Exploring the Impact of Onobrychis cornuta and Veratrum lobelianum Extracts on C. elegans: Implications for MAPK Modulation, Germline Development, and Antitumor Properties
Abstract
1. Introduction
2. Results
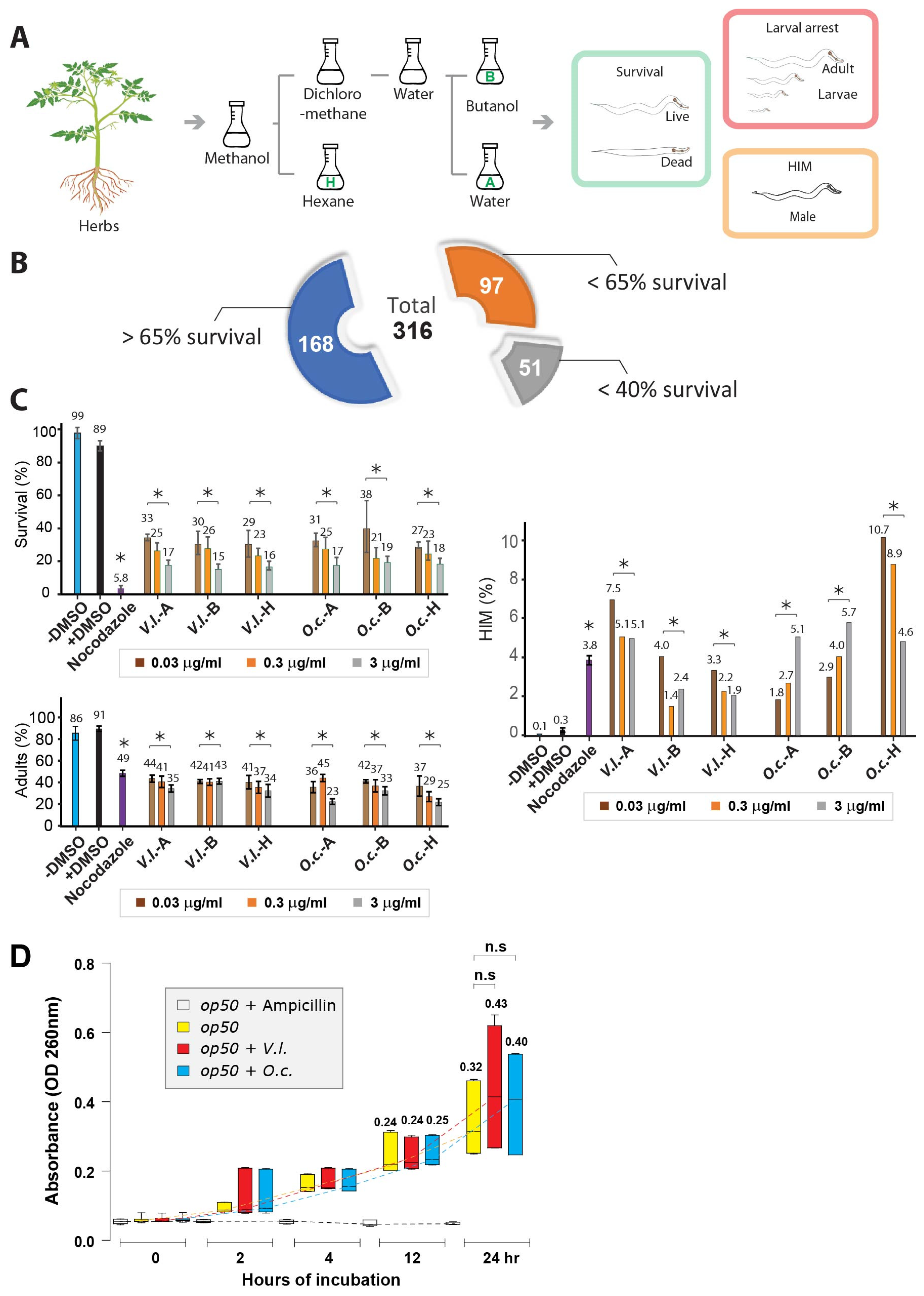
2.1. Herbal Extracts Unveil Nematocidal Potency
2.2. Dose-Dependent Nematocidal Effects of Herbal Extracts: Correlation and Phenotypic Observations
2.3. Impact of Herb Extracts on Nematode Survival and Bacterial Growth
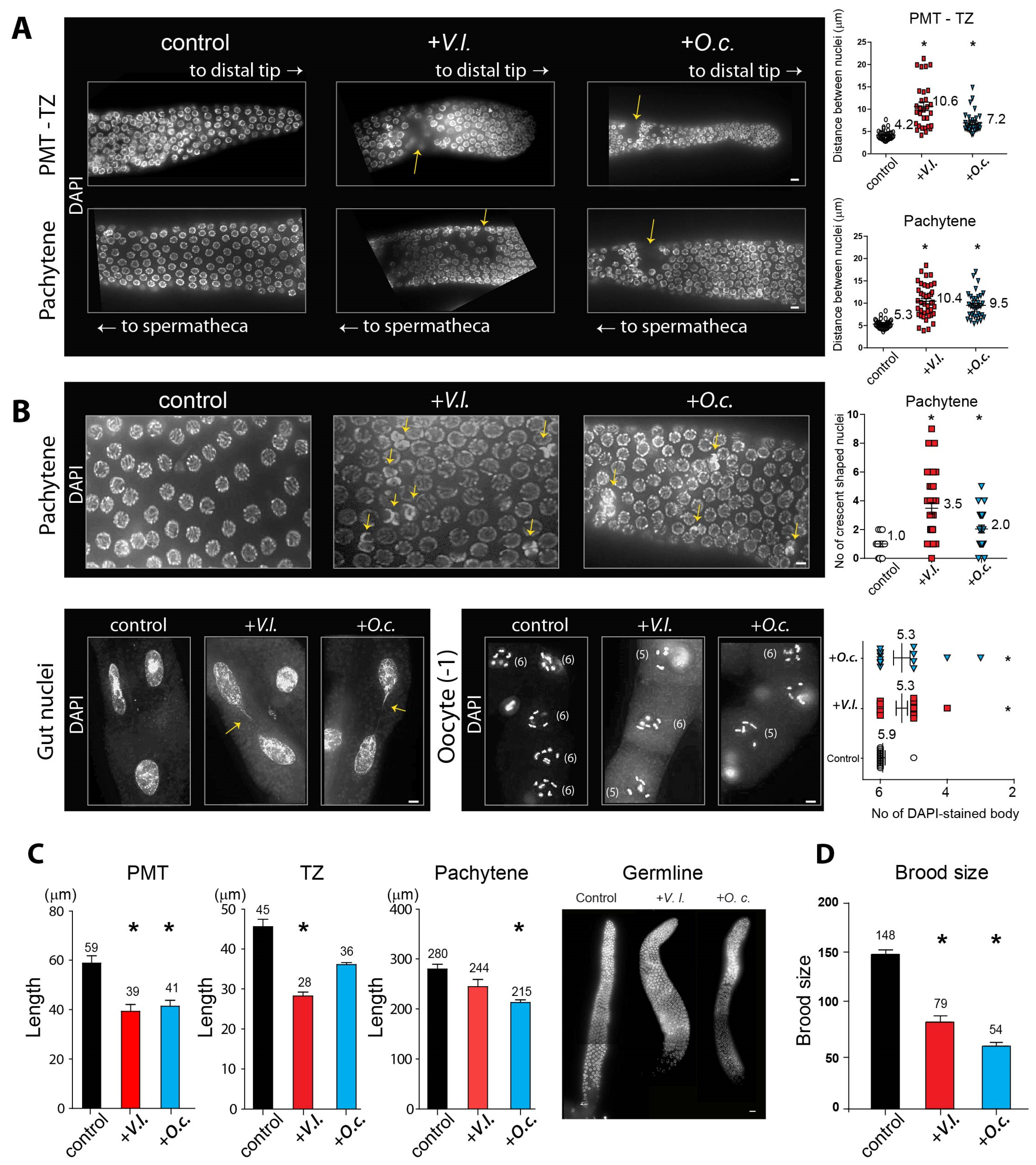
2.4. Herbal Extracts Cause Defective Germline Progression
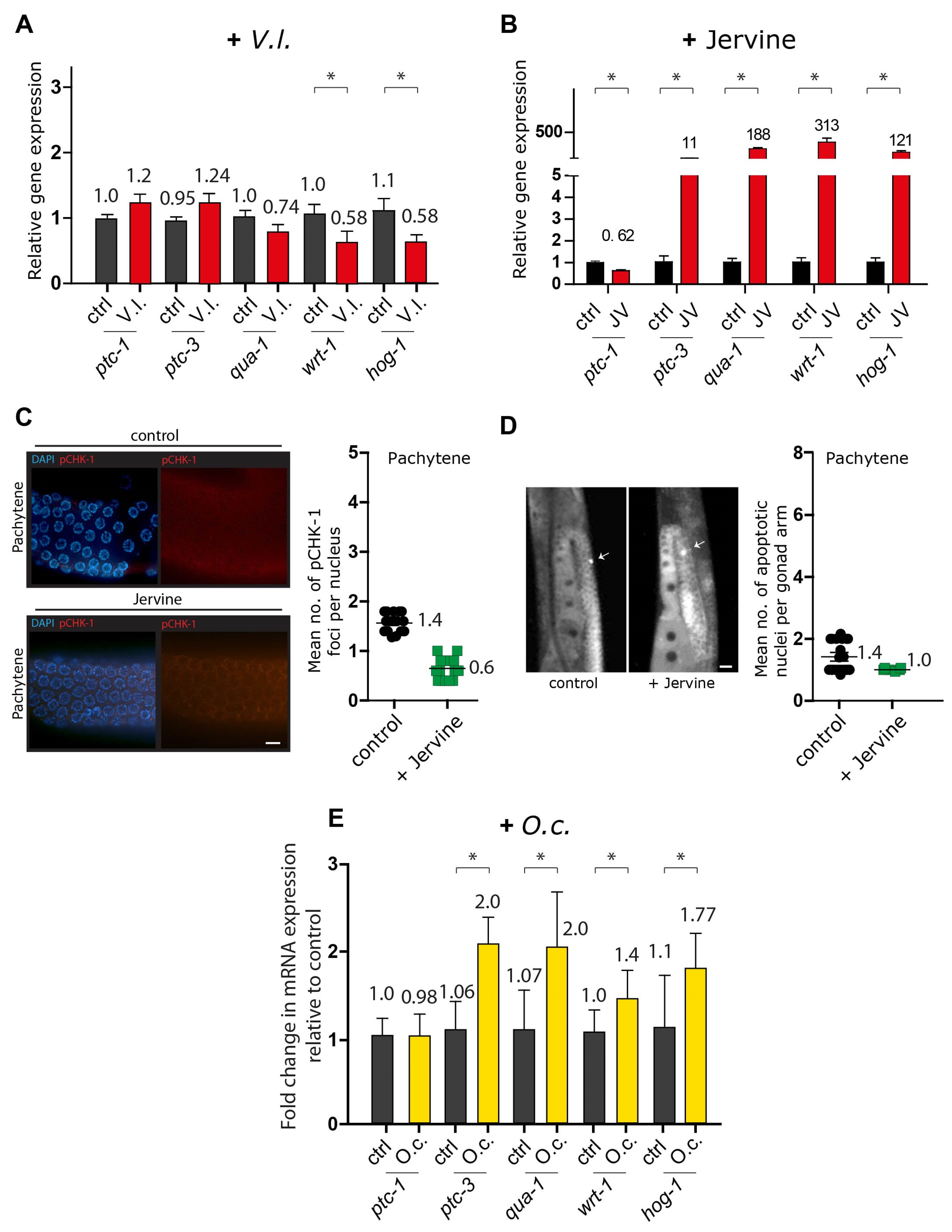
2.5. Herbal Extracts Activate DNA Damage Checkpoint Pathways: ATM, ATR, CHK1, and Apoptosis
2.6. LC–MS Analysis Identified Anticancer Compounds
2.7. V. lobelianum and the Hedgehog Pathway
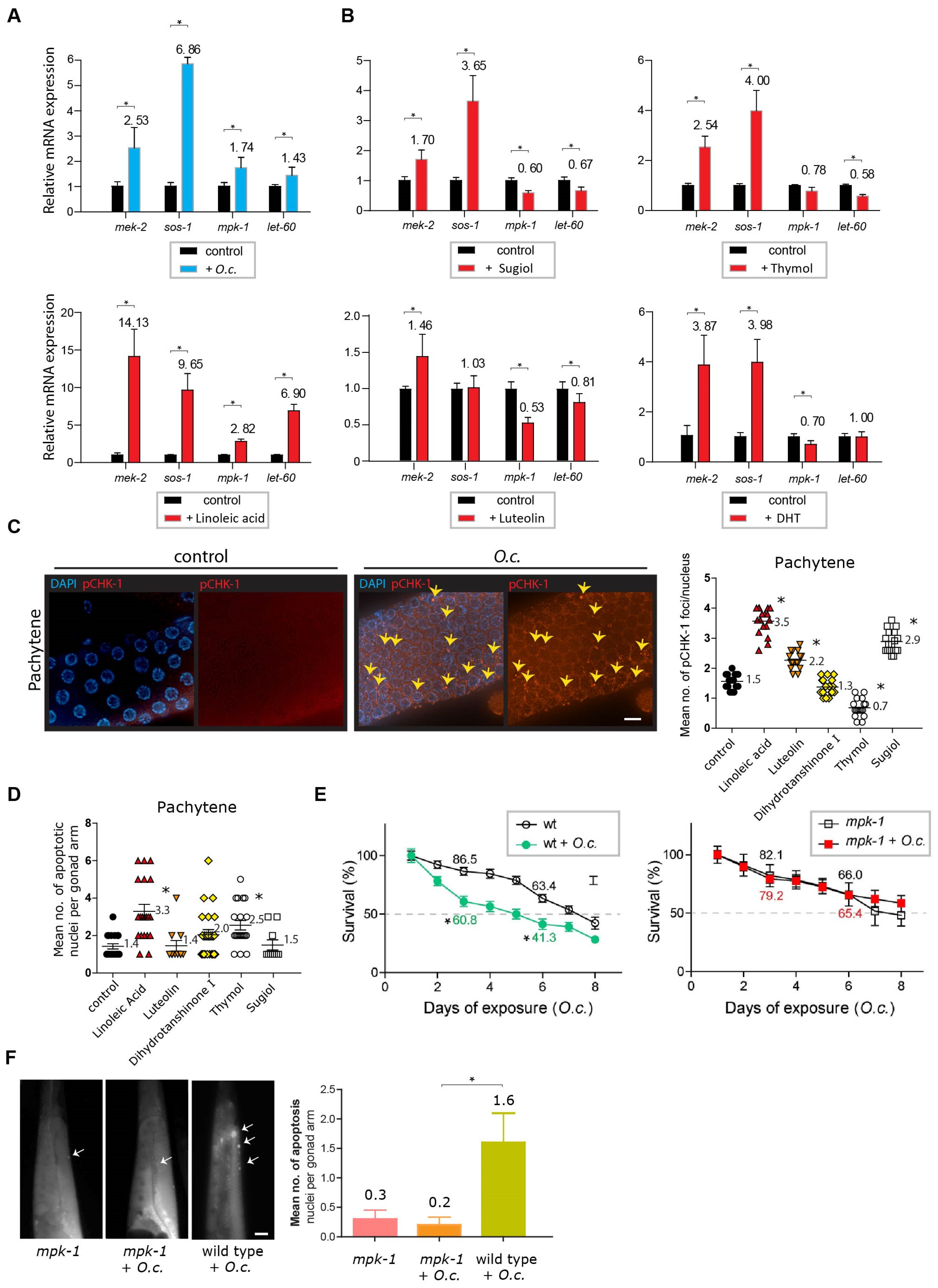
2.8. O. cornuta and the MAPK Kinase Pathway
3. Discussion
3.1. V. lobelianum
3.2. O. cornuta
3.3. V. lobelianum and O. cornuta Herbal Extracts: Balancing Promise and Toxicity
4. Materials and Methods
4.1. Strains and Alleles
4.2. Herb Extraction
4.3. Survival, Larval Arrest/Lethality and HIM
4.4. Cumulative Survival
4.5. Preparation of Worm Lysates for Mass Spectrometric Analysis
4.6. LC–MS Analysis
4.7. Monitoring the Growth of E. coli
4.8. Immunofluorescence Staining
4.9. Quantitative Analysis of pCHK-1 Foci
4.10. Quantitation of Germline Apoptosis
4.11. Quantitative Real-Time PCR (qPCR)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Report, M.A. Medical Foods Market Size, Share & Trends Analysis Report By Route of Administration (Oral, Enteral), By Product (Pills, Powder, Liquid), By Application, By Sales Channel, And Segment Forecasts, 2022–2030. 2020. Available online: https://www.grandviewresearch.com/industry-analysis/medical-foods-market (accessed on 13 December 2023).
- Shaul, N.C.; Jordan, J.M.; Falsztyn, I.B.; Baugh, L.R. Insulin/IGF-dependent Wnt signaling promotes formation of germline tumors and other developmental abnormalities following early-life starvation in Caenorhabditis elegans. Genetics 2022, 223, iyac173. [Google Scholar] [CrossRef] [PubMed]
- Lui, D.Y.; Colaiacovo, M.P. Meiotic development in Caenorhabditis elegans. Adv. Exp. Med. Biol. 2013, 757, 133–170. [Google Scholar] [PubMed]
- Duronio, R.J.; O’Farrell, P.H.; Sluder, G.; Su, T.T. Sophisticated lessons from simple organisms: Appreciating the value of curiosity-driven research. Dis. Models Mech. 2017, 10, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, F.; Guo, W.; Zhang, J.; Xiao, L.; Li, H.; Jia, W.; Huang, Z.; Shi, R.; Xiang, Y.; et al. Caenorhabditis elegans in Chinese medicinal studies: Making the case for aging and neurodegeneration. Rejuvenation Res. 2014, 17, 205–208. [Google Scholar] [CrossRef]
- Matsunami, K. Frailty and Caenorhabditis elegans as a Benchtop Animal Model for Screening Drugs Including Natural Herbs. Front. Nutr. 2018, 5, 111. [Google Scholar] [CrossRef]
- Kobet, R.A.; Pan, X.; Zhang, B.; Pak, S.C.; Asch, A.S.; Lee, M.H. Caenorhabditis elegans: A Model System for Anti-Cancer Drug Discovery and Therapeutic Target Identification. Biomol. Ther. 2014, 22, 371–383. [Google Scholar] [CrossRef]
- David, D.C.; Ollikainen, N.; Trinidad, J.C.; Cary, M.P.; Burlingame, A.L.; Kenyon, C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010, 8, e1000450. [Google Scholar] [CrossRef]
- Moliner, C.; López, V.; Barros, L.; Dias, M.I.; Ferreira, I.C.; Langa, E.; Gómez-Rincón, C. Rosemary flowers as edible plant foods: Phenolic composition and antioxidant properties in Caenorhabditis elegans. Antioxidants 2020, 9, 811. [Google Scholar] [CrossRef]
- Sayed, S.M.; Siems, K.; Schmitz-Linneweber, C.; Luyten, W.; Saul, N. Enhanced Healthspan in Caenorhabditis elegans Treated with Extracts from the Traditional Chinese Medicine Plants Cuscuta chinensis Lam. and Eucommia ulmoides Oliv. Front. Pharmacol. 2021, 12, 604435. [Google Scholar] [CrossRef]
- Anjaneyulu, J.; Vidyashankar, R.; Godbole, A. Differential effect of Ayurvedic nootropics on C. elegans models of Parkinson’s disease. J. Ayurveda Integr. Med. 2020, 11, 440–447. [Google Scholar] [CrossRef]
- Hodgkin, J.; Horvitz, H.R.; Brenner, S. Nondisjunction Mutants of the Nematode CAENORHABDITIS ELEGANS. Genetics 1979, 91, 67–94. [Google Scholar] [CrossRef] [PubMed]
- Cinquin, O.; Crittenden, S.L.; Morgan, D.E.; Kimble, J. Progression from a stem cell-like state to early differentiation in the C. elegans germ line. Proc. Natl. Acad. Sci. USA 2010, 107, 2048–2053. [Google Scholar] [CrossRef] [PubMed]
- Dernburg, A.F.; McDonald, K.; Moulder, G.; Barstead, R.; Dresser, M.; Villeneuve, A.M. Meiotic recombination in C. elegans initiates by a conserved mechanism and is dispensable for homologous chromosome synapsis. Cell 1998, 94, 387–398. [Google Scholar] [CrossRef] [PubMed]
- Gisselsson, D. Classification of chromosome segregation errors in cancer. Chromosoma 2008, 117, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Csankovszki, G.; Collette, K.; Spahl, K.; Carey, J.; Snyder, M.; Petty, E.; Patel, U.; Tabuchi, T.; Liu, H.; McLeod, I.; et al. Three distinct condensin complexes control C. elegans chromosome dynamics. Curr. Biol. 2009, 19, 9–19. [Google Scholar] [CrossRef]
- Girard, C.; Roelens, B.; Zawadzki, K.A.; Villeneuve, A.M. Interdependent and separable functions of Caenorhabditis elegans MRN-C complex members couple formation and repair of meiotic DSBs. Proc. Natl. Acad. Sci. USA 2018, 115, E4443–E4452. [Google Scholar] [CrossRef]
- Hofmann, E.R.; Milstein, S.; Boulton, S.J.; Ye, M.; Hofmann, J.J.; Stergiou, L.; Hengartner, M.O. Caenorhabditis elegans HUS-1 is a DNA damage checkpoint protein required for genome stability and EGL-1-mediated apoptosis. Curr. Biol. 2002, 12, 1908–1918. [Google Scholar] [CrossRef]
- Kim, H.M.; Colaiacovo, M.P. ZTF-8 Interacts with the 9-1-1 Complex and Is Required for DNA Damage Response and Double-Strand Break Repair in the C. elegans Germline. PLoS Genet. 2014, 10, e1004723. [Google Scholar] [CrossRef]
- Marechal, A.; Zou, L. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb. Perspect. Biol. 2013, 5, a012716. [Google Scholar] [CrossRef]
- Kim, H.M.; Colaiacovo, M.P. New Insights into the Post-Translational Regulation of DNA Damage Response and Double-Strand Break Repair in Caenorhabditis elegans. Genetics 2015, 200, 495–504. [Google Scholar] [CrossRef][Green Version]
- Gartner, A.; Milstein, S.; Ahmed, S.; Hodgkin, J.; Hengartner, M.O. A conserved checkpoint pathway mediates DNA damage--induced apoptosis and cell cycle arrest in C. elegans. Mol. Cell 2000, 5, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.U.; Ali, R.A.; Choudhary, M.I.; Sener, B.; Turkoz, S. New Steroidal Alkaloids from Rhizomes of Veratrum album. J. Nat. Prod. 1992, 55, 565–570. [Google Scholar] [CrossRef]
- Taldaev, A.; Terekhov, R.P.; Melnik, E.V.; Belova, M.V.; Kozin, S.V.; Nedorubov, A.A.; Ramenskaya, G.V. Insights into the Cardiotoxic Effects of Veratrum Lobelianum Alkaloids: Pilot Study. Toxins 2022, 14, 490. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Li, H.L.; Shen, Y.H.; Jin, H.Z.; Yan, S.K.; Liu, X.H.; Zhang, W.D. Antitumor and antiplatelet activity of alkaloids from veratrum dahuricum. Phytother. Res. 2010, 24, 821–826. [Google Scholar] [CrossRef]
- Tang, J.; Li, H.-L.; Shen, Y.-H.; Jin, H.-Z.; Yan, S.-K.; Liu, R.-H.; Zhang, W.-D. Antitumor activity of extracts and compounds from the rhizomes of Veratrum dahuricum. Phytother. Res. 2008, 22, 1093–1096. [Google Scholar] [CrossRef]
- Chen, J.; Wen, B.; Wang, Y.; Wu, S.; Zhang, X.; Gu, Y.; Wang, Z.; Wang, J.; Zhang, W.; Yong, J. Jervine exhibits anticancer effects on nasopharyngeal carcinoma through promoting autophagic apoptosis via the blockage of Hedgehog signaling. Biomed. Pharmacother. 2020, 132, 110898. [Google Scholar] [CrossRef]
- Burglin, T.R.; Kuwabara, P.E. Homologs of the Hh signalling network in C. elegans. The C. elegans Research Community, WormBook. 2006, pp. 1–14. Available online: http://www.wormbook.org/chapters/www_homologsHhsignalnetwork/homologsHhsignalnetwork.html (accessed on 13 December 2023).
- Raleigh, D.R.; Reiter, J.F. Misactivation of Hedgehog signaling causes inherited and sporadic cancers. J. Clin. Invest. 2019, 129, 465–475. [Google Scholar] [CrossRef]
- Wu, Y.; Han, M.; Guan, K.L. MEK-2, a Caenorhabditis elegans MAP kinase kinase, functions in Ras-mediated vulval induction and other developmental events. Genes Dev. 1995, 9, 742–755. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Lackner, M.R.; Kim, S.K. Genetic analysis of the Caenorhabditis elegans MAP kinase gene mpk-1. Genetics 1998, 150, 103–117. [Google Scholar] [CrossRef]
- Liao, W.J.; Yuan, Y.M.; Zhang, D.Y. Biogeography and evolution of flower color in Veratrum (Melanthiaceae) through inference of a phylogeny based on multiple DNA markers. Plant Syst. Evol. 2007, 267, 177–190. [Google Scholar] [CrossRef]
- Melnik, E.; Belova, M.; Tyurin, I.; Ramenskaya, G. Quantitative Content Parameter in the Standardization of Veratrum Aqua, Veratrum Lobelianum Bernh. Based Drug Drug Dev. Regist. 2021, 10, 107–113. [Google Scholar] [CrossRef]
- Yakan, S.; Aydin, T.; Gulmez, C.; Ozden, O.; Eren Erdogan, K.; Daglioglu, Y.K.; Cakir, A. The protective role of jervine against radiation-induced gastrointestinal toxicity. J. Enzym. Inhib. Med. Chem. 2019, 34, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Zomlefer, W.B.; Williams, N.H.; Whitten, W.M.; Judd, W.S. Generic circumscription and relationships in the tribe Melanthieae (Liliales, Melanthiaceae), with emphasis on Zigadenus: Evidence from ITS and trnL-F sequence data. Am. J. Bot. 2001, 88, 1657–1669. [Google Scholar] [CrossRef] [PubMed]
- Zomlefer, W.B.; Whitten, W.M.; Williams, N.H.; Judd, W.S. An Overview of Veratrum s.l. (Liliales: Melanthiaceae) and an Infrageneric Phylogeny Based on ITS Sequence Data. Syst. Bot. 2003, 28, 250–269. [Google Scholar]
- Erkovan, H.I.; Gullap, M.K.; Erkovan, S.; Koc, A. Horned sainfoin (onobrychis cornuta (L.) desv.): Is it an amusing or nuisance plant for steppe rangelands? Ecol. Saf. 2016, 10, 11. [Google Scholar]
- Joudi, L.; Bibalani, G.H. Exploration of medicinal species of Fabaceae, Lamiaceae and Asteraceae families in Ilkhji region, Eastern Azerbaijan Province (Northwestern Iran). J. Med. Plants Res. 2010, 4, 1081–1084. [Google Scholar]
- Burcham, P.C. Genotoxic lipid peroxidation products: Their DNA damaging properties and role in formation of endogenous DNA adducts. Mutagenesis 1998, 13, 287–305. [Google Scholar] [CrossRef]
- Inouye, S. Site-specific cleavage of double-strand DNA by hydroperoxide of linoleic acid. FEBS Lett. 1984, 172, 231–234. [Google Scholar] [CrossRef]
- Nakayama, T.; Kaneko, M.; Kodama, M. Detection of DNA Damage in Cultured Human Fibroblasts Induced by Methyl Linoleate Hydroperoxide. Agric. Biol. Chem. 1986, 50, 261–262. [Google Scholar]
- Ueda, K.; Kobayashi, S.; Morita, J.; Komano, T. Site-specific DNA damage caused by lipid peroxidation products. Biochim Biophys Acta 1985, 824, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Beeharry, N.; Lowe, J.E.; Hernandez, A.R.; Chambers, J.A.; Fucassi, F.; Cragg, P.J.; Green, I.C. Linoleic acid and antioxidants protect against DNA damage and apoptosis induced by palmitic acid. Mutat. Res. 2003, 530, 27–33. [Google Scholar] [PubMed]
- Chang, C.; Hopper, N.A.; Sternberg, P.W. Caenorhabditis elegans SOS-1 is necessary for multiple RAS-mediated developmental signals. EMBO J. 2000, 19, 3283–3294. [Google Scholar] [CrossRef] [PubMed]
- Flora of Israel and Adjacent Areas [Internet]. 2016. Available online: https://flora.org.il/en/plants/ (accessed on 13 December 2023).
- Lee, S.T.; Panter, K.E.; Gaffield, W.; Stegelmeier, B.L. Development of an enzyme-linked immunosorbent assay for the veratrum plant teratogens: Cyclopamine and jervine. J. Agric. Food Chem. 2003, 51, 582–586. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Tian, S.; Meng, Q.; Kim, H.M. Histone Demethylase AMX-1 Regulates Fertility in a p53/CEP-1 Dependent Manner. Front. Genet. 2022, 13, 929716. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Tian, S.; Beese-Sims, S.E.; Chen, J.; Shin, N.; Colaiácovo, M.P.; Kim, H.-M. Histone demethylase AMX-1 is necessary for proper sensitivity to interstrand crosslink DNA damage. PLoS Genet. 2021, 17, e1009715. [Google Scholar] [CrossRef]
- Kim, H.M.; Colaiacovo, M.P. DNA Damage Sensitivity Assays in Caenorhabditis elegans. Bio-Protocol 2015, 5, e1487. [Google Scholar] [CrossRef]
- Webster, C.M.; Deline, M.L.; Watts, J.L. Stress response pathways protect germ cells from omega-6 polyunsaturated fatty acid-mediated toxicity in Caenorhabditis elegans. Dev. Biol. 2013, 373, 14–25. [Google Scholar] [CrossRef]
- Shin, N.; Cuenca, L.; Karthikraj, R.; Kannan, K.; Colaiacovo, M.P. Assessing effects of germline exposure to environmental toxicants by high-throughput screening in C. elegans. PLoS Genet. 2019, 15, e1007975. [Google Scholar] [CrossRef]
- Son, M.S.; Taylor, R.K. Growth and maintenance of Escherichia coli laboratory strains. Curr. Protoc. Microbiol. 2012, 27, 5A 4. [Google Scholar]
- Penuelas-Urquides, K.; Villarreal-Trevino, L.; Silva-Ramirez, B.; Rivadeneyra-Espinoza, L.; Said-Fernandez, S.; de Leon, M.B. Measuring of Mycobacterium tuberculosis growth. A correlation of the optical measurements with colony forming units. Braz. J. Microbiol. 2013, 44, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Colaiácovo, M.P.; MacQueen, A.J.; Martinez-Perez, E.; McDonald, K.; Adamo, A.; La Volpe, A.; Villeneuve, A.M. Synaptonemal complex assembly in C. elegans is dispensable for loading strand-exchange proteins but critical for proper completion of recombination. Dev. Cell 2003, 5, 463–474. [Google Scholar] [CrossRef]
- Kelly, K.O.; Dernburg, A.F.; Stanfield, G.M.; Villeneuve, A.M. Caenorhabditis elegans msh-5 is required for both normal and radiation-induced meiotic crossing over but not for completion of meiosis. Genetics 2000, 156, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Hernando, G.; Turani, O.; Bouzat, C. Caenorhabditis elegans muscle Cys-loop receptors as novel targets of terpenoids with potential anthelmintic activity. PLoS Negl. Trop. Dis. 2019, 13, e0007895. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Justiniano, R.; Zhang, D.D.; Wondrak, G.T. The Nrf2-inducers tanshinone I and dihydrotanshinone protect human skin cells and reconstructed human skin against solar simulated UV. Redox Biol. 2013, 1, 532–541. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, L.; Zhu, X.; Qin, Y.; Yu, C.; Jiang, N.; Liu, Y. Luteolin promotes pathogen resistance in Caenorhabditis elegans via DAF-2/DAF-16 insulin-like signaling pathway. Int. Immunopharmacol. 2023, 115, 109679. [Google Scholar] [CrossRef]
- Jin, M.S.; Oldham, M.L.; Zhang, Q.; Chen, J. Crystal structure of the multidrug transporter P-glycoprotein from Caenorhabditis elegans. Nature 2012, 490, 566–569. [Google Scholar] [CrossRef]



| No | Compound | Rt | [M+H]+ | Fragments | Herbs | Types |
|---|---|---|---|---|---|---|
| 1 | Luteolin-7-O-Rucoside | 12.091 | 594.1658 | 286.0477, 301.0713, 463.1224 | V.l. and O.c. | Flavonoids |
| 2 | Thymol | 20.594 | 151.1123 | 77.0408, 91.0574, 105.0714, 107.0540 | V.l. and O.c. | Terpenoids |
| 3 | Dihydrocarvone | 26.597 | 153.1276 | 152.1122 | V.l. and O.c. | Terpenoids |
| 4 | Carvyl acetate | 17.201 | 195.1379 | 153.1283 | V.l. and O.c. | Terpenoids |
| 5 | Menthyl Acetate | 27.991 | 199.1694 | 144.0785, 111.1197, 55.0596, 69.0730 | V.l. and O.c. | Terpenoids |
| 6 | Luteolin | 1.36 | 287.0549 | 168.0056, 140.0109 | V.l. and O.c. | Flavonoids |
| 7 | 2,4,3′,5′-tetrahydroxystilbene | 21.186 | 245.0784 | 228.0723, 180.9145, 140.9176 | V.l. and O.c. | Phenolic compounds |
| 8 | Pilloin | 1.125 | 315.0803 | 182.0429 | V.l. and O.c. | Carotenoid |
| 9 | Linoleic acid | 28.325 | 281.2475 | 248.9901, 151.0287 | V.l. and O.c. | Fatty acids |
| 10 | Homoplantain | 12.761 | 463.1234 | 301.0711, 286.0476 | V.l. and O.c. | Alkaloids |
| 11 | Resveratrol | 23.73 | 229.0858 | 151.0394 | V.l. | Phenolic compounds |
| 12 | Diosmetin | 12.762 | 301.0707 | 286.0476, 168.0044 | V.l. | Lignin |
| 13 | Ferruginol | 27.55 | 287.2376 | 173.1332, 93.0721 | V.l. | Prostaglandins |
| 14 | Tilianin | 18.678 | 447.1293 | 294.1053, 259.1366, 105.0362 | V.l. | Flavonoids |
| 15 | Vitexin | 12.589 | 433.1134 | 286.0474 | V.l. | Flavonoids |
| 16 | Vitexin-2″-O-rhamnoside | 10.538 | 579.1711 | 301.1366, 285.0770 | V.l. | Flavonoids |
| 17 | Vitexin-4″-O-glucoside | 12.091 | 595.1658 | 463.1240, 301.0713, 286.0477 | V.l. | Flavonoids |
| 18 | Naringin | 13.299 | 581.1863 | 273.0768 | V.l. | Flavonoids |
| 19 | Jervine | 11.59 | 426.3007 | 313.2138 | V.l. | Alkaloids |
| 20 | Sugiol | 23.15 | 301.216 | 259.1685, 163.0752 | O.c. | Terpenoids |
| 21 | Dihydrotanshinone I | 19.354 | 279.0933 | 152.1122 | O.c. | Tanshinones |
| 22 | Aucubin | 26.472 | 347.1333 | 216.9976, 129.0189 | O.c. | Iridoids |
| 23 | Paclitaxel | 5.34 | 297.77 | 279.0874 | O.c. | Iridoids |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Q.; Pathak, N.; Ren, X.; Borris, R.P.; Kim, H.-M. Exploring the Impact of Onobrychis cornuta and Veratrum lobelianum Extracts on C. elegans: Implications for MAPK Modulation, Germline Development, and Antitumor Properties. Nutrients 2024, 16, 8. https://doi.org/10.3390/nu16010008
Meng Q, Pathak N, Ren X, Borris RP, Kim H-M. Exploring the Impact of Onobrychis cornuta and Veratrum lobelianum Extracts on C. elegans: Implications for MAPK Modulation, Germline Development, and Antitumor Properties. Nutrients. 2024; 16(1):8. https://doi.org/10.3390/nu16010008
Chicago/Turabian StyleMeng, Qinghao, Nishit Pathak, Xiaojing Ren, Robert P. Borris, and Hyun-Min Kim. 2024. "Exploring the Impact of Onobrychis cornuta and Veratrum lobelianum Extracts on C. elegans: Implications for MAPK Modulation, Germline Development, and Antitumor Properties" Nutrients 16, no. 1: 8. https://doi.org/10.3390/nu16010008
APA StyleMeng, Q., Pathak, N., Ren, X., Borris, R. P., & Kim, H.-M. (2024). Exploring the Impact of Onobrychis cornuta and Veratrum lobelianum Extracts on C. elegans: Implications for MAPK Modulation, Germline Development, and Antitumor Properties. Nutrients, 16(1), 8. https://doi.org/10.3390/nu16010008








