 g/day for 8 weeks in a double-blind RCT and found a benefit on total cholesterol, but not LDL or HDL cholesterol [86].
One single-blind crossover study found that a single dose of 2 g of fennel as a tea decreased appetite in healthy women, but did not impact food consumption [87].
3.4. Fenugreek
There were twenty studies looking at fenugreek, mainly for its impact on blood glucose, insulin and lipids, in healthy individuals [87,91,92,95], diabetics [89,90,93,94,96,97,98,99,100,101,103,104,105,106], individuals with coronary artery disease [137] and adults with hyperlipidaemia/hypercholesterolaemia [88,102]. Quantities ranged from 2 g up to 100 g, with the majority of studies using 10–15 g/day. Effects on blood insulin, glucose and lipids were promising, with 11 out of 14 studies showing significant positive effects on blood glucose, 7 out of 8 studies finding significant changes in insulin and 6 out of 8 studies significantly improving blood lipids, regardless of dose used. However, excluding low-quality studies reduced the number of studies indicating a benefit on blood glucose (to 4 out of 5 studies) and blood lipid levels (to 1 out of 2 studies).
3.5. Garlic
There were ten clinical studies (four double-blind RCTs and six single-blind or randomised clinical studies) looking at the benefits of garlic. Both clinical trials that looked at the effect of garlic on blood pressure (BP) found benefits when participants consumed 20 g or 100 mg/kg bodyweight of fresh garlic daily [107,111]. Most of the studies investigated the impact of garlic on blood lipids. One clinical study was carried out on overweight smokers [112]. Four clinical studies looked at platelet function [110], cholesterol [108,109], immunity and cancer markers [168] in healthy individuals. Two studies looked at multiple outcomes in patients with MetS [107,115], one study investigated NAFLD [114] and two studies looked at individuals with hyperlipidaemia [111,116]. For the interventional studies, doses ranged from 1.6 g to 40 g, with higher doses of fresh garlic compared with dried garlic powder. All but two of the clinical studies looked at blood lipids and seven out of eight found a benefit. Fresh garlic in doses of 100 mg/kg body weight, 20 g or 40 g/day significantly reduced triglycerides in three studies [107,108,111], while two studies found that 1.6 g/day of dried garlic reduced triglycerides [113,115]. Total cholesterol was significantly reduced by 1.6, 2 or 3 g/day dried garlic and 20 g or 40 g of fresh garlic [108,109,111,113,116]. Only one study of overweight participants at risk of cardiovascular disease found no impact of 2.1 g of garlic on blood lipids [112].
3.6. Ginger
Out of a total of 24 studies on ginger, 6 were carried out on healthy individuals to look at blood clotting [119], energy intake and appetite [138], thermoregulatory function or thermogenesis and appetite [118,120], anthropometric measurements [128,133], anthropometric measurements and blood glucose and fats [130] and cardiovascular risk factors [137]. There were 11 studies on patients with type 2 diabetes looking at the impact of ginger on blood sugar, insulin and blood lipids [126,134,135], metabolic health and inflammation [125,132], fasting blood glucose and insulin sensitivity [127,129], blood glucose, insulin and inflammation [121], vascular function [122], anthropometric measurements and blood pressure [84] and anthropometric measurements and inflammation [124]. One study looked at the effects of 1.5 g of ginger on anthropometric measurements and insulin resistance in women with PCOS [83]. One study looked at the effect of 3 g/day on blood lipids in individuals with hyperlipidaemia [136]. Two studies looked at the impact of ginger on liver function, anthropometric measurements, blood sugar and inflammatory markers in individuals with NAFLD [117,123]. One looked at the impact of 1 g of ginger daily in obese children with NAFLD [117], while, in the other study, adults were given 1.5 g/day [123]. A pilot study investigated the impact of 1 g/day of ginger on thyroid symptom score, anthropometric measurements, blood glucose and blood lipids in hypothyroid patients [131].
Ginger was used in doses that ranged from 1 g to 10 g of dried powdered ginger for a single dose or up to 12 weeks daily, apart from one study that used 15 g of fresh ginger or 40 g of cooked ginger [119] and another study that used 20 g of fresh ginger [138]. There did not appear to be a correlation between dose and efficacy. All seven studies that investigated the impact of ginger (doses of 1.5–3 g/day) on insulin found a benefit, ten out of twelve found positive effects on blood glucose (doses of 1.2–3 g/day), six out of seven found a positive effect on blood lipids, such as total cholesterol, LDL and triglycerides (doses of 1–3 g) and four out of five studies looking at inflammatory markers (doses of 1.5–3 g) found a benefit. The studies were mainly of high quality (22 out of 24) according to Jadad scoring, with 18 double-blind RCTs, 3 randomized cross-over studies and 3 placebo-controlled study.
3.7. Nigella Seeds
There were 17 studies on nigella seeds, mainly investigating their effect on anthropometric measurements, blood glucose, insulin and lipids. One double-blind RCT was carried out on healthy male volunteers [141]. A large double-blind RCT looked at the impact of 1.5 g/day of nigella seeds in 250 healthy men with MetS [165]. One double-blind RCT was carried out on men with obesity [153]. Two studies were carried out with thyroiditis patients [147,150], four in individuals with MetS [139,140,143,152], three in patients with hyperlipidemia/hypercholesterolaemia [144,146,154], three in patients with type 2 diabetes [142,145,151] and two in patients with NAFLD [148,149]. The studies on nigella seeds used between 500 mg and 3 g/day for durations ranging from 4 weeks to 1 year.
Nigella seeds improved anthropometric measurements such as BMI and weight in three out of seven studies (at doses of 2–3 g/day), improved blood glucose in five out of ten studies (at doses of 500 mg–2 g/day), insulin (at 2 g/day) in three out of four studies, blood lipids in seven out of eleven studies (at 500 mg–2 g/day) and inflammatory markers in one out of four studies (at a dose of 2 g/day). Of the 17 studies, 13 were high-quality according to Jadad.
3.8. Rosemary, Sage and Turmeric
The one high-quality, double-blind RCT on rosemary found no impact on liver enzymes, anthropometric measurements, fasting blood glucose, insulin and blood lipids from 4 g/day for 8 weeks in patients with NAFLD [155].
One low-quality, non-randomised cross-over study found that drinking 600 mL of sage tea daily for 4 weeks improved lipid profile but had no effect on blood glucose in healthy female volunteers aged 40–50 years [156].
Five out of the eleven studies on turmeric looked at patients with type 2 diabetes [157,159,161,162,164]. One turmeric study was carried out on healthy volunteers to investigate glycaemic effect [158]. Two other studies were carried out on individuals who were stated to be overweight, obese or prediabetic, with no other health issues [166,167]. Two studies looked at the effect of turmeric on NAFLD [163,169]. The studies used between 1 and 3 g/day for between 4 and 12 weeks, or single doses of 1 g [166] and 6 g [158].
All studies were of a high quality according to Jadad. Three out of five studies investigating anthropometric measurements, such as weight and BMI, found some positive effect from turmeric (at a dose of 2.1 g/day). Four out of seven studies found improvements in blood glucose levels (at doses of 2.1–2 g/day), one out of two studies found improvements in insulin (from a dose of 2 g/day) and five out of six studies found improvements in blood lipids such as triglycerides, total cholesterol and LDL (at doses of 2.1–2.4 g/day). Out of three studies looking at inflammatory markers, two found beneficial effects from 2.1 to 2.4 g/day of turmeric powder in capsules.
3.9. Herb/Spice Efficacy
Figure 2 identifies the main health markers measured and whether effects were seen for each of the herbs and spices in all studies and only in high-quality studies. Blood glucose and insulin were the most commonly measured markers, followed by blood lipids, then inflammatory markers. Only including high-quality studies did not make a big difference to the pattern of responses seen for glucose, insulin or inflammatory markers. However, the benefits of fenugreek and garlic on blood lipids were not apparent when only high-quality studies were considered.
3.10. Adverse Effects
No adverse effects were reported for any of the studies at the doses used.
3.11. Study Quality
Studies were scored for quality using Jadad (details on the scores given are provided in Supplementary Table S2). There were 40 low-quality studies (28% of the scored studies), 97 high quality studies (66% of the scored studies), and 4 studies for which there was not enough information to score them. Of the 142 studies, 81 were double-blind RCTs, 30 were cross-over clinical studies, 30 were single-blind or parallel clinical studies and 1 was a cross-sectional observational study.
4. Discussion
The aim of this scoping review was to assess the clinical evidence available for culinary doses of herbs and spices, what doses are used and in which health conditions, with a view to identifying areas that need further research. In this review, there were a total of 142 studies looking at the effects of black pepper, chilli, cardamom, cinnamon, cloves, coriander, cumin, fennel, fenugreek, garlic, ginger, nigella seed, rosemary, sage and turmeric on metabolic health. Cinnamon, fenugreek and ginger showed the most promise in controlling blood glucose and insulin. Cinnamon, ginger, nigella seed and turmeric were most promising in terms of having beneficial effects on blood lipid levels. Cardamom, ginger and turmeric showed promise for reducing systemic inflammation due to a decrease in inflammatory markers.
Some herbs/spices were more likely to be researched in a specific population or in an investigation of a specific metabolic biomarker. This either indicates increased efficacy or traditional associations and observations stimulating more research in these areas. Findings from in vitro studies or in vivo animal research may also have prompted researchers to focus on a particular herb and effect. For example, despite not having a traditional association with inflammation, the anti-inflammatory effects of cardamom have been found in an animal model [160].
Cardamom was only investigated in individuals with disease associated with MetS and no studies were carried out in healthy individuals. Most studies investigated the impact of cardamom on inflammatory markers. MetS involves increased inflammation; therefore, changes in inflammatory markers are more likely to be observed in those with MetS than healthy individuals. Preclinical research has identified the potential of cardamom for use in inflammation and hyperlipidaemia. Cardamom reduced swelling and downregulated inflammatory cytokines such as cyclo-oxygenase-2 (COX-2) in an animal model [160]. This confirms the finding in this scoping review that cardamom has anti-inflammatory activity. A terpenoid compound from cardamom, 1,8-cineole, prevented lipid oxidation in vitro and lowered serum lipid levels in zebrafish, while cardamom oil at a dose of 3 g/kg reduced total cholesterol, LDL-cholesterol and triglycerides in Wistar rats [170]. This scoping review did not identify any clinical studies showing a beneficial effect of cardamom on blood lipids, which may reflect a failure of animal studies to relate to effects in humans, or may be due to the difference in dose or formulation.
Almost all the studies on chilli were carried out in healthy individuals. Chilli did not have marked effects on the main insulin, glucose, lipids and inflammatory biomarkers, which may reflect the use of healthy populations for most studies, the doses used or difficulty in blinding chilli consumption in food. It showed more promise for impacting thermogenesis, metabolic rate and appetite. The key active compound in chilli is capsaicin and this binds to transient receptor potential vanilloid receptor 1 (TRPV1) to activate metabolic modulators such as peroxisome proliferator activated receptor (PPAR)α and glucagon-like peptide (GLP)1 [171]. Capsaicin has also been found to decrease ghrelin secretion [171], which would explain its appetite-suppressing effects.
The majority of studies on cinnamon were carried out in patients with diabetes, indicating the strong association of this spice with blood sugar control, e.g., references [172,173,174]. This impact on blood sugar is thought to be due to an insulin-mimetic effect and via the inhibition of digestive enzymes, such as α-amylase, in the gastrointestinal tract [172,174]. Cinnamon activates both PPARα and PPARγ, which would explain its effect on glycaemia [175]. However, there was considerable heterogeneity in the effect of cinnamon on blood sugar and blood insulin across the different studies in this review. Cinnamon was more likely to positively impact blood sugar and insulin in healthy individuals than those with type 2 diabetes. Seven out of nine studies (77%) looking at the impact of cinnamon on blood sugar in healthy individuals found a benefit, while only seven out of fifteen studies (47%) looking at the impact of cinnamon on blood sugar in diabetic patients found a benefit. This suggests that it could be of preventative and therapeutic benefit; however, due to the heterogeneity of the results, systematic reviews isolating the impact of the dose and the health of the participants on outcomes would be interesting. There are a number of meta-analyses of cinnamon in diabetes [172,176,177,178,179,180,181,182]; however, there did not appear to be any assessing blood glucose control in healthy individuals.
The effects of cinnamon on blood sugar, insulin and lipids appeared to be quite mixed from both this scoping review and published meta-analyses. Well-researched areas, such as is found with cinnamon, often highlight heterogenous results. This indicates that further research is needed to separate out the factors that impact efficacy, such as dose, presence in a food matrix, population or duration of study. Yu et al. found that dosage did impact efficacy, with a dose of less than 1.2 g significantly reducing fasting blood glucose, when pooled results found no benefit [175]. There have been at least eight meta-analyses of clinical trials of cinnamon looking at LDL-cholesterol. Six found decreases in LDL-cholesterol [175,176,180,183,184,185], but two found no impact on LDL-cholesterol [186,187]. Yu et al. found that the effect of cinnamon on LDL-C was influenced by dose [175], which may explain some of the heterogeneity.
Fennel has been used traditionally for its digestive properties [188], but it has also been found to have anti-inflammatory and antihyperlipidaemic activity [20]. Bae et al. found that fennel did not impact food consumption; however, it did reduce appetite, indicating that further research might be beneficial [87].
The impact of fenugreek was investigated in patients with diabetes in 14 studies and in healthy individuals in 6 studies. The effects on blood glucose and insulin were mainly positive whether in a healthy or diseased population; however, high-quality studies were less likely to find benefits. Larger doses of fenugreek tended to be used, as the beneficial effect was usually considered to be from the soluble fibre in the seeds [189], although some studies suggest an effect of other compounds such as flavonoids, saponins and the alkaloid trigonelline [190,191]. As a relatively mild-flavoured spice, larger amounts are palatable in the diet. The studies using larger quantities incorporated the seed powder into a food matrix, such as being baked into bread; therefore, these were considered culinary doses and were included.
Nine meta-analyses on fenugreek in MetS or associated conditions have been published in the last ten years [189,190,191,192,193,194,195,196,197]. All of those that assessed effects on blood glucose found a benefit, but concerns were raised about the quality of the clinical trials. A review of fenugreek in blood pressure found a dose-dependent effect, with doses greater than 15 g/day for longer than 12 weeks being effective [192]. Neelakantan et al. also found that dose impacted the effect of fenugreek on glycaemia, with doses higher than 5 g being effective [189].
Studies on garlic looked almost exclusively at blood lipid levels, whether in healthy populations or those with metabolic disorders. Garlic has a strong association with cardiovascular health and cholesterol levels [198,199,200]; however, much of the published research has looked at the effect of standardised garlic extracts, rather than its consumption in food. Systematic reviews and umbrella reviews have identified strong potential for garlic in hyperlipidaemia, hypertension and inflammation [198,199,200].
This review has confirmed the benefits of garlic for hyperlipidaemia and indicates that concentrated extracts may not be necessary in order to benefit from some of the positive health effects from garlic, as both lower and higher doses showed efficacy. There was no relationship between dose and size of effect; for example, the reductions in total cholesterol from 2, 20 and 40 g of garlic compared with control were 82, 19 and 51 mg/dL, respectively [108,111,116]. Both low- and high-quality studies, according to Jadad score, showed effectiveness. Garlic and its phytochemicals have been found to have anti-hyperlipidaemic activity via 3-hydroxy-3-methylglutaryl CoA (HMG CoA) inhibition and reduction in cholesterol synthesis; hypotensive activity via angiotensin-converting enzyme (ACE) inhibition, the downregulation of angiotensin II and the stimulation of nitric oxide; and anti-inflammatory/anti-atherosclerotic effects via COX inhibition, the decreased synthesis of thromboxane B2, the decreased production of leukotriene C4 and a reduction in LDL oxidation [201]. There are feasible mechanisms of action for the findings and evidence for the benefits of garlic is building.
Most of the ginger studies in healthy populations looked at thermoregulatory function and appetite rather than metabolic biomarkers of glucose, insulin or lipids. The obvious sensorial heating effects on the body from ginger can explain this choice of research. The activation of TRPV1 by pungent principles in spices such as ginger leads to a sensation of heat in the mouth upon consumption and has been suggested to have a thermogenic effect [202]. Studies in diabetic patients appeared largely positive for blood glucose, insulin and lipid levels. Whether this is via the activation of TRPV1 receptors, via anti-inflammatory effects through the inhibition of COX and lipoxygenase or via another mechanism remains to be investigated.
Both nigella seeds and turmeric were investigated in a range of health conditions, with no predominance of either healthy individuals or those with type 2 diabetes. This reflects the broad range of uses of these herbs/spices in traditional medicine.
The studies on nigella seeds were heterogenous in terms of the populations investigated and effects seen. The health effects are broad and many traditional medicine systems consider it to be a panacea [203,204,205], which has led to a lack of focus for research into its benefits. However, the phytochemical thymoquinone, found in the essential oil of the seed, has been identified as being responsible for some of the health benefits [203]. This may indicate there is likely to be more benefit from the use of nigella seed oil than the seeds. A meta-analysis by Daryabeygi-Khotbehsara et al. found that there was a reduction in triglycerides by nigella seed oil, but not the seeds [206]. Sahebkar et al. found that nigella seed powder was more effective than the oil for reducing blood pressure [207], while Askari et al. found that the oil was more effective than the powder for blood glucose control [208]. It is likely that thymoquinone and other terpenoids are responsible for supporting healthy blood lipid and glucose levels, but other phytochemicals not found in the oil are responsible for the hypotensive effect. This indicates the importance of assessing the effect of culinary uses separately from that of concentrated food supplements or extracts. Future research focusing on whether dose and formulation impact on the effects and efficacy would be of interest.
There are at least 10 meta-analyses on the use of nigella seeds for MetS and associated conditions [206,207,208,209,210,211,212,213,214,215]. These all find benefits of nigella seeds on anthropometric measurements such as body weight, blood lipids, glucose control and inflammation, apart from a review of studies on patients with NAFLD, which found mixed results on blood lipids, inflammatory markers and glucose control [214].
Most studies on turmeric found a beneficial effect, with the most promising areas being inflammation and blood lipids. A meta-review on the health benefits of turmeric by Rolfe et al. identified osteoarthritis and MetS to be the most promising areas of research [216]. Turmeric is well researched for its use in inflammatory conditions such as osteoarthritis; however, most research focuses on high-dose curcumin extracts due to the poor bioavailability of curcumin [217]. Therefore, it is interesting that the relatively low doses of 2 g were found to have some effect in this review. Sahebkar found in a meta-analysis that overall turmeric reduced CRP, but that bioavailability-improved preparations of curcuminoids were superior [218], so it may be that higher doses are preferable but not essential.
Nearly all of the spices investigated, but none of the herbs, had some evidence to support their use in culinary doses for the prevention or treatment of MetS and associated disorders. Four mixed herb/spice intervention studies found metabolic benefits from Italian herb seasoning mixes or mixes containing Mediterranean herbs rosemary, basil, thyme, oregano and parsley [8,10,13,14]; however, this scoping review has found a lack of studies to confirm the effects of individual herbs mint, parsley, thyme, rosemary, sage and oregano. These plants are particularly rich sources of volatile oils; therefore, research investigating the antimicrobial properties of the essential oil is abundant, e.g., references [219,220]. In vitro antimicrobial research is relatively easy to carry out, which may explain why other properties of these herbs have not been investigated to date. In addition to volatile oils, as is the case with the spices cinnamon, turmeric and ginger, these herbs are also good sources of polyphenols [24]. Further research into the general health benefits of adding these herbs to the diet would be beneficial.
Considering the widespread use of black pepper in food, it was surprising that there was only one study investigating the impact of this popular condiment for health. It may be that flavour prevents the use of large enough quantities in food for this spice to be of benefit. One study of black pepper was excluded as it investigated the use of a water extract made from 20 g of black pepper, which was not representative of culinary use [15]. It was not clear from the methodology what final dose was consumed. However, if it was a comparable dose to that used by Gregersen et al. [138], then the two studies found opposite effects on appetite, with Zanzer et al. finding an effect of black pepper on appetite, but Gregersen et al. finding none. Black pepper contains piperine, which is recognised as a phytochemical that enhances the absorption of other food components [221], as well as some prescription drugs [222]. The benefits of black pepper could be largely due to its ability to improve the bioavailability of polyphenols and other phytochemicals in herb/spice mixtures.
Changes in insulin, blood sugar and blood lipids were the most common biomarkers to be investigated. Effective glycaemic control, whether via eating foods with a low glycaemic index or adding in herbs/spices and other phytochemical-containing plant foods that help to reduce the glycaemic index of foods, is crucial for the management and prevention of diabetes, as well as the reduction in cardiometabolic risk factors in diabetics [223]. In terms of coronary heart disease risk, only LDL cholesterol has been proven in formal clinical trials to be a biomarker that can be considered a causative factor. Other factors, such as HDL cholesterol, triacylglycerol, vascular function and oxidative damage, require further evidence before their measurement can be considered predictive [224]. This scoping review found promise for cardamom, cinnamon, fenugreek, garlic, ginger, nigella seeds and turmeric, as there were positive findings from at least five different studies for one or more of these biomarkers. However, whether this translates to clinically significant effects for patients with MetS remains to be tested.
Limitations and Future Directions
Heterogeneity in the methodology is a major limiting factor in both interpreting the results of this scoping review and the many systematic reviews that have been carried out in this area. As herbs/spices are complex in terms of phytochemistry, as well as effect, and there are not commonly accepted doses, comparisons across different studies are challenging.
The doses used in different studies varied more greatly for herbs/spices which can be used both fresh and dried, such as ginger, garlic and chilli. The difficulty in comparing fresh with dried herbs/spices could be overcome in the future by ensuring that a phytochemical analysis of fresh versus dried samples is carried out. Methodologies that account for differences such as these will add value and enable the clearer comparison of one study with another. Most dried herbs/spices were used in doses of between 1 and 6 g, which is representative of the amounts usually used in cooking (however, the doses were usually chosen based on what amounts had been found to be beneficial in previous studies). A notable exception was fenugreek, which was used in higher doses for the additional fibre benefits. It is possible that investigating higher doses of other herbs/spices may also indicate greater benefit, but this would have to be weighed against palatability.
The combination of multiple herbs and spices is likely to have greater beneficial effect than any individual herb or spice, due to the greater quantity and variety of phytochemicals. A limitation of this scoping review is that focusing on individual herbs prevents comparisons being made with the efficacy of herb/spice mixes. Future research could compare the impact of adding a single herb/spice with that from the synergy of a mix of herbs and spices in the diet, providing a rich variety of phytochemicals.
The duration of the clinical trials is also likely to be a major limiting factor in determining whether consuming herbs and spices in the diet is likely to have a noticeable effect on MetS and cardiovascular health. Although the duration of the studies included in this review did not appear to impact on whether the effect was positive or not, any dietary intervention for preventative health needs to be assessed over a longer period, which adds considerable cost and complexity to trials. Many of these diseases develop over a long period of time, so the next step should be to measure changes in biomarkers associated with them over longer periods. Identifying metabolite markers that indicate an increased consumption of specific herbs may be of use, as has been suggested for measuring the intake of polyphenol-rich foods [225].
The health benefits of herbs/spices are likely due to their phytochemical content and complex interactions between these molecules, other dietary components, the microbiome and the gut wall. Phytochemicals, and polyphenols specifically, have been found to impact carbohydrate absorption and metabolism, gut bacteria populations and the uptake of glucose into muscle and adipose tissue [226]. None of the studies incorporated an analysis of the phytochemical content of the herbs/spices used, which would have been a useful addition to tease out the mechanisms or to ascertain which bioactive phytochemicals are driving the effects. The impact of any dietary intervention is dependent on multiple other factors, such as the rest of the diet, participants’ stress levels and physical activity; therefore, teasing out the true impact of individual herbs/spices remains a challenge.
5. Conclusions
Overall, this scoping review has highlighted that there is evidence for the beneficial effect of culinary doses of cardamom, cinnamon, chilli, fenugreek, garlic, ginger, nigella seeds and turmeric in the prevention and treatment of MetS and its associated disorders. Cardamom, ginger and turmeric appear to have the most potential for inflammation linked to MetS, garlic, ginger and turmeric for blood lipids and cinnamon, ginger and fenugreek for blood glucose control. Future research needs to address which factors are most important to unlocking these benefits: the food matrix; the combinations of different herbs/spices; the duration of consumption; and how herb/spice intake interacts with other important dietary and lifestyle changes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nu15234867/s1, Table S1: Excluded papers; Table S2: JADAD scoring.
Author Contributions
Conceptualization, M.M., S.M. and V.R.; methodology, M.M. and V.R.; validation, M.M. and V.R.; formal analysis, M.M. and V.R.; investigation, M.M. and V.R.; data curation, M.M.; writing—original draft preparation, M.M.; writing—review and editing, M.M., A.R.-M., S.M. and V.R.; visualization, M.M. and A.R.-M.; supervision, V.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in FigShare at https://doi.org/10.6084/m9.figshare.24411967.
Acknowledgments
Elizabeth Opara provided advice on the interpretation of the data.
Conflicts of Interest
Authors M.M., V.R. and S.M. are employed by Pukka Herbs Ltd.
References
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef] [PubMed]
- Alkhatib, D.H.; Jaleel, A.; Tariq, M.N.M.; Feehan, J.; Apostolopoulos, V.; Cheikh Ismail, L.; Stojanovska, L.; Al Dhaheri, A.S. The role of bioactive compounds from dietary spices in the management of metabolic syndrome: An overview. Nutrients 2021, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Pallauf, K.; Giller, K.; Huebbe, P.; Rimbach, G. Nutrition and healthy ageing: Calorie restriction or polyphenol-rich “MediterrAsian” diet? Oxid. Med. Cell Longev. 2013, 2013, 707421. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, Z.; Golzarand, M.; Mirmiran, P.; Saadati, N.; Azizi, F. The association of dietary phytochemical index and cardiometabolic risk factors in adults: Tehran Lipid and Glucose Study. J. Hum. Nutr. Diet. 2013, 26 (Suppl. 1), 145–153. [Google Scholar] [CrossRef]
- Dzah, C.S.; Asante-Donyinah, D.; Letsyo, E.; Dzikunoo, J.; Adams, Z.S. Dietary polyphenols and obesity: A review of polyphenol effects on lipid and glucose metabolism, mitochondrial homeostasis, and starch digestibility and absorption. Plant Foods Hum. Nutr. 2022, 78, 1–12. [Google Scholar] [CrossRef]
- Haldar, S.; Chia, S.C.; Henry, C.J. Polyphenol-rich curry made with mixed spices and vegetables increases postprandial plasma GLP-1 concentration in a dose-dependent manner. Eur. J. Clin. Nutr. 2018, 72, 297–300. [Google Scholar] [CrossRef]
- Huang, Y.; Tsai, M.F.; Thorat, R.S.; Xiao, D.; Zhang, X.; Sandhu, A.K.; Edirisinghe, I.; Burton-Freeman, B.M. Endothelial function and postprandial glucose control in response to test-meals containing herbs and spices in adults with overweight/obesity. Front. Nutr. 2022, 9, 811433. [Google Scholar] [CrossRef]
- Kroff, J.; Hume, D.J.; Pienaar, P.; Tucker, R.; Lambert, E.V.; Rae, D.E. The metabolic effects of a commercially available chicken peri-peri (African bird’s eye chilli) meal in overweight individuals. Br. J. Nutr. 2017, 117, 635–644. [Google Scholar] [CrossRef]
- McCrea, C.E.; West, S.G.; Kris-Etherton, P.M.; Lambert, J.D.; Gaugler, T.L.; Teeter, D.L.; Sauder, K.A.; Gu, Y.; Glisan, S.L.; Skulas-Ray, A.C. Effects of culinary spices and psychological stress on postprandial lipemia and lipase activity: Results of a randomized crossover study and in vitro experiments. J. Transl. Med. 2015, 13, 7. [Google Scholar] [CrossRef]
- Nakayama, H.; Tsuge, N.; Sawada, H.; Masamura, N.; Yamada, S.; Satomi, S.; Higashi, Y. A single consumption of curry improved postprandial endothelial function in healthy male subjects: A randomized, controlled crossover trial. Nutr. J. 2014, 13, 67. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Petersen, K.S.; Kris-Etherton, P.M.; Rogers, C.J. Spices in a high-saturated-fat, high-carbohydrate meal reduce postprandial proinflammatory cytokine secretion in men with overweight or obesity: A 3-period, crossover, randomized controlled trial. J. Nutr. 2020, 150, 1600–1609. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Rogers, C.J.; West, S.G.; Proctor, D.N.; Kris-Etherton, P.M. The effect of culinary doses of spices in a high-saturated fat, high-carbohydrate meal on postprandial lipemia and endothelial function: A randomized, controlled, crossover pilot trial. Food Funct. 2020, 11, 3191–3200. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.S.; Davis, K.M.; Rogers, C.J.; Proctor, D.N.; West, S.G.; Kris-Etherton, P.M. Herbs and spices at a relatively high culinary dosage improves 24-hour ambulatory blood pressure in adults at risk of cardiometabolic diseases: A randomized, crossover, controlled-feeding study. Am. J. Clin. Nutr. 2021, 114, 1936–1948. [Google Scholar] [CrossRef]
- Zanzer, Y.C.; Plaza, M.; Dougkas, A.; Turner, C.; Östman, E. Black pepper-based beverage induced appetite-suppressing effects without altering postprandial glycaemia, gut and thyroid hormones or gastrointestinal well-being: A randomized crossover study in healthy subjects. Food Funct. 2018, 9, 2774–2786. [Google Scholar] [CrossRef]
- Peters, M.D.J.; Marnie, C.; Colquhoun, H.; Garritty, C.M.; Hempel, S.; Horsley, T.; Langlois, E.V.; Lillie, E.; O’Brien, K.K.; Tunçalp, Ö.; et al. Scoping reviews: Reinforcing and advancing the methodology and application. Syst. Rev. 2021, 10, 263. [Google Scholar] [CrossRef]
- Cacchione, P.Z. The evolving methodology of scoping reviews. Clin. Nurs. Res. 2016, 25, 115–119. [Google Scholar] [CrossRef]
- Agarwal, A.K. Spice up your life: Adipose tissue and inflammation. J. Lipids 2014, 2014, 182575. [Google Scholar] [CrossRef]
- Akhter, S. Low to no cost remedies for the management of diabetes mellitus; global health concern. J. Diabetes Metab. Disord. 2021, 20, 951–962. [Google Scholar] [CrossRef]
- Bower, A.; Marquez, S.; de Mejia, E.G. The health benefits of selected culinary herbs and spices found in the traditional mediterranean diet. Crit. Rev. Food Sci. Nutr. 2016, 56, 2728–2746. [Google Scholar] [CrossRef]
- Deekshith, C.; Jois, M.; Radcliffe, J.; Thomas, J. Effects of culinary herbs and spices on obesity: A systematic literature review of clinical trials. J. Funct. Foods 2021, 81, 104449. [Google Scholar] [CrossRef]
- Gupta, K.; Testa, H.; Greenwood, T.; Kostek, M.; Haushalter, K.; Kris-Etherton, P.M.; Petersen, K.S. The effect of herbs and spices on risk factors for cardiometabolic diseases: A review of human clinical trials. Nutr. Rev. 2022, 80, 400–427. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.I. Culinary herbs and spices: What can human studies tell us about their role in the prevention of chronic non-communicable diseases? J. Sci. Food Agric. 2019, 99, 4511–4517. [Google Scholar] [CrossRef] [PubMed]
- Opara, E.I.; Chohan, M. Culinary herbs and spices: Their bioactive properties, the contribution of polyphenols and the challenges in deducing their true health benefits. Int. J. Mol. Sci. 2014, 15, 19183–19202. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Olivo, S.A.; Macedo, L.G.; Gadotti, I.C.; Fuentes, J.; Stanton, T.; Magee, D.J. Scales to assess the quality of randomized controlled trials: A systematic review. Phys. Ther. 2008, 88, 156–175. [Google Scholar] [CrossRef]
- Verma, S.K.; Jain, V.; Katewa, S.S. Blood pressure lowering, fibrinolysis enhancing and antioxidant activities of cardamom (Elettaria cardamomum). Indian J. Biochem. Biophys. 2009, 46, 503–506. [Google Scholar]
- Kazemi, S.; Yaghooblou, F.; Siassi, F.; Rahimi Foroushani, A.; Ghavipour, M.; Koohdani, F.; Sotoudeh, G. Cardamom supplementation improves inflammatory and oxidative stress biomarkers in hyperlipidemic, overweight, and obese pre-diabetic women: A randomized double-blind clinical trial. J. Sci. Food Agric. 2017, 97, 5296–5301. [Google Scholar] [CrossRef]
- Aghasi, M.; Koohdani, F.; Qorbani, M.; Nasli-Esfahani, E.; Ghazi-Zahedi, S.; Khoshamal, H.; Keshavarz, A.; Sotoudeh, G. Beneficial effects of green cardamom on serum SIRT1, glycemic indices and triglyceride levels in patients with type 2 diabetes mellitus: A randomized double-blind placebo controlled clinical trial. J. Sci. Food Agric. 2019, 99, 3933–3940. [Google Scholar] [CrossRef]
- Daneshi-Maskooni, M.; Keshavarz, S.A.; Qorbani, M.; Mansouri, S.; Alavian, S.M.; Badri-Fariman, M.; Jazayeri-Tehrani, S.A.; Sotoudeh, G. Green cardamom supplementation improves serum irisin, glucose indices, and lipid profiles in overweight or obese non-alcoholic fatty liver disease patients: A double-blind randomized placebo-controlled clinical trial. BMC Complement. Altern. Med. 2019, 19, 59. [Google Scholar] [CrossRef]
- Ghazi Zahedi, S.; Koohdani, F.; Qorbani, M.; Nasli-Esfahani, E.; Aghasi, M.; Khoshamal, H.; Keshavarz, A.; Sotoudeh, G. Effects of green cardamom supplementation on serum levels of Hs-CRP, dimethylarginine, nitric oxide and blood pressure in patients with type 2 diabetes: A randomized, double-blind, placebo controlled, clinical trial. J. Herb. Med. 2022, 32, 100555. [Google Scholar] [CrossRef]
- Ghazi Zahedi, S.; Koohdani, F.; Qorbani, M.; Nasli-Esfahani, E.; Aghasi, M.; Khoshamal, H.; Sotoudeh, G. The effects of Elettaria cardamom supplementation on inflammatory markers and vascular function in patients with type 2 diabetes mellitus: A mechanism -based randomized clinical trial. J. Herb. Med. 2021, 25, 100403. [Google Scholar] [CrossRef]
- Daneshi-Maskooni, M.; Keshavarz, S.A.; Qorbani, M.; Mansouri, S.; Alavian, S.M.; Badri-Fariman, M.; Jazayeri-Tehrani, S.A.; Sotoudeh, G. Green cardamom increases Sirtuin-1 and reduces inflammation in overweight or obese patients with non-alcoholic fatty liver disease: A double-blind randomized placebo-controlled clinical trial. Nutr. Metab. 2018, 15, 63. [Google Scholar] [CrossRef]
- Cheshmeh, S.; Elahi, N.; Ghayyem, M.; Mosaieby, E.; Moradi, S.; Pasdar, Y.; Tahmasebi, S.; Moradinazar, M. Effect of green cardamom on the expression of genes implicated in obesity and diabetes among obese women with polycystic ovary syndrome: A double blind randomized controlled trial. Genes. Nutr. 2022, 17, 17. [Google Scholar] [CrossRef] [PubMed]
- Cheshmeh, S.; Ghayyem, M.; Khamooshi, F.; Heidarzadeh-Esfahani, N.; Rahmani, N.; Hojati, N.; Mosaieby, E.; Moradi, S.; Pasdar, Y. Green cardamom plus low-calorie diet can decrease the expression of inflammatory genes among obese women with polycystic ovary syndrome: A double-blind randomized clinical trial. Eat. Weight. Disord. 2022, 27, 821–830. [Google Scholar] [CrossRef]
- Clegg, M.E.; Golsorkhi, M.; Henry, C.J. Combined medium-chain triglyceride and chilli feeding increases diet-induced thermogenesis in normal-weight humans. Eur. J. Nutr. 2013, 52, 1579–1585. [Google Scholar] [CrossRef]
- Ahuja, K.D.; Robertson, I.K.; Geraghty, D.P.; Ball, M.J. Effects of chili consumption on postprandial glucose, insulin, and energy metabolism. Am. J. Clin. Nutr. 2006, 84, 63–69. [Google Scholar] [CrossRef]
- Ahuja, K.D.; Ball, M.J. Effects of daily ingestion of chilli on serum lipoprotein oxidation in adult men and women. Br. J. Nutr. 2006, 96, 239–242. [Google Scholar] [CrossRef]
- Ahuja, K.D.; Robertson, I.K.; Geraghty, D.P.; Ball, M.J. The effect of 4-week chilli supplementation on metabolic and arterial function in humans. Eur. J. Clin. Nutr. 2007, 61, 326–333. [Google Scholar] [CrossRef]
- Reinbach, H.C.; Martinussen, T.; Møller, P. Effects of hot spices on energy intake, appetite and sensory specific desires in humans. Food Qual. Prefer. 2010, 21, 655–661. [Google Scholar] [CrossRef]
- Kang, C.; Zhang, Y.; Zhu, X.; Liu, K.; Wang, X.; Chen, M.; Wang, J.; Chen, H.; Hui, S.; Huang, L.; et al. Healthy subjects differentially respond to dietary capsaicin correlating with specific gut enterotypes. J. Clin. Endocrinol. Metab. 2016, 101, 4681–4689. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.J.; Qin, Y.; Wang, L.; Zeng, Y.; Chang, H.; Wang, J.; Chen, S.-H.; Zhang, Q.-Y.; Zhu, J.-D.; Zhou, Y.; et al. Capsaicin-containing chili improved postprandial hyperglycemia, hyperinsulinemia, and fasting lipid disorders in women with gestational diabetes mellitus and lowered the incidence of large-for-gestational-age newborns. Clin. Nutr. 2016, 35, 388–393. [Google Scholar] [CrossRef]
- Chaiyasit, K.; Khovidhunkit, W.; Wittayalertpanya, S. Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai. 2009, 92, 108–113. [Google Scholar] [PubMed]
- Janssens, P.L.; Hursel, R.; Martens, E.A.; Westerterp-Plantenga, M.S. Acute effects of capsaicin on energy expenditure and fat oxidation in negative energy balance. PLoS ONE 2013, 8, e67786. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.L.; Hursel, R.; Westerterp-Plantenga, M.S. Capsaicin increases sensation of fullness in energy balance, and decreases desire to eat after dinner in negative energy balance. Appetite 2014, 77, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T.P.; Blannin, A.K. Changes in glucose tolerance and insulin sensitivity following 2 weeks of daily cinnamon ingestion in healthy humans. Eur. J. Appl. Physiol. 2009, 105, 969–976. [Google Scholar] [CrossRef]
- Vanschoonbeek, K.; Thomassen, B.J.; Senden, J.M.; Wodzig, W.K.; van Loon, L.J. Cinnamon supplementation does not improve glycemic control in postmenopausal type 2 diabetes patients. J. Nutr. 2006, 136, 977–980. [Google Scholar] [CrossRef]
- Solomon, T.P.; Blannin, A.K. Effects of short-term cinnamon ingestion on in vivo glucose tolerance. Diabetes Obes. Metab. 2007, 9, 895–901. [Google Scholar] [CrossRef]
- Hlebowicz, J.; Hlebowicz, A.; Lindstedt, S.; Björgell, O.; Höglund, P.; Holst, J.J.; Darwiche, G.; Almér, L.-O. Effects of 1 and 3 g cinnamon on gastric emptying, satiety, and postprandial blood glucose, insulin, glucose-dependent insulinotropic polypeptide, glucagon-like peptide 1, and ghrelin concentrations in healthy subjects. Am. J. Clin. Nutr. 2009, 89, 815–821. [Google Scholar] [CrossRef]
- Markey, O.; McClean, C.M.; Medlow, P.; Davison, G.W.; Trinick, T.R.; Duly, E.; Shafat, A. Effect of cinnamon on gastric emptying, arterial stiffness, postprandial lipemia, glycemia, and appetite responses to high-fat breakfast. Cardiovasc. Diabetol. 2011, 10, 78. [Google Scholar] [CrossRef]
- Wickenberg, J.; Lindstedt, S.; Berntorp, K.; Nilsson, J.; Hlebowicz, J. Ceylon cinnamon does not affect postprandial plasma glucose or insulin in subjects with impaired glucose tolerance. Br. J. Nutr. 2012, 107, 1845–1849. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.L.; Bowden, R.G.; Willoughby, D.S. Cassia cinnamon supplementation reduces peak blood glucose responses but does not improve insulin resistance and sensitivity in young, sedentary, obese women. J. Diet. Suppl. 2016, 13, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Sahib, A.S. Anti-diabetic and antioxidant effect of cinnamon in poorly controlled type-2 diabetic Iraqi patients: A randomized, placebo-controlled clinical trial. J. Intercult. Ethnopharmacol. 2016, 5, 108–113. [Google Scholar] [CrossRef]
- Magistrelli, A.; Chezem, J.C. Effect of ground cinnamon on postprandial blood glucose concentration in normal-weight and obese adults. J. Acad. Nutr. Diet. 2012, 112, 1806–1809. [Google Scholar] [CrossRef]
- Bernardo, M.A.; Silva, M.L.; Santos, E.; Moncada, M.M.; Brito, J.; Proença, L.; Singh, J.; de Mesquita, M.F. Effect of cinnamon tea on postprandial glucose concentration. J. Diabetes Res. 2015, 2015, 913651. [Google Scholar] [CrossRef] [PubMed]
- Hlebowicz, J.; Darwiche, G.; Björgell, O.; Almér, L.O. Effect of cinnamon on postprandial blood glucose, gastric emptying, and satiety in healthy subjects. Am. J. Clin. Nutr. 2007, 85, 1552–1556. [Google Scholar] [CrossRef]
- Atkinson, F.S.; Stockmann, K.S.; Ek, K.L.; Brand-Miller, J.C. Cassia but not cinnamon reduces postprandial glucose and insulin responses to oatmeal in lean, young adults. Asia Pacific J. Clin. Nutr. 2008, 17, S137. [Google Scholar]
- Kort, D.H.; Lobo, R.A. Preliminary evidence that cinnamon improves menstrual cyclicity in women with polycystic ovary syndrome: A randomized controlled trial. Am. J. Obstet. Gynecol. 2014, 211, e1–e6. [Google Scholar] [CrossRef]
- Crawford, P. Effectiveness of cinnamon for lowering hemoglobin A1C in patients with type 2 diabetes: A randomized, controlled trial. J. Am. Board. Fam. Med. 2009, 22, 507–512. [Google Scholar] [CrossRef]
- Suppapitiporn, S.; Kanpaksi, N.; Suppapitiporn, S. The effect of cinnamon cassia powder in type 2 diabetes mellitus. J. Med. Assoc. Thai. 2006, 89 (Suppl. 3), S200–S205. [Google Scholar]
- Blevins, S.M.; Leyva, M.J.; Brown, J.; Wright, J.; Scofield, R.H.; Aston, C.E. Effect of cinnamon on glucose and lipid levels in non insulin-dependent type 2 diabetes. Diabetes Care 2007, 30, 2236–2237. [Google Scholar] [CrossRef] [PubMed]
- Askari, F.; Rashidkhani, B.; Hekmatdoost, A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr. Res. 2014, 34, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Talaei, B.; Amouzegar, A.; Sahranavard, S.; Hedayati, M.; Mirmiran, P.; Azizi, F. Effects of cinnamon consumption on glycemic indicators, advanced glycation end products, and antioxidant status in type 2 diabetic patients. Nutrients 2017, 9, 991. [Google Scholar] [CrossRef]
- Mirmiran, P.; Davari, M.; Hashemi, R.; Hedayati, M.; Sahranavard, S.; Bahreini, S.; Tavakoly, R.; Talaei, B. A randomized controlled trial to determining the effect of cinnamon on the plasma levels of soluble forms of vascular adhesion molecules in type 2 diabetes mellitus. Eur. J. Clin. Nutr. 2019, 73, 1605–1612. [Google Scholar] [CrossRef]
- Khan, A.A.; Begum, W. Efficacy of Darchini in the management of polycystic ovarian syndrome: A randomized clinical study. J. Herb. Med. 2019, 15, 100249. [Google Scholar] [CrossRef]
- Davari, M.; Hashemi, R.; Mirmiran, P.; Hedayati, M.; Sahranavard, S.; Bahreini, S.; Tavakoly, R.; Talaei, B. Effects of cinnamon supplementation on expression of systemic inflammation factors, NF-kB and sirtuin-1 (SIRT1) in type 2 diabetes: A randomized, double blind, and controlled clinical trial. Nutr. J. 2020, 19, 1. [Google Scholar] [CrossRef]
- Altschuler, J.A.; Casella, S.J.; MacKenzie, T.A.; Curtis, K.M. The effect of cinnamon on A1C among adolescents with type 1 diabetes. Diabetes Care 2007, 30, 813–816. [Google Scholar] [CrossRef]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Glycated haemoglobin and blood pressure-lowering effect of cinnamon in multi-ethnic Type 2 diabetic patients in the UK: A randomized, placebo-controlled, double-blind clinical trial. Diabet. Med. 2010, 27, 1159–1167. [Google Scholar] [CrossRef]
- Wainstein, J.; Stern, N.; Heller, S.; Boaz, M. Dietary cinnamon supplementation and changes in systolic blood pressure in subjects with type 2 diabetes. J. Med. Food 2011, 14, 1505–1510. [Google Scholar] [CrossRef]
- Zahedifar, A.; Khodashenas, M.; Bijari, B.; Zahedifar, F. Effects of cinnamon on fasting blood sugar and hemoglobin A1C in patients with type II diabetes mellitus: A randomized clinical trial. [In Persian]. J. Mazandaran Univ. Med. Sci. 2018, 27, 80–88. [Google Scholar]
- Zahmatkesh, M.; Fallah Huseini, H.; Hajiaghaee, R.; Heidari, M.; Mehrafarin, A.; Tavakoli-far, B. The effects of Cinnamomum zeylanicum J. Presl on blood glucose level in patients with type 2 diabetes, a double-blind clinical trial. J. Med. Plants 2012, 11 (Suppl. 8), 258–263. [Google Scholar]
- Borzoei, A.; Rafraf, M.; Niromanesh, S.; Farzadi, L.; Narimani, F.; Doostan, F. Effects of cinnamon supplementation on antioxidant status and serum lipids in women with polycystic ovary syndrome. J. Tradit. Complement. Med. 2017, 8, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Borzoei, A.; Rafraf, M.; Asghari-Jafarabadi, M. Cinnamon improves metabolic factors without detectable effects on adiponectin in women with polycystic ovary syndrome. Asia Pac. J. Clin. Nutr. 2018, 27, 556–563. [Google Scholar]
- Mirfeizi, M.; Mehdizadeh Tourzani, Z.; Mirfeizi, S.Z.; Asghari Jafarabadi, M.; Rezvani, H.R.; Afzali, M. Controlling type 2 diabetes mellitus with herbal medicines: A triple-blind randomized clinical trial of efficacy and safety. J. Diabetes. 2016, 8, 647–656. [Google Scholar] [CrossRef]
- Mirmiranpour, H.; Huseini, H.F.; Derakhshanian, H.; Khodaii, Z.; Tavakoli-Far, B. Effects of probiotic, cinnamon, and synbiotic supplementation on glycemic control and antioxidant status in people with type 2 diabetes; a randomized, double-blind, placebo-controlled study. J. Diabetes Metab. Disord. 2019, 19, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Safdar, M.; Ali Khan, M.M.; Khattak, K.N.; Anderson, R.A. Cinnamon improves glucose and lipids of people with type 2 diabetes. Diabetes Care 2003, 26, 3215–3218. [Google Scholar] [CrossRef]
- Gupta Jain, S.; Puri, S.; Misra, A.; Gulati, S.; Mani, K. Effect of oral cinnamon intervention on metabolic profile and body composition of Asian Indians with metabolic syndrome: A randomized double -blind control trial. Lipids Health Dis. 2017, 16, 113. [Google Scholar] [CrossRef]
- Lira Neto, J.C.G.; Damasceno, M.M.C.; Ciol, M.A.; de Freitas, R.W.J.F.; de Araújo, M.F.M.; Teixeira, C.R.S.; Carvalho, G.C.N.; Lisboa, K.W.S.C.; Marques, R.L.L.; Alencar, A.M.P.G.; et al. Efficacy of cinnamon as an adjuvant in reducing the glycemic biomarkers of type 2 diabetes mellitus: A three-month, randomized, triple-blind, placebo-controlled clinical trial. J. Am. Nutr. Assoc. 2022, 41, 266–274. [Google Scholar] [CrossRef]
- Zare, R.; Nadjarzadeh, A.; Zarshenas, M.M.; Shams, M.; Heydari, M. Efficacy of cinnamon in patients with type II diabetes mellitus: A randomized controlled clinical trial. Clin. Nutr. 2019, 38, 549–556. [Google Scholar] [CrossRef]
- Hajimonfarednejad, M.; Nimrouzi, M.; Heydari, M.; Zarshenas, M.M.; Raee, M.J.; Jahromi, B.N. Insulin resistance improvement by cinnamon powder in polycystic ovary syndrome: A randomized double-blind placebo controlled clinical trial. Phytother. Res. 2018, 32, 276–283. [Google Scholar] [CrossRef]
- Soares, A.P.D.C.; de Faria, N.C.; Graciano, G.F.; Dos Santos, A.L.S.; Valenzuela, V.D.C.; Toulson Davisson Correia, M.I.; Cosenza, G.P.; Anastácio, L.R. Cinnamon infusion reduces satiety and increases energy intake: A randomized crossover trial. Ann. Nutr. Metab. 2022, 78, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Mettler, S.; Schwarz, I.; Colombani, P.C. Additive postprandial blood glucose-attenuating and satiety-enhancing effect of cinnamon and acetic acid. Nutr. Res. 2009, 29, 723–727. [Google Scholar] [CrossRef] [PubMed]
- Dastgheib, M.; Barati-Boldaji, R.; Bahrampour, N.; Taheri, R.; Borghei, M.; Amooee, S.; Mohammadi-Sartang, M.; Wong, A.; Babajafari, S.; Mazloomi, S.M. A comparison of the effects of cinnamon, ginger, and metformin consumption on metabolic health, anthropometric indices, and sexual hormone levels in women with poly cystic ovary syndrome: A randomized double-blinded placebo-controlled clinical trial. Front. Nutr. 2022, 9, 1071515. [Google Scholar] [CrossRef] [PubMed]
- Azimi, P.; Ghiasvand, R.; Feizi, A.; Hosseinzadeh, J.; Bahreynian, M.; Hariri, M.; Khosravi-Boroujeni, H. Effect of cinnamon, cardamom, saffron and ginger consumption on blood pressure and a marker of endothelial function in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Blood Press. 2016, 25, 133–140. [Google Scholar] [CrossRef]
- Zare, R.; Heshmati, F.; Fallahzadeh, H.; Nadjarzadeh, A. Effect of cumin powder on body composition and lipid profile in overweight and obese women. Complement. Ther. Clin. Pract. 2014, 20, 297–301. [Google Scholar] [CrossRef]
- Pishdad, S.; Nadjarzadeh, A.; Salehi Abargouei, A.; Karimi Nazari, E.; Papoli, M. Effect of cumin and cinnamon on lipid profile in middle-aged women with dyslipidemia: A double blind, randomized controlled clinical trial. Progr. Nutr. 2018, 20, 232–237. [Google Scholar]
- Bae, J.; Kim, J.; Choue, R.; Lim, H. Fennel (Foeniculum vulgare) and fenugreek (Trigonella foenum-graecum) tea drinking suppresses subjective short-term appetite in overweight women. Clin. Nutr. Res. 2015, 4, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, P.; Rajyalakshmi, P. Hypocholesterolemic effect of germinated fenugreek seeds in human subjects. Plant Foods Hum. Nutr. 1999, 53, 359–365. [Google Scholar] [CrossRef]
- Sharma, R.D.; Raghuram, T.C.; Rao, N.S. Effect of fenugreek seeds on blood glucose and serum lipids in type I diabetes. Eur. J. Clin. Nutr. 1990, 44, 301–306. [Google Scholar]
- Madar, Z.; Abel, R.; Samish, S.; Arad, J. Glucose-lowering effect of fenugreek in non-insulin dependent diabetics. Eur. J. Clin. Nutr. 1988, 42, 51–54. [Google Scholar]
- Kiss, R.; Szabó, K.; Gesztelyi, R.; Somodi, S.; Kovács, P.; Szabó, Z.; Németh, J.; Priksz, D.; Kurucz, A.; Juhász, B.; et al. Insulin-sensitizer effects of fenugreek seeds in parallel with changes in plasma MCH levels in healthy volunteers. Int. J. Mol. Sci. 2018, 19, 771. [Google Scholar] [CrossRef] [PubMed]
- Gopalpura, P.B.; Jayanthi, C.; Dubey, S. Effect of Trigonella foenum-graecum seeds on the glycemic index of food: A clinical evaluation. Int. J. Diabetes Dev. Ctries. 2007, 27, 41–45. [Google Scholar] [CrossRef]
- Kassaian, N.; Azadbakht, L.; Forghani, B.; Amini, M. Effect of fenugreek seeds on blood glucose and lipid profiles in type 2 diabetic patients. Int. J. Vitam. Nutr. Res. 2009, 79, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Losso, J.N.; Holliday, D.L.; Finley, J.W.; Martin, R.J.; Rood, J.C.; Yu, Y.; Greenway, F.L. Fenugreek bread: A treatment for diabetes mellitus. J. Med. Food. 2009, 12, 1046–1049. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.D.; Ismail, A.A.; Rosli, W.I. Reduction of postprandial blood glucose in healthy subjects by buns and flatbreads incorporated with fenugreek seed powder. Eur. J. Nutr. 2016, 55, 2275–2280. [Google Scholar] [CrossRef]
- Najdi, R.A.; Hagras, M.M.; Kamel, F.O.; Magadmi, R.M. A randomized controlled clinical trial evaluating the effect of Trigonella foenum-graecum (fenugreek) versus glibenclamide in patients with diabetes. Afr. Health Sci. 2019, 19, 1594–1601. [Google Scholar] [CrossRef]
- Tavakoly, R.; Maracy, M.R.; Karimifar, M.; Entezari, M.H. Does fenugreek (Trigonella foenum-graecum) seed improve inflammation, and oxidative stress in patients with type 2 diabetes mellitus? A parallel group randomized clinical trial. Eur. J. Integr. Med. 2018, 18, 13–17. [Google Scholar] [CrossRef]
- Sharma, R.D.; Sarkar, A.; Hazra, D.K.; Misra, B.; Singh, J.B.; Maheshwari, B.B.; Sharma, S.K. Hypolipidaemic effect of fenugreek seeds: A chronic study in non-insulin dependent diabetic patients. Phytother. Res. 1996, 10, 332–334. [Google Scholar] [CrossRef]
- Bhadauria, S.S.; Kushwah, A. Fenugreek seeds as a therapeutic supplement for patients with noninsulin dependent diabetes mellitus: A cross-sectional study. J. Clin. Diagn. Res. 2021, 15, BC21–BC23. [Google Scholar] [CrossRef]
- Hadi, A.; Arab, A.; Hajianfar, H.; Talaei, B.; Miraghajani, M.; Babajafari, S.; Marx, W.; Tavakoly, R. The effect of fenugreek seed supplementation on serum irisin levels, blood pressure, and liver and kidney function in patients with type 2 diabetes mellitus: A parallel randomized clinical trial. Complement. Ther. Med. 2020, 49, 102315. [Google Scholar] [CrossRef]
- Geberemeskel, G.A.; Debebe, Y.G.; Nguse, N.A. Antidiabetic effect of fenugreek seed powder solution (Trigonella foenum-graecum L.) on hyperlipidemia in diabetic patients. J. Diabetes Res. 2019, 2019, 8507453. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, E.; Zareiy, S.; Zavoshy, R.; Noroozi, M.; Jahanihashemi, H.; Ardalani, H. Fenugreek: A therapeutic complement for patients with borderline hyperlipidemia: A randomised, double-blind, placebo-controlled, clinical trial. Adv. Integr. Med. 2017, 4, 31–35. [Google Scholar] [CrossRef]
- Rafraf, M.; Malekiyan, M.; Asghari-Jafarabadi, M.; Aliasgarzadeh, A. Effect of fenugreek seeds on serum metabolic factors and adiponectin levels in type 2 diabetic patients. Int. J. Vitam. Nutr. Res. 2014, 84, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Hassani, S.S.; Arezodar, F.F.; Esmaeili, S.S.; Gholami-Fesharaki, M. The effect of combined therapy with fenugreek and nutrition training based on iranian traditional medicine on FBS, HGA1C, BMI, and waist circumference in type 2 diabetic patients: A randomized double-blinded clinical trial. J. Adv. Med. Biomed. Res. 2019, 27, 37–42. [Google Scholar] [CrossRef]
- Hassani, S.S.; Fallahi Arezodar, F.; Esmaeili, S.S.; Gholami-Fesharaki, M. Effect of fenugreek use on fasting blood glucose, glycosylated hemoglobin, body mass index, waist circumference, blood pressure and quality of life in patients with type 2 diabetes mellitus: A randomized, double-blinded, placebo-controlled clinical trials. Galen. Med. J. 2019, 8, e1432. [Google Scholar] [CrossRef]
- Sharma, R.D. Effect of fenugreek seeds and leaves on blood glucose and serum insulin responses in human subjects. Nutr. Res. 1986, 6, 1353–1364. [Google Scholar] [CrossRef]
- Choudhary, P.R.; Jani, R.D.; Sharma, M.S. Effect of raw crushed garlic (Allium sativum L.) on components of metabolic syndrome. J. Diet Suppl. 2018, 15, 499–506. [Google Scholar] [CrossRef]
- Bakhsh, R.; Chughtai, M.I. Influence of garlic on serum cholesterol, serum triglycerides, serum total lipids and serum glucose in human subjects. Food/Nahrung 1984, 28, 159–163. [Google Scholar] [CrossRef]
- Roberts, K.; Jahner, D.K.W.; Buddington, R.K. Influence of garlic supplementation on human fecal flora and serum lipid levels. FASEB J. 1998, 12, A876. [Google Scholar]
- Scharbert, G.; Kalb, M.L.; Duris, M.; Marschalek, C.; Kozek-Langenecker, S.A. Garlic at dietary doses does not impair platelet function. Anesth. Analg. 2007, 105, 1214–1218. [Google Scholar] [CrossRef]
- Aslani, N.; Entezari, M.H.; Askari, G.; Maghsoudi, Z.; Maracy, M.R. Effect of garlic and lemon juice mixture on lipid profile and some cardiovascular risk factors in people 30–60 years old with moderate hyperlipidaemia: A randomized clinical trial. Int. J. Prev. Med. 2016, 7, 95. [Google Scholar] [PubMed]
- van Doorn, M.B.; Espirito Santo, S.M.; Meijer, P.; Kamerling, I.M.; Schoemaker, R.C.; Dirsch, V.; Vollmar, A.; Haffner, T.; Gebhardt, R.; Cohen, A.F.; et al. Effect of garlic powder on C-reactive protein and plasma lipids in overweight and smoking subjects. Am. J. Clin. Nutr. 2006, 84, 1324–1329. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Mohammad Hosseini Azar, M.R.; Alizadeh, M. Effect of garlic powder supplementation on hepatic steatosis, liver enzymes and lipid profile in patients with non-alcoholic fatty liver disease: A double-blind randomised controlled clinical trial. Br. J. Nutr. 2020, 124, 450–456. [Google Scholar] [CrossRef]
- Sangouni, A.A.; Mohammad Hosseini Azar, M.R.; Alizadeh, M. Effects of garlic powder supplementation on insulin resistance, oxidative stress, and body composition in patients with non-alcoholic fatty liver disease: A randomized controlled clinical trial. Complement. Ther. Med. 2020, 51, 102428. [Google Scholar] [CrossRef] [PubMed]
- Sangouni, A.A.; Alizadeh, M.; Jamalzehi, A.; Parastouei, K. Effects of garlic powder supplementation on metabolic syndrome components, insulin resistance, fatty liver index, and appetite in subjects with metabolic syndrome: A randomized clinical trial. Phytother. Res. 2021, 35, 4433–4441. [Google Scholar] [CrossRef]
- Zeb, F.; Safdar, M.; Fatima, S.; Khan, S.; Alam, S.; Muhammad, M.; Syed, A.; Habib, F.; Shakoor, H. Supplementation of garlic and coriander seed powder: Impact on body mass index, lipid profile and blood pressure of hyperlipidemic patients. Pak. J. Pharm. Sci. 2018, 31, 1935–1941. [Google Scholar] [PubMed]
- Kamari, N.; Moradinazar, M.; Qasemi, M.; Khosravy, T.; Samadi, M.; Abdolahzad, H. Combination of the effect of ginger and anti-inflammatory diet on children with obesity with nonalcoholic fatty liver disease: A randomized clinical trial. Food Sci. Nutr. 2023, 11, 1846–1859. [Google Scholar] [CrossRef]
- Miyamoto, M.; Matsuzaki, K.; Katakura, M.; Hara, T.; Tanabe, Y.; Shido, O. Oral intake of encapsulated dried ginger root powder hardly affects human thermoregulatory function, but appears to facilitate fat utilization. Int. J. Biometeorol. 2015, 59, 1461–1474. [Google Scholar] [CrossRef]
- Janssen, P.L.; Meyboom, S.; van Staveren, W.A.; de Vegt, F.; Katan, M.B. Consumption of ginger (Zingiber officinale roscoe) does not affect ex vivo platelet thromboxane production in humans. Eur. J. Clin. Nutr. 1996, 50, 772–774. [Google Scholar]
- Mansour, M.S.; Ni, Y.M.; Roberts, A.L.; Kelleman, M.; Roychoudhury, A.; St-Onge, M.P. Ginger consumption enhances the thermic effect of food and promotes feelings of satiety without affecting metabolic and hormonal parameters in overweight men: A pilot study. Metabolism 2012, 61, 1347–1352. [Google Scholar] [CrossRef]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Zarezadeh, M.; Saedisomeolia, A.; Khorshidi, M.; Kord Varkane, H.; Makhdoomi Arzati, M.; Abdollahi, M.; Yekaninejad, M.S.; Hashemi, R.; Effatpanah, M.; Honarvar, N.M. Asymmetric dimethylarginine and soluble inter-cellular adhesion molecule-1 serum levels alteration following ginger supplementation in patients with type 2 diabetes: A randomized double-blind, placebo-controlled clinical trial. J. Complement. Integr. Med. 2018, 16, 20180019. [Google Scholar] [CrossRef] [PubMed]
- Rafie Hosseini, S.A.; Hajiani, E.; Saki Malehi, A.; Mard, S.A. Effect of ginger powder supplementation in patients with non-alcoholic fatty liver disease: A randomized clinical trial. Clin. Exp. Gastroenterol. 2020, 13, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh Honarvar, N.; Zarezadeh, M.; Khorshidi, M.; Makhdoomi Arzati, M.; Yekaninejad, M.S.; Abdollahi, M.; Effatpanah, M.; Hashemi, R.; Saedisomeolia, A. The effect of an oral ginger supplementation on NF-κB concentration in peripheral blood mononuclear cells and anthropomorphic data of patients with type 2 diabetes: A randomized double-blind, placebo-controlled clinical trial. Complement. Ther. Med. 2019, 42, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Mahluji, S.; Ostadrahimi, A.; Mobasseri, M.; Ebrahimzade Attari, V.; Payahoo, L. Anti-inflammatory effects of Zingiber officinale in type 2 diabetic patients. Adv. Pharm. Bull. 2013, 3, 273–276. [Google Scholar]
- Mahluji, S.; Attari, V.E.; Mobasseri, M.; Payahoo, L.; Ostadrahimi, A.; Golzari, S.E. Effects of ginger (Zingiber officinale) on plasma glucose level, HbA1c and insulin sensitivity in type 2 diabetic patients. Int. J. Food Sci. Nutr. 2013, 64, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Mozaffari-Khosravi, H.; Talaei, B.; Jalali, B.A.; Najarzadeh, A.; Mozayan, M.R. The effect of ginger powder supplementation on insulin resistance and glycemic indices in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Complement. Ther. Med. 2014, 22, 9–16. [Google Scholar] [CrossRef]
- Ebrahimzadeh Attari, V.; Asghari Jafarabadi, M.; Zemestani, M.; Ostadrahimi, A. Effect of Zingiber officinale supplementation on obesity management with respect to the uncoupling protein 1 -3826A>G and ß3-adrenergic receptor Trp64Arg polymorphism. Phytother. Res. 2015, 29, 1032–1039. [Google Scholar] [CrossRef]
- Hajimoosayi, F.; Jahanian Sadatmahalleh, S.; Kazemnejad, A.; Pirjani, R. Effect of ginger on the blood glucose level of women with gestational diabetes mellitus (GDM) with impaired glucose tolerance test (GTT): A randomized double-blind placebo-controlled trial. BMC Complement. Med. Ther. 2020, 20, 116. [Google Scholar] [CrossRef]
- Ebrahimzadeh Attari, V.; Mahluji, S.; Asghari Jafarabadi, M.; Ostadrahimi, A. Effects of supplementation with ginger (Zingiber officinale Roscoe) on serum glucose, lipid profile and oxidative stress in obese women: A randomized, placebo-controlled clinical trial. Pharm. Sci. 2015, 21, 184–191. [Google Scholar] [CrossRef]
- Ashraf, H.; Heydari, M.; Shams, M.; Zarshenas, M.M.; Tavakoli, A.; Sayadi, M. Efficacy of ginger supplementation in relieving persistent hypothyroid symptoms in patients with controlled primary hypothyroidism: A pilot randomized, double-blind, placebo-controlled clinical trial. Evid. Based Complement. Alt. Med. 2022, 2022, 5456855. [Google Scholar] [CrossRef] [PubMed]
- Arablou, T.; Aryaeian, N.; Valizadeh, M.; Sharifi, F.; Hosseini, A.; Djalali, M. The effect of ginger consumption on glycemic status, lipid profile and some inflammatory markers in patients with type 2 diabetes mellitus. Int. J. Food Sci. Nutr. 2014, 65, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Ebrahimzadeh Attari, V.; Ostadrahimi, A.; Asghari Jafarabadi, M.; Mehralizadeh, S.; Mahluji, S. Changes of serum adipocytokines and body weight following Zingiber officinale supplementation in obese women: A RCT. Eur. J. Nutr. 2016, 55, 2129–2136. [Google Scholar] [CrossRef]
- Carvalho, G.C.N.; Lira-Neto, J.C.G.; Araújo, M.F.M.; Freitas, R.W.J.F.; Zanetti, M.L.; Damasceno, M.M.C. Effectiveness of ginger in reducing metabolic levels in people with diabetes: A randomized clinical trial. Rev. Lat. Am. Enfermagem. 2020, 28, e3369. [Google Scholar] [CrossRef] [PubMed]
- Khandouzi, N.; Shidfar, F.; Rajab, A.; Rahideh, T.; Hosseini, P.; Mir Taheri, M. The effects of ginger on fasting blood sugar, hemoglobin a1c, apolipoprotein B, apolipoprotein a-I and malondialdehyde in type 2 diabetic patients. Iran. J. Pharm. Res. 2015, 14, 131–140. [Google Scholar] [PubMed]
- Alizadeh-Navaei, R.; Roozbeh, F.; Saravi, M.; Pouramir, M.; Jalali, F.; Moghadamnia, A.A. Investigation of the effect of ginger on the lipid levels. A double blind controlled clinical trial. Saudi Med. J. 2008, 29, 1280–1284. [Google Scholar] [PubMed]
- Bordia, A.; Verma, S.K.; Srivastava, K.C. Effect of ginger (Zingiber officinale Rosc.) and fenugreek (Trigonella foenumgraecum L.) on blood lipids, blood sugar and platelet aggregation in patients with coronary artery disease. Prostaglandins Leukot. Essent. Fatty Acids. 1997, 56, 379–384. [Google Scholar] [CrossRef]
- Gregersen, N.T.; Belza, A.; Jensen, M.G.; Ritz, C.; Bitz, C.; Hels, O.; Frandsen, E.; Mela, D.J.; Astrup, A. Acute effects of mustard, horseradish, black pepper and ginger on energy expenditure, appetite, ad libitum energy intake and energy balance in human subjects. Br. J. Nutr. 2013, 109, 556–563. [Google Scholar] [CrossRef]
- Mohtashami, A. Effects of bread with Nigella Sativa on blood glucose, blood pressure and anthropometric indices in patients with metabolic syndrome. Clin. Nutr. Res. 2019, 8, 138–147. [Google Scholar] [CrossRef]
- Mohtashami, A.; Mahaki, B.; Azadbakht, L.; Entezari, M.H. Effects of bread with Nigella sativa on lipid profiles, apolipoproteins and inflammatory factor in metabolic syndrome patients. Clin. Nutr. Res. 2016, 5, 89–95. [Google Scholar] [CrossRef]
- Pelegrin, S.; Galtier, F.; Chalançon, A.; Gagnol, J.P.; Barbanel, A.M.; Pélissier, Y.; Larroque, M.; Lepape, S.; Faucanié, M.; Gabillaud, I.; et al. Effects of Nigella sativa seeds (black cumin) on insulin secretion and lipid profile: A pilot study in healthy volunteers. Br. J. Clin. Pharmacol. 2019, 85, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Bamosa, A.O.; Kaatabi, H.; Lebdaa, F.M.; Elq, A.M.; Al-Sultanb, A. Effect of Nigella sativa seeds on the glycemic control of patients with type 2 diabetes mellitus. Indian J. Physiol. Pharmacol. 2010, 54, 344–354. [Google Scholar] [PubMed]
- Ibrahim, R.M.; Hamdan, N.S.; Ismail, M.; Saini, S.M.; Abd Rashid, S.N.; Abd Latiff, L.; Mahmud, R. Protective effects of Nigella sativa on metabolic syndrome in menopausal women. Adv. Pharm. Bull. 2014, 4, 29–33. [Google Scholar] [PubMed]
- Ibrahim, R.M.; Hamdan, N.S.; Mahmud, R.; Imam, M.U.; Saini, S.M.; Rashid, S.N.; Ghafar, S.A.; Latiff, L.A.; Ismail, M. A randomised controlled trial on hypolipidemic effects of Nigella sativa seeds powder in menopausal women. J. Transl. Med. 2014, 12, 82. [Google Scholar] [CrossRef]
- Badar, A.; Kaatabi, H.; Bamosa, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Alkhadra, A.; Al-Almaie, S. Effect of Nigella sativa supplementation over a one-year period on lipid levels, blood pressure and heart rate in type-2 diabetic patients receiving oral hypoglycemic agents: Nonrandomized clinical trial. Ann. Saudi Med. 2017, 37, 56–63. [Google Scholar] [CrossRef]
- Qidwai, W.; Hamza, H.B.; Qureshi, R.; Gilani, A. Effectiveness, safety, and tolerability of powdered Nigella sativa (kalonji) seed in capsules on serum lipid levels, blood sugar, blood pressure, and body weight in adults: Results of a randomized, double-blind controlled trial. J. Altern. Complement. Med. 2009, 15, 639–644. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Dehghan, P.; Tajmiri, S.; Abbasi, M.M. The effects of Nigella sativa on thyroid function, serum vascular endothelial growth factor (VEGF)-1, Nesfatin-1 and anthropometric features in patients with Hashimoto’s thyroiditis: A randomized controlled trial. BMC Complement. Altern. Med. 2016, 16, 471. [Google Scholar] [CrossRef]
- Darand, M.; Darabi, Z.; Yari, Z.; Saadati, S.; Hedayati, M.; Khoncheh, A.; Hosseini-Ahangar, B.; Alavian, S.M.; Hekmatdoost, A. Nigella sativa and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Results from a randomized, double-blind, placebo-controlled, clinical trial. Complement. Ther. Med. 2019, 44, 204–209. [Google Scholar]
- Darand, M.; Darabi, Z.; Yari, Z.; Hedayati, M.; Shahrbaf, M.A.; Khoncheh, A.; Hosseini-Ahangar, B.; Alavian, S.M.; Hekmatdoost, A. The effects of black seed supplementation on cardiovascular risk factors in patients with nonalcoholic fatty liver disease: A randomized, double-blind, placebo-controlled clinical trial. Phytother. Res. 2019, 33, 2369–2377. [Google Scholar] [CrossRef]
- Farhangi, M.A.; Tajmiri, S. The effects of powdered black cumin seeds on markers of oxidative stress, intracellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1 in patients with Hashimoto’s thyroiditis. Clin. Nutr. ESPEN 2020, 37, 207–212. [Google Scholar] [CrossRef]
- Kaatabi, H.; Bamosa, A.O.; Badar, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Al-Khadra, A.; Al-Almaie, S. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: Placebo controlled participant blinded clinical trial. PLoS ONE 2015, 10, e0113486. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.; Khodakarami, F.; Feizabad, E.; Ghaemi, M. The effects of Nigella sativa on anthropometric and biochemical indices in postmenopausal women with metabolic syndrome. Endocrine 2020, 69, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Datau, E.A.; Wardhana Surachmanto, E.E.; Pandelaki, K.; Langi, J.A. Efficacy of Nigella sativa on serum free testosterone and metabolic disturbances in central obese male. Acta Med. Indones. 2010, 42, 130–134. [Google Scholar] [PubMed]
- Sabzghabaee, A.M.; Dianatkhah, M.; Sarrafzadegan, N.; Asgary, S.; Ghannadi, A. Clinical evaluation of Nigella sativa seeds for the treatment of hyperlipidemia: A randomized, placebo controlled clinical trial. Med. Arch. 2012, 66, 198–200. [Google Scholar] [CrossRef] [PubMed]
- Akbari, S.; Sohouli, M.H.; Ebrahimzadeh, S.; Ghanaei, F.M.; Hosseini, A.F.; Aryaeian, N. Effect of rosemary leaf powder with weight loss diet on lipid profile, glycemic status, and liver enzymes in patients with nonalcoholic fatty liver disease: A randomized, double-blind clinical trial. Phytother. Res. 2022, 36, 2186–2196. [Google Scholar] [CrossRef] [PubMed]
- Sá, C.M.; Ramos, A.A.; Azevedo, M.F.; Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Sage tea drinking improves lipid profile and antioxidant defences in humans. Int. J. Mol. Sci. 2009, 10, 3937–3950. [Google Scholar] [CrossRef]
- Darmian, M.A.; Hoseini, R.; Amiri, E.; Golshani, S. How combined and separate aerobic training and turmeric supplementation alter lipid profile and glycemic status? A clinical trial in middle-aged females with type 2 diabetes and hyperlipidemia. Int. Cardiovasc. Res. J. 2021, 15, e118791. [Google Scholar]
- Wickenberg, J.; Ingemansson, S.L.; Hlebowicz, J. Effects of Curcuma longa (turmeric) on postprandial plasma glucose and insulin in healthy subjects. Nutr. J. 2010, 9, 43. [Google Scholar] [CrossRef]
- Maithili Karpaga Selvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Sripradha, R. Efficacy of turmeric as adjuvant therapy in type 2 diabetic patients. Indian J. Clin. Biochem. 2015, 30, 180–186. [Google Scholar] [CrossRef]
- Kandikattu, H.K.; Rachitha, P.; Jayashree, G.V.; Krupashree, K.; Sukhith, M.; Majid, A.; Amruta, N.; Khanum, F. Anti-inflammatory and anti-oxidant effects of cardamom (Elettaria repens (Sonn.) Baill) and its phytochemical analysis by 4D GCXGC TOF-MS. Biomed. Pharmacother. 2017, 91, 191–201. [Google Scholar] [CrossRef]
- Darmian, M.A.; Hoseini, R.; Amiri, E.; Golshani, S. Downregulated hs-CRP and MAD, upregulated GSH and TAC, and improved metabolic status following combined exercise and turmeric supplementation: A clinical trial in middle-aged women with hyperlipidemic type 2 diabetes. J. Diabetes Metab. Disord. 2022, 21, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Adab, Z.; Eghtesadi, S.; Vafa, M.R.; Heydari, I.; Shojaii, A.; Haqqani, H.; Arablou, T.; Eghtesadi, M. Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytother. Res. 2019, 33, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Navekar, R.; Rafraf, M.; Ghaffari, A.; Asghari-Jafarabadi, M.; Khoshbaten, M. Turmeric supplementation improves serum glucose indices and leptin levels in patients with nonalcoholic fatty liver diseases. J. Am. Coll. Nutr. 2017, 36, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, A.; Selvarajan, S.; Kamalanathan, S.; Kadhiravan, T.; Prasanna Lakshmi, N.C.; Adithan, S. Effect of Curcuma longa on vascular function in native Tamilians with type 2 diabetes mellitus: A randomized, double-blind, parallel arm, placebo-controlled trial. Phytother. Res. 2019, 33, 1898–1911. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.; Islam, N.; Anila, N.; Gilani, A.H. Clinical efficacy of the co-administration of Turmeric and Black seeds (Kalongi) in metabolic syndrome—A double blind randomized controlled trial—TAK-MetS trial. Complement. Ther. Med. 2015, 23, 165–174. [Google Scholar] [CrossRef]
- Lee, M.S.; Wahlqvist, M.L.; Chou, Y.C.; Fang, W.H.; Lee, J.T.; Kuan, J.C.; Liu, H.-Y.; Lu, T.-M.; Xiu, L.; Hsu, C.-C.; et al. Turmeric improves post-prandial working memory in pre-diabetes independent of insulin. Asia Pac. J. Clin. Nutr. 2014, 23, 581–591. [Google Scholar]
- Nieman, D.C.; Cialdella-Kam, L.; Knab, A.M.; Shanely, R.A. Influence of red pepper spice and turmeric on inflammation and oxidative stress biomarkers in overweight females: A metabolomics approach. Plant Foods Hum. Nutr. 2012, 67, 415–421. [Google Scholar] [CrossRef]
- Charron, C.S.; Dawson, H.D.; Albaugh, G.P.; Solverson, P.M.; Vinyard, B.T.; Solano-Aguilar, G.I.; Molokin, A.; Novotny, J.A. A single meal containing raw, crushed garlic influences expression of immunity- and cancer-related genes in whole blood of humans. J. Nutr. 2015, 145, 2448–2455. [Google Scholar] [CrossRef]
- Jarhahzadeh, M.; Alavinejad, P.; Farsi, F.; Husain, D.; Rezazadeh, A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: A randomized double blind clinical trial. Diabetol. Metab. Syndr. 2021, 13, 112. [Google Scholar] [CrossRef]
- Yahyazadeh, R.; Ghasemzadeh Rahbardar, M.; Razavi, B.M.; Karimi, G.; Hosseinzadeh, H. The effect of Elettaria cardamomum (cardamom) on the metabolic syndrome: Narrative review. Iran. J. Basic. Med. Sci. 2021, 24, 1462–1469. [Google Scholar]
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in metabolic syndrome. Nutrients 2018, 10, 630. [Google Scholar] [CrossRef] [PubMed]
- Akilen, R.; Tsiami, A.; Devendra, D.; Robinson, N. Cinnamon in glycaemic control: Systematic review and meta analysis. Clin. Nutr. 2012, 31, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mandal, A.; Kant, R.; Jachak, S.; Jagzape, M. Is cinnamon efficacious for glycaemic control in type-2 diabetes mellitus? J. Pak. Med. Assoc. 2020, 70, 2065–2069. [Google Scholar] [PubMed]
- Silva, M.L.; Bernardo, M.A.; Singh, J.; de Mesquita, M.F. Cinnamon as a complementary therapeutic approach for dysglycemia and dyslipidemia control in type 2 diabetes mellitus and its molecular mechanism of action: A review. Nutrients 2022, 14, 2773. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Lu, K.; Cao, X.; Xia, H.; Wang, S.; Sun, G.; Chen, L.; Liao, W. The effect of cinnamon on glycolipid metabolism: A dose-response meta-analysis of randomized controlled trials. Nutrients 2023, 15, 2983. [Google Scholar] [CrossRef]
- Allen, R.W.; Schwartzman, E.; Baker, W.L.; Coleman, C.I.; Phung, O.J. Cinnamon use in type 2 diabetes: An updated systematic review and meta-analysis. Ann. Fam. Med. 2013, 11, 452–459. [Google Scholar] [CrossRef]
- Baker, W.L.; Gutierrez-Williams, G.; White, C.M.; Kluger, J.; Coleman, C.I. Effect of cinnamon on glucose control and lipid parameters. Diabetes Care 2008, 31, 41–43. [Google Scholar] [CrossRef]
- Davis, P.A.; Yokoyama, W. Cinnamon intake lowers fasting blood glucose: Meta-analysis. J. Med. Food 2011, 14, 884–889. [Google Scholar] [CrossRef]
- Deyno, S.; Eneyew, K.; Seyfe, S.; Tuyiringire, N.; Peter, E.L.; Muluye, R.A.; Tolo, C.U.; Ogwang, P.E. Efficacy and safety of cinnamon in type 2 diabetes mellitus and pre-diabetes patients: A meta-analysis and meta-regression. Diabetes Res. Clin. Pract. 2019, 156, 107815. [Google Scholar] [CrossRef]
- Jamali, N.; Kazemi, A.; Saffari-Chaleshtori, J.; Samare-Najaf, M.; Mohammadi, V.; Clark, C.C.T. The effect of cinnamon supplementation on lipid profiles in patients with type 2 diabetes: A systematic review and meta-analysis of clinical trials. Complement. Ther. Med. 2020, 55, 102571. [Google Scholar] [CrossRef]
- Leach, M.J.; Kumar, S. Cinnamon for diabetes mellitus. Cochrane Database Syst. Rev. 2012, 2012, CD007170. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Jayawardana, R.; Galappaththy, P.; Constantine, G.R.; de Vas Gunawardana, N.; Katulanda, P. Efficacy and safety of ‘true’ cinnamon (Cinnamomum zeylanicum) as a pharmaceutical agent in diabetes: A systematic review and meta-analysis. Diabet. Med. 2012, 29, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Heydarpour, F.; Hemati, N.; Hadi, A.; Moradi, S.; Mohammadi, E.; Farzaei, M.H. Effects of cinnamon on controlling metabolic parameters of polycystic ovary syndrome: A systematic review and meta-analysis. J. Ethnopharmacol. 2020, 254, 112741. [Google Scholar] [CrossRef]
- Kutbi, E.H.; Sohouli, M.H.; Fatahi, S.; Lari, A.; Shidfar, F.; Aljhdali, M.M.; Alhoshan, F.M.; Elahi, S.S.; Almusa, H.A.; Abu-Zaid, A. The beneficial effects of cinnamon among patients with metabolic diseases: A systematic review and dose-response meta-analysis of randomized-controlled trials. Crit. Rev. Food Sci. Nutr. 2022, 62, 6113–6131. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Huang, W.; He, M.; Yue, R. Effects of cinnamon supplementation on lipid profiles among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis. Complement. Ther. Clin. Pract. 2022, 49, 101625. [Google Scholar] [CrossRef] [PubMed]
- Krittanawong, C.; Isath, A.; Scott, C.Z.; Wang, Z.; Kaplin, S.; Jneid, H.; Lavie, C.J.; Virani, S.S. Association between cinnamon consumption and risk of cardiovascular health: A systematic review and meta-analysis. Am. J. Med. 2022, 135, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Maierean, S.M.; Serban, M.C.; Sahebkar, A.; Ursoniu, S.; Serban, A.; Penson, P.; Banach, M.; Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. The effects of cinnamon supplementation on blood lipid concentrations: A systematic review and meta-analysis. J. Clin. Lipidol. 2017, 11, 1393–1406. [Google Scholar] [CrossRef]
- Valussi, M. Functional foods with digestion-enhancing properties. Int. J. Food Sci. Nutr. 2012, 63 (Suppl. 1), 82–89. [Google Scholar] [CrossRef]
- Neelakantan, N.; Narayanan, M.; de Souza, R.J.; van Dam, R.M. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: A meta-analysis of clinical trials. Nutr. J. 2014, 13, 7. [Google Scholar] [CrossRef]
- Askarpour, M.; Alami, F.; Campbell, M.S.; Venkatakrishnan, K.; Hadi, A.; Ghaedi, E. Effect of fenugreek supplementation on blood lipids and body weight: A systematic review and meta-analysis of randomized controlled trials. J. Ethnopharmacol. 2020, 253, 112538. [Google Scholar] [CrossRef]
- Shabil, M.; Bushi, G.; Bodige, P.K.; Maradi, P.S.; Patra, B.P.; Padhi, B.K.; Khubchandani, J. Effect of fenugreek on hyperglycemia: A systematic review and meta-analysis. Medicina 2023, 59, 248. [Google Scholar] [CrossRef] [PubMed]
- Amini, M.R.; Payandeh, N.; Sheikhhossein, F.; Pourreza, S.; Ghalandari, H.; Askarpour, M.; Hekmatdoost, A. The effects of fenugreek seed consumption on blood pressure: A systematic review and meta-analysis of randomized controlled trials. High. Blood Press. Cardiovasc. Prev. 2023, 30, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.G.D.S.; Alencar, M.B.; Dos Santos, A.N.; da Paixão, D.C.B.; Sandes, F.L.F.; Andrade, B.; Castro, Y.; de Andrade, J.S. Effect of saffron and fenugreek on lowering blood glucose: A systematic review with meta-analysis. Phytother. Res. 2023, 37, 2092–2101. [Google Scholar] [CrossRef] [PubMed]
- Fakhr, L.; Chehregosha, F.; Zarezadeh, M.; Chaboksafar, M.; Tarighat-Esfanjani, A. Effects of fenugreek supplementation on the components of metabolic syndrome: A systematic review and dose-response meta-analysis of randomized clinical trials. Pharmacol. Res. 2023, 187, 106594. [Google Scholar] [CrossRef]
- Gong, J.; Fang, K.; Dong, H.; Wang, D.; Hu, M.; Lu, F. Effect of fenugreek on hyperglycaemia and hyperlipidemia in diabetes and prediabetes: A meta-analysis. J. Ethnopharmacol. 2016, 194, 260–268. [Google Scholar] [CrossRef]
- Heshmat-Ghahdarijani, K.; Mashayekhiasl, N.; Amerizadeh, A.; Teimouri Jervekani, Z.; Sadeghi, M. Effect of fenugreek consumption on serum lipid profile: A systematic review and meta-analysis. Phytother. Res. 2020, 34, 2230–2245. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Khosropanah, M.H.; Ayati, Z.; Chang, D.; Nasli-Esfahani, E.; Ayati, M.H.; Namazi, N. The effects of fenugreek on cardiometabolic risk factors in adults: A systematic review and meta-analysis. Complement. Ther. Med. 2020, 52, 102416. [Google Scholar] [CrossRef]
- Gadidala, S.K.; Johny, E.; Thomas, C.; Nadella, M.; Undela, K.; Adela, R. Effect of garlic extract on markers of lipid metabolism and inflammation in coronary artery disease (CAD) patients: A systematic review and meta-analysis. Phytother. Res. 2023, 37, 2242–2254. [Google Scholar] [CrossRef]
- Schwingshackl, L.; Missbach, B.; Hoffmann, G. An umbrella review of garlic intake and risk of cardiovascular disease. Phytomedicine 2016, 23, 1127–1133. [Google Scholar] [CrossRef]
- Varshney, R.; Budoff, M.J. Garlic and Heart Disease. J. Nutr. 2016, 146, 416S–421S. [Google Scholar] [CrossRef]
- Sobenin, I.A.; Myasoedova, V.A.; Iltchuk, M.I.; Zhang, D.W.; Orekhov, A.N. Therapeutic effects of garlic in cardiovascular atherosclerotic disease. Chin. J. Nat. Med. 2019, 17, 721–728. [Google Scholar] [CrossRef] [PubMed]
- Westerterp-Plantenga, M.; Diepvens, K.; Joosen, A.M.; Bérubé-Parent, S.; Tremblay, A. Metabolic effects of spices, teas, and caffeine. Physiol. Behav. 2006, 89, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Dalli, M.; Bekkouch, O.; Azizi, S.E.; Azghar, A.; Gseyra, N.; Kim, B. Nigella sativa L. phytochemistry and pharmacological activities: A review (2019–2021). Biomolecules 2021, 12, 20. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Imran, M.; Ul-Haq, I.; Živković, J.; Abu-Reidah, I.M.; Sen, S.; Taheri, Y.; Acharya, K.; Azadi, H.; et al. Nigella plants—Traditional uses, bioactive phytoconstituents, preclinical and clinical studies. Front. Pharmacol. 2021, 12, 625386. [Google Scholar] [CrossRef] [PubMed]
- Daryabeygi-Khotbehsara, R.; Golzarand, M.; Ghaffari, M.P.; Djafarian, K. Nigella sativa improves glucose homeostasis and serum lipids in type 2 diabetes: A systematic review and meta-analysis. Complement. Ther. Med. 2017, 35, 6–13. [Google Scholar] [CrossRef]
- Sahebkar, A.; Soranna, D.; Liu, X.; Thomopoulos, C.; Simental-Mendia, L.E.; Derosa, G.; Maffioli, P.; Parati, G. A systematic review and meta-analysis of randomized controlled trials investigating the effects of supplementation with Nigella sativa (black seed) on blood pressure. J. Hypertens. 2016, 34, 2127–2135. [Google Scholar] [CrossRef]
- Askari, G.; Rouhani, M.H.; Ghaedi, E.; Ghavami, A.; Nouri, M.; Mohammadi, H. Effect of Nigella sativa (black seed) supplementation on glycemic control: A systematic review and meta-analysis of clinical trials. Phytother. Res. 2019, 33, 1341–1352. [Google Scholar] [CrossRef]
- Hallajzadeh, J.; Milajerdi, A.; Mobini, M.; Amirani, E.; Azizi, S.; Nikkhah, E.; Bahadori, B.; Sheikhsoleimani, R.; Mirhashemi, S.M. Effects of Nigella sativa on glycemic control, lipid profiles, and biomarkers of inflammatory and oxidative stress: A systematic review and meta-analysis of randomized controlled clinical trials. Phytother. Res. 2020, 34, 2586–2608. [Google Scholar] [CrossRef]
- Kavyani, Z.; Musazadeh, V.; Golpour-Hamedani, S.; Moridpour, A.H.; Vajdi, M.; Askari, G. The effect of Nigella sativa (black seed) on biomarkers of inflammation and oxidative stress: An updated systematic review and meta-analysis of randomized controlled trials. Inflammopharmacology 2023, 31, 1149–1165. [Google Scholar] [CrossRef]
- Montazeri, R.S.; Fatahi, S.; Sohouli, M.H.; Abu-Zaid, A.; Santos, H.O.; Găman, M.A.; Shidfar, F. The effect of Nigella sativa on biomarkers of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Food Biochem. 2021, 45, e13625. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.M.; Sheikhi, A.; Varkaneh, H.K.; Zarezadeh, M.; Rahmani, J.; Milajerdi, A. Effect of Nigella sativa supplementation on obesity indices: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2018, 38, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Namazi, N.; Larijani, B.; Ayati, M.H.; Abdollahi, M. The effects of Nigella sativa L. on obesity: A systematic review and meta-analysis. J. Ethnopharmacol. 2018, 219, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Zhang, L.; Tao, J.; Wei, Z. Effect of Nigella sativa in the treatment of nonalcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2021, 35, 4183–4193. [Google Scholar] [CrossRef]
- Tavakoly, R.; Arab, A.; Vallianou, N.; Clark, C.C.T.; Hadi, A.; Ghaedi, E.; Ghavami, A. The effect of Nigella sativa L. supplementation on serum C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 45, 149–155. [Google Scholar] [CrossRef]
- Rolfe, V.; Mackonochie, M.; Mills, S.; MacLennan, E. Turmeric/curcumin health outcomes: A meta-review of systematic reviews. Eur. J. Integr. Med. 2020, 40, 101252. [Google Scholar] [CrossRef]
- Dei Cas, M.; Ghidoni, R. Dietary curcumin: Correlation between bioavailability and health potential. Nutrients 2019, 11, 2147. [Google Scholar] [CrossRef]
- Sahebkar, A. Are curcuminoids effective C-reactive protein-lowering agents in clinical practice? Evidence from a meta-analysis. Phytother. Res. 2014, 28, 633–642. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial activity of basil, oregano, and thyme essential oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- El-Kased, R.F.; El-Kersh, D.M. GC-MS profiling of naturally extracted essential oils: Antimicrobial and beverage preservative actions. Life 2022, 12, 1587. [Google Scholar] [CrossRef]
- Wightman, E.L.; Reay, J.L.; Haskell, C.F.; Williamson, G.; Dew, T.P.; Kennedy, D.O. Effects of resveratrol alone or in combination with piperine on cerebral blood flow parameters and cognitive performance in human subjects: A randomised, double-blind, placebo-controlled, cross-over investigation. Br. J. Nutr. 2014, 112, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Ajazuddin; Alexander, A.; Qureshi, A.; Kumari, L.; Vaishnav, P.; Sharma, M.; Saraf, S.; Saraf, S. Role of herbal bioactives as a potential bioavailability enhancer for active pharmaceutical ingredients. Fitoterapia 2014, 97, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chiavaroli, L.; Lee, D.; Ahmed, A.; Cheung, A.; Khan, T.A.; Blanco, S.; Mirrahimi, A.; Jenkins, D.J.A.; Livesey, G.; Wolever, T.M.S.; et al. Effect of low glycaemic index or load dietary patterns on glycaemic control and cardiometabolic risk factors in diabetes: Systematic review and meta-analysis of randomised controlled trials. BMJ 2021, 374, n1651. [Google Scholar] [CrossRef] [PubMed]
- Mensink, R.P.; Aro, A.; Den Hond, E.; German, J.B.; Griffin, B.A.; ten Meer, H.-U.; Mutanen, M.; Pannemans, D.; Stahl, W. PASSCLAIM–Diet-related cardiovascular disease. Eur. J. Nutr. 2003, 42 (Suppl. 1), 1/6–1/27. [Google Scholar] [CrossRef] [PubMed]
- Edmands, W.M.; Ferrari, P.; Rothwell, J.A.; Rinaldi, S.; Slimani, N.; Barupal, D.K.; Biessy, C.; Jenab, M.; Clavel-Chapelon, F.; Fagherazzi, G.; et al. Polyphenol metabolome in human urine and its association with intake of polyphenol-rich foods across European countries. Am. J. Clin. Nutr. 2015, 102, 905–913. [Google Scholar] [CrossRef]
- Shahwan, M.; Alhumaydhi, F.; Ashraf, G.M.; Hasan, P.M.Z.; Shamsi, A. Role of polyphenols in combating type 2 diabetes and insulin resistance. Int. J. Biol. Macromol. 2022, 206, 567–579. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).